2. 中日友好医院呼吸与危重症医学科,北京 100029
2. Department of Respiratory and Critical Care Medicine, China-Japan Friendship Hospital, Beijing 100029, China
支气管哮喘是以慢性气道炎症为特征的一种异质性疾病,临床表现为随时间改变的气喘、胸闷和咳嗽,伴有可逆性的气流受限,影响全球3亿多的哮喘患者[1]。研究发现哮喘患者的外周血和肺组织中嗜酸性粒细胞(EOS)数量明显升高[2-3],释放的炎性介质损伤上皮细胞,诱导支气管收缩,粘液产生和增加血管通透性,还趋化并诱导2型辅助T淋巴细胞免疫应答和参与气道重塑,且数量的多少与哮喘的严重程度呈正相关[4-5]。促进EOS的凋亡有助于气道炎症的消退,糖皮质激素是支气管哮喘的主要治疗药物,除广泛的抗炎机制外,还能够促进EOS的凋亡[6]。细胞凋亡受多种基因的调控,其中Bcl-2和Fas被认为是细胞凋亡的重要调控基因[7-8]。青蒿琥酯是抗疟疾药物,是具有倍半萜内酯结构的青蒿素衍生物。大量研究发现青蒿琥酯除了抗疟疾以外,还具有抗炎[9]、抗菌[10]、抗肿瘤[11]和抗纤维化[12]的作用。近年来发现青蒿琥酯能够减少哮喘小鼠支气管肺泡灌洗液(BALF)和肺组织中EOS的数量,从而改善哮喘的气道炎症和气道高反应性,但EOS减少的机制尚未阐明。本实验通过观察青蒿琥酯对哮喘小鼠EOS凋亡及相关基因Fas和Bcl-2表达的影响,初步探讨青蒿琥酯降低EOS和减轻气道炎症的机制。
1 材料和方法 1.1 动物无特定病原体(SPF)级6~8周龄雌性BALB/c小鼠,体质量16~18 g,购自北京维通利华公司。动物合格证号:NO.11400700355487。按随机数字表法分为对照组,哮喘组和青蒿琥酯组,每组10只。
1.2 主要材料及仪器卵原蛋白(OVA)、氢氧化铝(Sigma);青蒿琥酯注射液(桂林南药);APC-Cy7-CD45抗体,FITC-CD11b抗体和AF-647-siglce-F抗体(BD);细胞凋亡检测试,剂盒(友谊中联);Fas抗体(武汉三鹰);Bcl-2抗体(博士德)。
1.3 哮喘模型的建立按照参考文献进行动物造模[13],对照组在第0、14天给予腹腔注射生理盐水0.2 mL致敏,哮喘组和青蒿琥酯组腹腔注射OVA与氢氧化铝混悬液0.2 mL致敏(含OVA20 μg和氢氧化铝2 mg),对照组第21~28天给予无菌磷酸盐(PBS)10 mL进行雾化激发,哮喘组和青蒿琥酯组给予溶于无菌PBS的1%OVA10 mL进行雾化激发,每次30 min。对照组、模型组、青蒿琥酯组第21~28天每次雾化激发前1 h分别给予腹腔注射生理盐水、生理盐水、青蒿琥酯(30 mg/kg)[14-16]0.2 mL。
1.4 肺组织病理末次激发24 h后,取小鼠左肺组织固定在4%多聚甲醛,48 h后脱水、石蜡组织包埋、切片,每个切片大约4 μm厚,行HE染色,中性树胶封片。
1.5 流式细胞术末次激发24 h后,1%戊巴比妥钠腹腔注射麻醉,分离气管组织,结扎左肺,气管插管行BALF,加入抗体APC-cy7-CD451 μg(5 μL),FITC-CD11b 1 μg(2 μL),AF-647-siglec-F 1 μg(5 μL),4 ℃孵育30 min。离心200×g 5 min;去上清,留沉淀;加入1.5 mL的1×PBS;离心200×g 5 min;去上清,留沉淀;结合缓冲液重新悬浮细胞,取细胞悬液于5 mL流式管中,加入5 μLAnnexin V-PE,混匀后于室温避光孵育5 min;加入10 μL的7-AAD溶液;在1 h内流式细胞仪分析。
