2. 南方医科大学南方医院影像中心,广东 广州 510515
2. Medical Imaging Center, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China
肺结核在不同的病期其CT影像表现多种多样,其中CT上呈现为肺实变肿块的活动性肺结核与孤立性肺癌很难鉴别[1-2]。两者的治疗方案、预后完全不同,肺结核经规律抗结核药物治疗并必要时辅以手术切除则可治愈,但孤立性肺癌通常需采取手术治疗和/或放化疗才能达到较好的治疗效果[3-4],因此治疗前准确鉴别这两种疾病非常重要。18氟-脱氧葡萄糖(18F-FDG)正电子发射型断层/计算机断层扫描(PET/CT)显像是恶性肿瘤诊断、鉴别诊断、分期及疗效评价的重要显像技术,可以准确地显示、定量病变的葡萄糖代谢状态[5-7]。在鉴别诊断中,大多数研究一般仅根据病灶对18F-FDG的摄取高低来判断病变的良恶性[8-13],但文献报道,活动性肺结核与肺癌一样均可表现为18F-FDG高摄取,无法单纯根据病灶18F-FDG摄取高低将两者准确区分[14-15]。
PET/CT是PET和CT的整合型显像技术,既能提供PET代谢信息,又能通过CT将病灶的解剖学细节清楚地显示,将PET和CT两种影像所提供的多种信息相结合,可能比单纯地根据18F-FDG摄取高低更能准确地鉴别肿块型活动性肺结核和孤立性肺癌,但这在国内外尚未见深入研究。因此,本研究回顾性地分析了138例肺癌和肿块型活动性结核病灶的PET/CT显像结果,通过PET/CT的形态学和功能学多因素分析,探索、建立鉴别两者的新方法及可行性。
1 资料和方法 1.1 研究对象肺结核与肺癌的纳入标准:肺癌与肺结核病灶均经穿刺活检或手术获得病理学诊断依据;病灶在CT上表现为结节状或者块状,病灶直径小于或接近3 cm,但不超过3.5 cm。
肺结核患者排除标准:病灶为非活动性结核;病灶呈现为片状、散在、非实性病灶;患者接受过抗结核治疗。肺癌患者排除标准:病灶为磨玻璃样或混杂密度结节;病灶呈现为片状、散在、非实性病灶;患者接受过针对肿瘤的治疗;发生淋巴结转移或远处转移。其他排除标准:同时有肺癌和结核并存者;患者的临床数据不完整;经后处理不能提取完整的代谢参数。
按照上述纳入及排除标准收集2013年1月~2018年10月在本中心行18F-FDG PET/CT检查的患者,共收集到肺结核患者74例,其中男49例,女25例,年龄23~ 77岁(平均57.73岁),59例经手术病理确认,15例经活检病理确认,根据临床和影像学表现诊断为活动性肺结核。肺癌患者64例,其中男26例,女38例,年龄28~75岁(平均57.81岁),57例经手术病理确认,7例经活检病理确认,均为腺癌。
1.2 18F-FDG PET/CT显像138例患者均采用Biograph mCTx (Siemens)扫描仪进行PET/CT检查。18F-FDG由PETtrace回旋加速器(GE)和18F-FDG化学合成模块(GE)自动合成,放化纯度>95%。
患者空腹6 h以上,平静状态下按质量通过三通管经静脉注射18F-FDG 270~410 MBq (5.55 MBq/kg),暗室内静卧约1 h,排尿后行PET/CT显像。显像包括CT平扫及PET发射扫描,扫描范围从大腿中段至颅顶,必要时加扫双下肢。Biograph mCTx采集参数:CT电压120 kV、电流为自动毫安、螺距0.55、球管单圈旋转时间为1.0 s,层厚为3 mm;PET发射扫描采用三维采集,2 min/床位。
PET重建采用有序子集最大期望值迭代法(OSEM),图像衰减校正采用CT扫描数据。CT图像采用标准法重建,重建层厚为2.0 mm (Biograph mCTx)。将PET和CT图像传送到Syngo MMWP工作站,进行帧对帧图像对位融合。
1.3 图像分析及相关指标所有PET/CT图像均由2名有经验的PET/CT诊断医师进行独立评价,再由1名有丰富临床经验的PET/ CT高年资诊断医师进行复核。对病灶在非增强CT图像上进行分析,分析时设定肺窗的窗宽为1200 HU,窗位为-600 HU;纵隔窗的窗宽为300 HU,窗位为40 HU。分析病灶的基本形态,如大小[以最长径表示(cm)]、边缘征象(分叶征、边缘是否光滑)、内部特征(空洞、空泡征),CT纵隔/肺窗直径比值。同时分析病灶的PET代谢特征,如病灶SUVmax、病灶代谢是否高于纵隔和病灶内是否存在明显放射性缺损。
SUVmax的测定通过在PET图像上针对病灶设感兴趣区间(ROI),然后由分析工作站自动计算出最大标准摄取值SUVmax。
1.4 统计学分析统计学分析由统计软件包SPSS 20.0版完成,计量资料用均数±标准差表示,计数资料用率表示。单因素分析采用χ2检验或两样本t检验。多因素分析用Logistic多元回归(前进法)分析。以P<0.05为差异有统计学意义。用ROC曲线评价PET/CT多元回归概率的诊断效率。