1.6 TUNEL法细胞凋亡原位检测本实验参照TUNEL试剂盒说明进行操作,主要步骤如下:(1)石蜡切片经二甲苯Ⅰ和二甲苯Ⅱ脱蜡及梯度酒精脱水,PBS漂洗5 min×3次;(2)pH6.0的柠檬酸缓冲液微波修复15 min,自然晾凉,蛋白酶K37 ℃消化30 min,PBS漂洗5 min×3次;(3)3%H2O2封闭液封闭10 min,PBS漂洗5 min×3次;(4)DNaseI反应液制作阳性对照片,反应10 min,PBS漂洗5 min×3次;(5)实验组和阳性对照片分别加入TdT酶反应液50 μL,37 ℃避光反应60 min,PBS漂洗5 min×3次;(6)加入StreptavidinHRP工作液,37 ℃避光反应30 min,PBS漂洗5 min×3次;(7)配制DAB工作液,加入适量的DAB工作液显色至阳性对照显色良好时终止呈色反应;(8)复染细胞核,中性树胶封片。
1.7 免疫组织化学染色(1)石蜡切片经二甲苯I和二甲苯II脱蜡及梯度酒精脱水,PBS缓冲液冲洗3 min×3次;(2)pH6.0的柠檬酸缓冲液微波修复15 min,自然晾凉,PBS缓冲液冲洗3 min×3次;(3)加入适量的内源性过氧化物酶阻断剂,室温孵育10 min,PBS缓冲液冲洗3 min×3次;(4)滴加Bcl-2(1:200)和Fas抗体(1:1600)湿盒孵育2 h,PBS缓冲液冲洗3 min×3次;(5)滴加适量的反应增强液,室温孵育20 min,PBS缓冲液冲洗3 min×3次;(6)滴加适量的增强酶标山羊抗兔IgG聚合物,室温孵育20 min,PBS缓冲液冲洗3 min×3次;(7)DAB显色30 s,终止显色反应;(8)苏木素复染细胞核,脱水、透明、中性树胶封片。
1.8 结果处理TUNEL法检测嗜酸性粒细胞凋亡,光镜下(×400)随机选取5个互不重叠视野,计数凋亡细胞,计算凋亡指数(AI)=阳性细胞(/阳性细胞+阴性细胞)×100%。光学显微镜下观察Fas、BCL-2阳性细胞表达情况,用image pro-plus图像分析软件测定肺组织Fas、Bcl-2表达的阳性细胞总面积(Area)和积分光密度(IOD)。
1.9 统计学方法实验数据用均数±标准差表示,应用SPSS 25.0统计软件对数据进行分析,多组间比较采用单因素方差分析,组间两两比较采用t检验,P<0.05为差异有统计学意义。
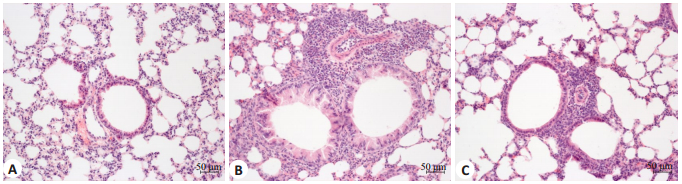
2 结果 2.1 肺组织形态学结果对照组小鼠肺组织切片支气管、肺组织结构大致正常,支气管周围和肺泡间隔未见明显炎性细胞浸润,哮喘组可见支气管壁增厚,支气管黏膜上皮水肿,变性,支气管内可见脱落的上皮细胞及分泌物,支气管周围和肺泡间隔可见大量炎性细胞和EOS浸润,青蒿琥酯组小鼠支气管黏膜水肿减轻,支气管周围和肺泡间隔炎症细胞和EOS较哮喘组明显减少(图 1)。
|
图 1 各组小鼠肺组织的病理变化 Fig.1 Pathological changes of the lung tissue in different groups (Original magnification:×200). A: In the control group, the lumen of the bronchus was smooth and the epithelium of the bronchus was intact with mucosa edema of the bronchus; no obvious inflammatory cell infiltration was seen around the bronchus or the alveolar septum; B: In asthmatic group, goblet cell hyperplasia and mucosal epithelial edema are observed with numerous inflammatory cells and eosinophils infiltrating the bronchus and the alveolar septum; C: In artesunate group, bronchial mucosa edema is lessened with reduced mucus secretion and significantly decreased inflammatory cells and eosinophils around the bronchus and the alveolar septum compared with asthmatic group. |
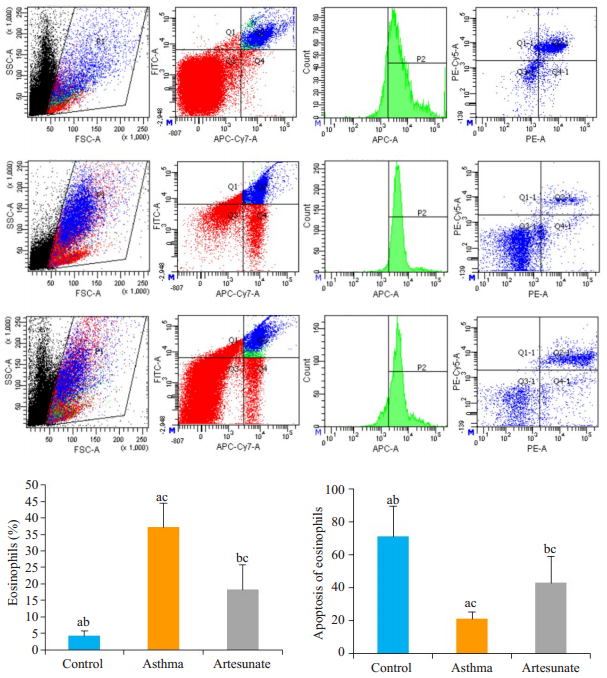
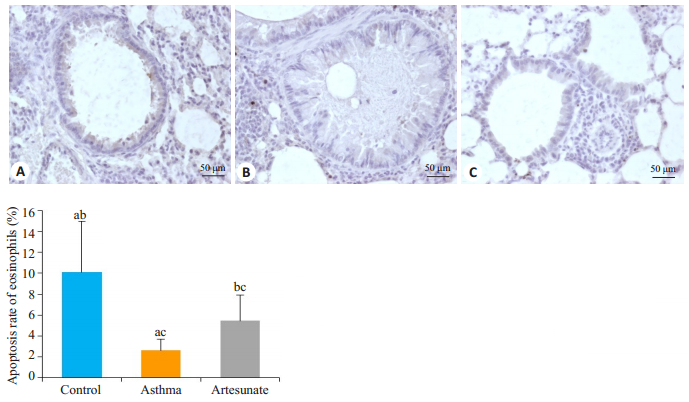
流式细胞术发现3组小鼠EOS的百分率和凋亡率均有差异,差异有统计学意义(F=15.348,39.517,均P<0.01)。哮喘组EOS百分率明显高于对照组(37.12±7.38 vs 4.26±1.55,t=-8.712,P<0.05),凋亡率低于对照组(21.15±4.04 vs 71.03±18.46,t=5.278,P<0.05)。与哮喘组比较,青蒿琥酯组EOS的百分率下降(18.26±7.40 vs 37.12±7.38,t=-3.803,P<0.05),而凋亡率升高(43.06±16.15 vs 21.15±4.04,t=2.615,P<0.05),差异有统计学意义(图 1)。肺组织TUNEL染色发现哮喘组EOS的凋亡率明显低于对照组(2.59±1.09 vs 10.11±4.91,t=5.597,P<0.05),而青蒿琥酯组EOS的凋亡率较哮喘组有所上升(5.45±2.44 vs 2.59±1.09,t=-3.999,P<0.05),差异有统计学意义(图 2)。
|
图 2 各组小鼠肺泡灌洗液流式细胞术检测Eos凋亡 Fig.2 Detection of apoptosis of EOS in the alveolar lavage fluid by flow cytometry. From the top to the bottom: control group, asthma group, and artesunate group; Asthma group compared with control group, aP < 0.05; Artesunate group compared with control group, bP < 0.05; Artesunate group compared with asthma group, cP < 0.05. |