进行Logistic分析时,把肺结核、肺癌作为因变量(肺结核=1,肺癌=0),把年龄(岁)、最长径(cm)、SUVmax等数值变量及病灶是否存在放射性缺损、代谢是否高于纵隔、是否存在分叶征、空洞、空泡征等二分类变量(无=0,有=1或者否=0,是=1)作为自变量。对上述PET/CT征象进行多元回归分析,建立Logistic回归方程并创建ROC曲线,以回归方程的概率值判定肺结核与肺癌的最佳临界值,并评价PET/CT多元回归方程的诊断效率。
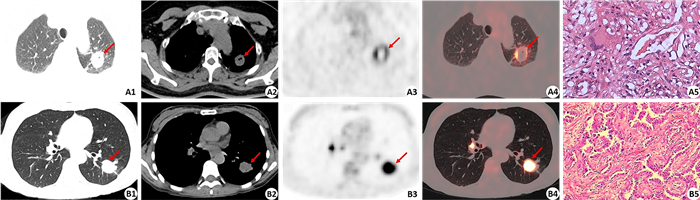
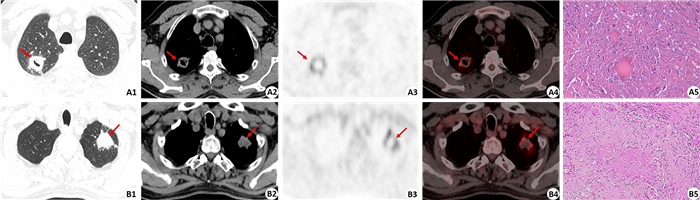
2 结果 2.1 肿块型活动性结核病灶的18F-FDG PET/CT图像表现74个活动性结核病灶中,病灶大小为(2.28±0.99)cm (表 1)。在PET图像上,所有病灶均可见18F-FDG摄取增高,其中52个(70.3%)病灶代谢明显增高(超过纵隔血池放射性),22个(29.7%)病灶代谢轻度增高(接近或稍低于纵隔血池放射性)。病灶的SUVmax为7.52±5.08,67个(90.5%)病灶的SUVmax大于2.5,7个(9.5%)病灶的SUVmax小于2.5。25个(33.8%)病灶内存在明显的放射性缺损(图 1、2)。在CT上,病灶呈现为边缘光滑者(图 3) 38个(51.4%),呈分叶状者27个(36.5%),病灶内有空泡征者12个(16.2%),有空洞者(图 1、2) 10个(13.5%),CT纵隔/肺窗直径比值为0.81±0.19。
| 表 1 肿块型肺活动性结核与肺癌的临床和18F-FDG PET/CT征象单因素分析 Tab.1 Univariate analysis of the clinical data and 18F-FDG PET/CT parameters of massive type active tuberculosis and lung cancer |
|
图 1 肿块型活动性肺结核和肺癌的病灶PET放射性缺损征象 Fig.1 Radioactive defect in massive type active tuberculosis and lung cancer. A1-A4: 18F-FDG PET/CT images of a massive type active tuberculosis in the upper lobe of the left lung, including CT images of the lung window and the mediastinal window, PET image and PET/CT fused image (CT lung window) (arrows indicate the lesion). The size of the lesion was 2.6 cm×2.0 cm×2.4 cm with moderate 18F-FDG uptake (SUVmax of 5.7). The lesion had a small cavity with smooth inner wall on CT image (A2) and a more obvious radioactive defect on PET image (A3). Histopathological examination confirmed the lesion as tuberculosis (A5) (HE staining, original magnification: ×400). B1-B4: 18F-FDG PET/CT images of lung cancer in the lower lobe of the left lung, including CT images of the lung window and the mediastinal window, PET image and PET/CT fused image (CT lung window) (arrow indicates the lesion). The size of the lesion was 2.6 cm×2.3 cm×2.1 cm with intense 18F-FDG uptake (SUVmax of 11.8). The lesion had no any cavity and the border was rough, lobulated and speculated on CT image (B2). There was no radioactive defect in the lesion on PET image (B3). Histopathological examination confirmed the lesion as adenocarcinoma (B5) (HE staining, ×200). |