|
图 3 各组小鼠肺组织TUNEL染色 Fig.3 TUNEL staining of the lung tissues of the mice (×400). A: Control group; B: Asthma group; C: Artesunate group. Asthma group compared with control group, aP < 0.05; Artesunate group compared with control group, bP < 0.05; Artesunate group compared with Asthma group, cP < 0.05. |
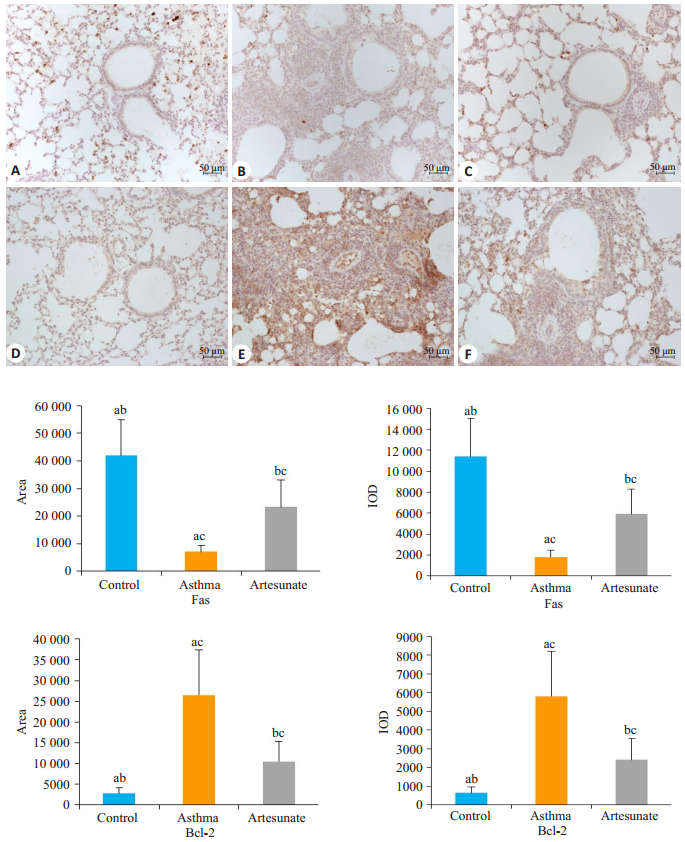
图 4可知,3组小鼠肺组织中Fas蛋白的阳性面积和IOD不同,差异有统计学意义(F=53.937,53.309,均P<0.05)。哮喘组和青蒿琥酯组的阳性面积和IOD均低于对照组(7067±2343和23 274±9852 vs 41 980±12 980,1811±630和5921±2389 vs 11 378±3640,t=8.774,3.762,8.583,4.102,均P<0.05)。青蒿琥酯组肺组织中Fas蛋白的阳性面积和IOD值高于哮喘组(23 274±9852 vs 7067±2343,5921±2389 vs 1811±630,t=-5.313,-5.528,均P<0.05),差异有统计学意义。与Fas蛋白表达相反,哮喘组肺组织中Bcl-2蛋白的表达量最高,青蒿琥酯组次之,正常组最少,3组之间肺组织Bcl-2的阳性面积和IOD有明显差异,差异具有统计学意义(F=24.712,23.771,均P<0.05)。与哮喘组比较,青蒿琥酯组Bcl-2的阳性面积(10 480±4892 vs 26 508±10 856,t=3.630,P<0.05)和IOD(2410±1139 vs 5805±2411,t=3.439,P<0.05)降低。
|
图 4 三组小鼠Fas蛋白(A~C)和Bcl-2蛋白(D~F)的表达情况 Fig.4 Expression of Fas protein (A, B, and C) and Bcl-2 protein (D, E, and F) in the 3 groups of mice (×200). The control group (A, D). The asthma group (B, E). The artesunate group (C, F) Asthma group compared with control group, aP < 0.05; Artesunate group compared with control group, bP < 0.05; Artesunate group compared with asthma group, cP < 0.05. |
EOS在哮喘的病理机制中起重要作用,其产生的细胞因子和炎性介质与其他介质和结构细胞相互作用共同促进哮喘的发生发展[17]。研究发现哮喘患者气道和肺组织中EOS明显增加,并通过炎症介质的组合和局部微环境的变化而寿命延长,且EOS凋亡降低与人类哮喘严重程度增加相关[18-20]。凋亡是一种非炎症性的死亡方式,形成的凋亡小体被巨噬细胞、树突细胞和上皮细胞清除[21-23]。研究发现糖皮质激素可增强EOS凋亡和随后的体外清除[24-25],即使对糖皮质激素反应不佳的哮喘患者,增加EOS的凋亡仍能有效改善哮喘的症状,提高控制率[26]。以后,哮喘治疗的重点可能是开发针对特定哮喘表型的药物,EOS炎症表型占据绝大多数,产生Th2极化的驱动因子并且通过作为抗原呈递细胞起作用,刺激Th细胞活化,增殖和产生IL-4,IL-5和IL-13等炎症因子,促进疾病的进展[17, 27]。因此,促进哮喘患者EOS的凋亡是有益的治疗方式。
本研究发现青蒿琥酯能够减轻哮喘小鼠肺组织的炎症状态,降低EOS浸润,这与之前的研究一致。但是EOS减少的机制是不清楚的,Cheng等[15]认为EOS减少可能是相关因子如IL-4,IL-5和IL-13等被抑制从而影响下游PI3k/Akt信号通路的结果,但尚未见到有关EOS凋亡方面的研究,我们假设青蒿琥酯参与了EOS的凋亡过程。流式检测结果发现,与哮喘组比较,青蒿琥酯组BALF中EOS的比例降低,同时EOS的凋亡率升高,提示青蒿琥酯促进了EOS的早期凋亡。中晚期细胞凋亡DNA发生断裂,在外源性末端脱氧核苷酸转移酶催化的反应中,将生物素配基的脱氧核苷酸特异地结合于DNA断裂的3'- OH末端,因此我们对肺组织进行TUNEL法检测,结果发现青蒿琥酯组EOS细胞的凋亡率高于哮喘组,实验表明青蒿琥酯能够促进哮喘小鼠EOS的早期和中晚期凋亡,从而减少了肺组织EOS的浸润。
细胞凋亡的调控机制是多方面的,主要通过两个相互关联的内在(线粒体依赖)和外在(线粒体非依赖)途径发生,通过将FasL连接到膜结合的Fas受体来启动,触发由Fas,FasL,Fas相关死亡域蛋白和procaspase 8组成的死亡诱导信号复合物的形成,导致EOS凋亡[28]。Oh等[29]研究发现激活Fas导致Ser194快速磷酸化,加速caspase 8和Bcl-2相互作用蛋白裂解有助于EOS的凋亡,从而改善哮喘的气道炎症和气道高反应状态。Bcl-2是与EOS凋亡相关的重要的抑凋亡基因,Bcl-2蛋白能够阻断细胞色素c的释放进而起到抗凋亡作用。给予Bcl-2抑制剂后,哮喘的气道炎症和气道高反应性能得到明显改善[30]。因此我们通过实验观察了Fas和Bcl-2的表达情况。结果显示哮喘组小鼠肺组织中Fas蛋白的表达量减少,而Bcl-2蛋白的表达量增多,提示EOS凋亡受损可能与促凋亡蛋白(Fas)的表达量减少和抗凋亡蛋白(Bcl-2)的增多有关。给予青蒿琥酯治疗后,Fas蛋白的表达量增多,Bcl-2蛋白的表达量降低,虽未达到对照组水平,但较哮喘组有明显的改善。本研究发现青蒿琥酯能够促进哮喘小鼠气道和肺组织EOS的凋亡,可能与促进Fas蛋白和抑制Bcl-2蛋白的表达有关,但具体的机制仍需进一步探讨。
综上,青蒿琥酯可能通过调节促凋亡基因Fas和抑凋亡基因Bcl-2的表达诱导EOS的凋亡,降低哮喘小鼠肺组织中EOS的数量,从而改善哮喘的炎症状态。
| [1] |
Boulet LP, Reddel HK, Bateman E, et al. Global initiative for asthma (GINA): 25 years late[J]. 2019. 54(2): Pil: 190058.