|
图 2 肿块型活动性肺结核病灶的PET放射性缺损征象 Fig.2 Radioactive defect in the massive type active tuberculosis. A1-A4: 18F-FDG PET/CT showed massive type active tuberculosis located in the upper lobe of the right lung, including CT image of the lung window, CT image of the mediastinal window, PET image and PET/CT fused image (CT lung window) (arrows indicate the lesion). The size of the lesion was 2.9 cm×2.6 cm×3.8 cm with moderate 18F-FDG uptake (SUVmax of 6.5). The lesion had a small cavity with smooth inner wall on CT image (A2) and a more obvious radioactive defect on PET image (A3). Histopathological examination confirmed the lesion as tuberculosis (A5) (HE staining, ×200). B1-B4: 18F-FDG PET/CT showed massive type active tuberculosis located in the upper lobe of the left lung, including CT image of lung window, CT image of mediastinal window, PET image and PET/CT fused image (CT lung window) (arrows indicate the lesion). The size of the lesion was 3.2 cm × 2.4 cm × 2.0 cm with moderate 18F-FDG uptake (SUVmax of 8.1). The lesion had no cavity on CT image (B2) but an obvious radioactive defect on PET image (B3). Histopathological examination confirmed the lesion as tuberculosis (B5) (HE staining, ×100). |
|
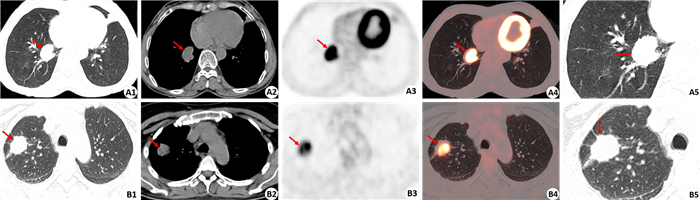
图 3 肿块型活动性肺结核和肺癌的病灶边缘征象 Fig.3 Margin characteristics of massive type active tuberculosis and lung cancer. A1-A5: 18F-FDG PET/CT images of a massive type active tuberculosis in the right lung, including CT image of the lung window, CT image of the mediastinal window, PET image, PET/CT fused image (CT lung window), and a closer observation of the CT image of the lung window (arrow: lesion). The size of lesion was 3.0 cm×2.7 cm×3.0 cm with intense 18F-FDG uptake (SUVmax of 9.1). CT images showed the lesion had a smooth margin without any lobulation (A1, A2, A5). B1-B5: 18F-FDG PET/CT images of lung cancer in the right lung, including CT image of the lung window, CT image of the mediastinal window, PET image, PET/CT fused image (CT lung window), and a closer observation of the CT image of the lung window (arrow: lesion). The size of lesion was 2.5 cm×2.0 cm×2.6 cm with intense 18F-FDG uptake (SUVmax of 8.9). CT images showed the lesion had a rough and lobulated margin (B1, B2, B5). |