|
| [2] |
Erkman J, Vaynblat A, Thomas K, et al. Airway and esophageal eosinophils in children with severe uncontrolled asthma[J]. Pediatr Pulmonol, 2018, 53(12): 1598-603. DOI:10.1002/ppul.24180 |
| [3] |
Mazzeo C, Cañas JA, Zafra MP, et al. Exosome secretion by eosinophils: A possible role in asthma pathogenesis[J]. J Allergy Clin Immunol, 2015, 135(6): 1603-13. DOI:10.1016/j.jaci.2014.11.026 |
| [4] |
Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations[J]. Am J Respir Crit Care Med, 2017, 195(3): 302-13. DOI:10.1164/rccm.201602-0419OC |
| [5] |
Shrimanker R, Keene O, Hynes G, et al. Prognostic and predictive value of blood eosinophil count, fractional exhaled nitric oxide, and their combination in severe asthma: a post Hoc analysis[J]. Am J Respir Crit Care Med, 2019, 200(10): 1308-12. DOI:10.1164/rccm.201903-0599LE |
| [6] |
Zhang X, Moilanen E, Kankaanranta H. Enhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasone[J]. Eur J Pharmacol, 2000, 406(3): 325-32. DOI:10.1016/S0014-2999(00)00690-7 |
| [7] |
Duez C, Tomkinson A, Shultz LD, et al. Fas deficiency delays the resolution of airway hyperresponsiveness after allergen sensitization and challenge[J]. JAllergy Clin Immunol, 2001, 108(4): 547-56. DOI:10.1067/mai.2001.118288 |
| [8] |
Lee HJ, Lee EK, Seo YE, et al. Roles of Bcl-2 and caspase-9 and-3 in CD30-induced human eosinophil apoptosis[J]. J Microbiol Immunol Infection, 2017, 50(2): 145-52. DOI:10.1016/j.jmii.2015.05.017 |
| [9] |
Sun Z, Ma Y, Chen F, et al. Artesunate ameliorates high glucoseinduced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway[J]. Chem Biol Interact, 2018, 293: 11-9. DOI:10.1016/j.cbi.2018.07.011 |
| [10] |
Yun YX, Ch en, Li LN, et al. Effect of artesunate supplementation on bacterial translocation and dysbiosis of gut microbiota in rats with liver cirrhosis[J]. World J Gastroenterol, 2016, 22(10): 2949-59. DOI:10.3748/wjg.v22.i10.2949 |
| [11] |
Von Hagens C, Walter-Sack I, Goeckenjan M, et al. Long-term addon therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2) J]. Phytomedicine, 2019, 54: 140-8.