单因素分析结果显示,肺结核病灶与肺癌病灶在SUVmax、代谢是否明显高于纵隔血池、患者年龄、病灶大小、是否存在空洞以及CT纵隔/肺窗比值等方面差异无统计学意义(P>0.05,表 1)。但在性别、有无放射性缺损、空泡征、边缘是否光滑和分叶征等差异有统计学意义(P<0.05,表 1)。肺结核多见于男性,而肺癌多见于女性,两者的性别差异有统计学意义(χ2=9.059,P=0.003,表 1)。
在PET征象上,肺结核组33.8%病灶存在放射性缺损(图 1),而肺癌组放射性缺损仅见于3.1%病灶,两者差异有统计学意义(χ2=20.498,P=0.000,表 1)。活动性结核病灶出现PET放射性缺损的发生率(33.8%)明显高于CT图像上的空洞征象发生率(13.5%),另外PET所显示的放射性缺损的面积也常较CT上所显示的空洞面积大(图 1、2)。在CT征象上,空泡征仅见于12个(16.2%)结核病灶,而见于24个(37.5%)肺癌病灶,差异有统计学意义(χ2=8.063,P=0.005)。38个(51.4%)结核病灶呈现为边缘光滑,而肺癌病灶边缘光滑者仅见于2个(3.1%)病灶(图 3),差异有统计学意义(χ2=38.777,P=0.000)。病灶边缘呈现分叶征见于58个(90.6%)肺癌病灶(图 3),而此征象仅见于27个(36.5%)结核病灶,差异有统计学意义(χ2=42.522,P=0.000)。
2.3 肿块型活动性结核病灶与肺癌病灶的18F-FDG PET/CT多因素分析及鉴别诊断模型的建立多因素分析结果显示,虽然在单因素分析中发现有5个变量在活动性结核和肺癌病灶两者差异有统计学意义,但只有4个变量在多因素分析中显示对区分活动性结核和肺癌有意义(P<0.05,表 2),空泡征对鉴别肺结核和肺癌无意义(P>0.05)。4个变量的OR值自强至弱分别为放射性缺损(OR=28.294)、边缘光滑(OR=17.226)、性别(OR=7.229)、和分叶征(OR=0.121,表 2)。
| 表 2 肿块型肺活动性结核与肺癌的临床和18F-FDG PET/CT征象多因素Logistic回归分析 Tab.2 Multivariate analysis of the clinical data and 18F-FDG PET/CT parameters of massive type active tuberculosis and lung cancer (a=0.05, Forward: LR) |
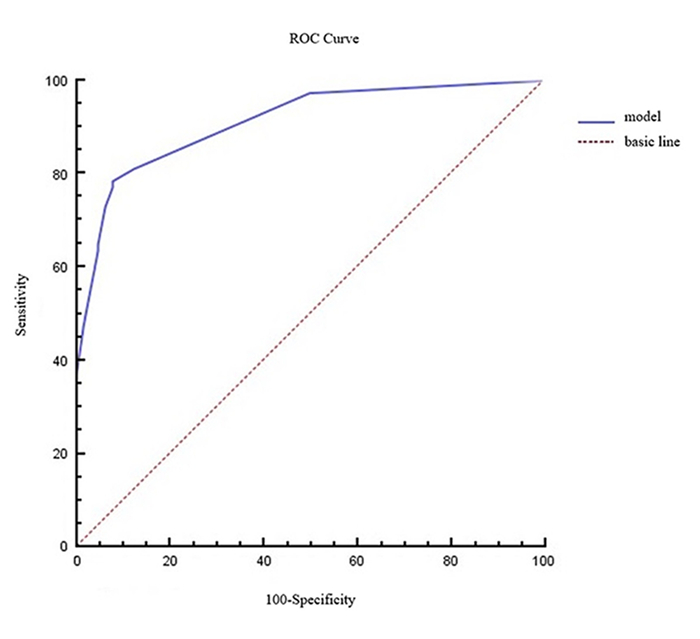
将此4个变量纳入Logistic回归方程,建立鉴别诊断活动性肺结核和肺癌的回归模型公式为:P=1/(1+e-x),P代表病灶为肺结核的概率,数值越接近1,肺结核的可能越大。X=-0.530 + 1.978 ×性别+ 3.343 ×放射性缺损+ 2.846×边缘光滑-2.116×分叶征。模型的拟合优度Nagelkerke R2=0.649,Hosmer-Lemeshow统计量为0.560,P=0.967,模型拟合良好。该模型的ROC曲线显示(图 4),ROC曲线下的面积为0.911 (95%CI: 0.864~ 0.959)。用于诊断肿块型活动性肺结核的灵敏度为78.4%(58/74),特异度为92.2%(59/64),准确率为84.8% (117/138),阳性预测值为92.1% (58/63),阴性预测值为78.7% (59/75)。
|
图 4 根据肿块型活动性肺结核预测模型建立的ROC曲线 Fig.4 ROC curve produced using the prediction model for massive active tuberculosis. The area under the ROC curve was 0.911 (95% CI: 0.864-0.959). |
本研究结果显示,仅根据病灶代谢增高程度,无论定量指标(SUVmax是否高于2.5) [11-13]还是定性指标(病灶代谢是否高于纵隔血池放射性) [16],均无法将肺癌与活动性肺结核病灶进行区分,这与文献报道一致[14]。但是本研究发现病灶内是否存在放射性缺损对鉴别诊断有较大的帮助。恶性肿瘤和活动性结核均需要大量的葡萄糖作为能量底物或作为组建新细胞的原材料,所以常能摄取大量18F-FDG而在PET图像上呈现为高代谢病灶[14],而坏死组织,由于无存活细胞而不摄取18F-FDG,则可在PET图像上呈现为放射性缺损[17-18]。由于两者差异明显,PET能灵敏地、清楚地显示高代谢病灶中的放射性缺损[17]。在活动性结核病灶中,PET图像上显示的放射性缺损代表病灶内存在干酪样坏死,这是活动性结核重要的病理学改变之一[17]。虽然肺癌病灶内也可因肿瘤中心缺血、缺氧而出现坏死,但常在病灶大时出现,而直径小于或接近3.0 cm的肿瘤出现明显组织坏死的发生率较低。本研究结果显示肺结核组33.8%病灶在PET图像上存在放射性缺损,而肺癌组出现放射性缺损者仅见于3.1%病灶,两者存在显著性差异(χ2=20.498,P=0.000)。这提示通过分析活动性结核病灶内因干酪样坏死而产生的明显放射性缺损有助于肺癌和肺结核病灶的鉴别诊断,这部分内容在既往的研究中未有文献详细论述。
本文多因素研究也显示,除了PET所显示的病灶内放射性缺损外,CT上显示的病灶边缘形态学改变(病灶边缘是否光滑、是否存在分叶征)也有助于鉴别肿块型活动性结核与肺癌。分叶征和“边缘光滑”征象是CT影像上病灶的重要边缘征象,病灶边缘呈现为分叶状还是光滑,在一定程度上反映病灶的生长异质性和对周围组织的侵袭、破坏能力。良性病变(如活动性结核)与肺癌的生物学特性存在明显的不同,导致两者的病灶边缘信息存在明显的不同。有研究也指出,与肺结核相比,原发性肺癌更可能表现出分叶征(67% vs 33%) [19]。但部分良性病变也可以出现,活动性结核在增殖过程中由于肺的间质和间隔以及次级肺小叶等因素的影响也会出现此征象,但其发生率相对较低。本研究结果显示,分叶征见于90.6%的肺癌病灶,而结核此征象仅见于36.5%病灶,差异有统计学意义(P=0.000)。与分叶征相反,病灶边缘光滑在CT征象上常提示良性病变[20],良性病变生长异质性相对较低,侵袭性也较低,难以突破周围物理阻隔,因此边缘常较光滑。有研究显示恶性的实性肺结节与病灶的大小,边缘棘样突起及不规则的形态有关[21]。有研究也认为肺结核病灶倾向于表现为圆形、多边形、边缘光滑,而原发型肺癌病灶倾向于表现为分叶征、边缘不规则伴毛刺[19]。本研究与上述研究相似,38个(51.4%)结核病灶呈现为边缘光滑,而肺癌病灶边缘光滑者仅见于2个(3.1%)病灶,两者差异有统计学意义(P=0.000)。
本研究发现性别在肺结核与肺癌组间的分布也有明显差异,肺结核组多见于男性,而肺癌组多见于女性,这与结核病和肺癌的流行病学统计结果是相符的。结核病的流行病学显示,全球约有90%的结核发生于成年人,其中64%为男性患者,男女比例约为2: 1。具有感染HIV病毒、营养不良、糖尿病、吸烟以及酗酒等危险因素的人患有结核的可能性更大,吸烟、酗酒以及职业暴露等因素会使男性比女性更易患结核[22]。而相反,肺癌的流行病学显示,周围型肺癌中以腺癌为主,而腺癌中女性发病率更高于男性[23],有报道在亚裔女性肺癌患者中,肺腺癌的比例甚至达到80% [24]。
虽然上述多个18F-FDG PET/CT征象在活动性结核和肺癌两组间存在差异,但是单一变量均无法较好地用于鉴别诊断。本研究中多因素研究及Logistic回归分析显示将多种变量相结合才更有助于活动性结核和肺癌的鉴别,从本研究所得的肿块型活动性结核的预测模型“P=1/(1+e-x),X=-0.530+1.978×性别+3.343×放射性缺损+2.846×边缘光滑-2.116×分叶征”中可以看出,在鉴别诊断中,各个征象的鉴别价值从强至弱分别为:放射性缺损>边缘光滑>性别>分叶征。采用本预测模型,可将近80%的活动性结核病灶与肺癌区分开来(诊断肿块型活动性肺结核的灵敏度为78.4%,特异度为92.2%)。该模型与其他预测模型略有不同[25-26],一是只选择两种临床最难鉴别的疾病,肺癌与肿块型活动性肺结核,目标更加具体和明确,而且所有病灶均取得组织病理学结果,而不是混杂有部分随访结果,使得研究的结果更加可靠、可信;二为不是单纯地研究PET图像上病灶的18F-FDG摄取高低对肿块型活动性结核和肺癌的鉴别价值,而是特别关注了病灶内组织坏死在PET图像上呈现的放射性缺损的意义,而其他文献研究和建立的模型里并没有引入该指标。本研究建立的预测模型也提示放射性缺损这个指标对鉴别这两者是最强、最有意义的。本研究的模型同时也将PET/CT显像所能获得到的CT重要征象纳入分析。综合分析这些与疾病相关的具有一定特征性的代谢和形态学征象,将可能更助于提高鉴别诊断的准确性。
本研究存在以下不足:(1)总体上病例数不足;(2)本研究只是单中心回顾性研究,难免出现病例选择偏移;(3)只选取最大径稍大于3.0 cm或小于3.0 cm的病灶,本文的结论是否适用于更大的病灶的鉴别尚不清楚。在将来,纳入更多病例、开展多中心前瞻性研究是非常有必要的。