|
| [12] |
Kong ZY, Liu R, Cheng YR. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway[J]. Biomed Pharmacother, 2019, 109: 2043-53. DOI:10.1016/j.biopha.2018.11.030 |
| [13] |
Reddy AT, Lakshmi SP, Reddy RC. Murine model of allergen induced asthma[J]. J Vis Exp, 2012(63): e3771. |
| [14] |
Cheng C, Ho WE, Goh FY, et al. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3- kinase/Akt pathway[J]. PLoS One, 2011, 6(6): e20932. DOI:10.1371/journal.pone.0020932 |
| [15] |
Cheng C, Ng DS, Chan TK, et al. Anti-allergic action of anti-malarial drug artesunate in experimental mast cell- mediated anaphylactic models[J]. Allergy, 2013, 68(2): 195-203. DOI:10.1111/all.12077 |
| [16] |
Luo Q, Lin J, Zhang L, et al. The anti-malaria drug artesunate inhibits cigarette smoke and ovalbumin concurrent exposure-induced airway inflammation and might reverse glucocorticoid insensitivity[J]. Int Immunopharmacol, 2015, 29(2): 235-45. |
| [17] |
Akuthota P, Wang HB, Spencer LA, et al. Immunoregulatory roles of eosinophils: a new look at a familiar cell[J]. Clin Exp Allergy, 2008, 38(8): 1254-63. DOI:10.1111/j.1365-2222.2008.03037.x |
| [18] |
Duncan CJ, Lawrie A, Blaylock MG, et al. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity[J]. Eur Respir J, 2003, 22(3): 484-90. DOI:10.1183/09031936.03.00109803a |
| [19] |
Pontin J, Blaylock MG, Walsh GM, et al. Sputum eosinophil apoptotic rate is positively correlated to exhaled nitric oxide in children[J]. Pediatr Pulmonol, 2008, 43(11): 1130-4. DOI:10.1002/ppul.20921 |
| [20] |
Woolley KL, Gibson PG, Carty K, et al. Eosinophil apoptosis and the resolution of airway inflammation in asthma[J]. Am J Respir Crit Care Med, 1996, 154(1): 237-43. DOI:10.1164/ajrccm.154.1.8680686 |
| [21] |
Juncadella IJ, Kadl A, Sharma AK, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation[J]. Nature, 2013, 493(7433): 547. DOI:10.1038/nature11714 |
| [22] |
Leitch AE, Duffin R, Haslett C, et al. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa[J]. Mucosal Immunol, 2008, 1(5): 350-63. DOI:10.1038/mi.2008.31 |
| [23] |
Poon IK, Lucas CD, Rossi AG, et al. Apoptotic cell clearance: basic biology and therapeutic potential[J]. Nat Rev Immunol, 2014, 14(3): 166-80. DOI:10.1038/nri3607 |
| [24] |
Druilhe A, Létuvé S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action[J]. Apoptosis, 2003, 8(5): 481-95. DOI:10.1023/A:1025590308147 |
| [25] |
Mccoll A, Bournazos S, Franz S, et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages[J]. J Immunol, 2009, 183(3): 2167-75. DOI:10.4049/jimmunol.0803503 |
| [26] |
Ilmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma[J]. Basic Clin Pharmacol Toxicol, 2014, 114(1): 109-17. DOI:10.1111/bcpt.12163 |
| [27] |
Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease[J]. Clin Exp Allergy, 2008, 38(5): 709-50. DOI:10.1111/j.1365-2222.2008.02958.x |
| [28] |
Tian BP, Li F, Li R, et al. Nanoformulated ABT-199 to effectively target Bcl-2 at mitochondrial membrane alleviates airway inflammation by inducing apoptosis[J]. Biomaterials, 2019, 192: 429-39. DOI:10.1016/j.biomaterials.2018.06.020 |
| [29] |
Oh J, Malter JS. Pin1-FADD interactions regulate Fas-Mediated apoptosis in activated eosinophils[J]. J Immunol, 2013, 190(10): 4937-45. DOI:10.4049/jimmunol.1202646 |
| [30] |
Tian BP, Xia LX, Bao ZQ, et al. Bcl-2 inhibitors reduce steroidinsensitive airway inflammation[J]. J Allergy Clin Immunol, 2017, 140(2): 418-30. DOI:10.1016/j.jaci.2016.11.027 |
 2020, Vol. 40
2020, Vol. 40