综上所述,本研究显示仅根据病灶的代谢增高程度,无论是定性指标还是定量指标,18F-FDG PET/CT难以准确地将肿块型活动性结核与肺癌病灶进行鉴别,将反映病灶内部信号(放射性缺损)、边缘信息(边缘是否光滑、有无分叶征)及性别等参数纳入多因素分析和Logistic回归方程而建立活动性肺结核的风险预测模型,可将约80%的肿块型活动性结核病灶与肺癌进行准确鉴别。虽然此预测模型尚难尽善尽美,但有助于明显减少误诊。本研究结果尚需经大量的临床实践进一步验证。
| [1] |
Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management[J]. Radiographics, 2017, 37(1): 52-72. DOI:10.1148/rg.2017160032 |
| [2] |
Klein JS, Braff S. Imaging evaluation of the solitary pulmonary nodule[J]. Clin Chest Med, 2008, 29(1): 15-28. |
| [3] |
刘晓飞, 欧阳晓辉, 苏家贵, 等. 18F-FDG PET/CT在孤立性肺癌与肺结核瘤中的诊断价值[J]. 中国医药导报, 2016, 13(4): 60-3. |
| [4] |
Rami-Porta R, Bolejack V, Goldstraw P. The new tumor, node, and metastasis staging system[J]. Semin Respir Crit Care Med, 2011, 32(1): 44-51. |
| [5] |
Vorster M, Sathekge MM, Bomanji J. Advances in imaging of tuberculosis: the role of 18F- FDG PET and PET/CT[J]. Curr Opin Pulm Med, 2014, 20(3): 287-93. DOI:10.1097/MCP.0000000000000043 |
| [6] |
Sathekge M, Maes A, D'asseler Y, et al. Nuclear medicine imaging in tuberculosis using commercially available radiopharmaceuticals[J]. Nucl Med Commun, 2012, 33(6): 581-90. DOI:10.1097/MNM.0b013e3283528a7c |
| [7] |
Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques[J]. Ann Nucl Med, 2011, 25(10): 681-700. DOI:10.1007/s12149-011-0521-z |
| [8] |
Hashimoto Y, Tsujikawa T, Kondo C, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5[J]. J Nucl Med, 2006, 47(3): 426-31. |
| [9] |
Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeo-xyglucose and SUV quantification[J]. Eur J Nucl Med Mol Imaging, 2010, 37(6): 1087-94. DOI:10.1007/s00259-010-1387-3 |
| [10] |
Yilmaz F, Tastekin G. Sensitivity of F-18-FDG PET in evaluation of solitary pulmonary nodules[J]. Int J Clin Exp Med, 2015, 8(1): 45-51. |
| [11] |
Nomori H, Watanabe K, Ohtsuka T, et al. Visual and semiquantitative analyses for F-18 fluorodeoxyglucose PET scanning in pulmonary nodules 1 cm to 3 cm in size[J]. Ann Thorac Surg, 2005, 79(3): 984-9. DOI:10.1016/j.athoracsur.2004.07.072 |
| [12] |
Groheux D, Quere G, Blanc E, et al. FDG PET-CT for solitary pulmonary nodule and lung cancer: literature review[J]. Diagn Interv Imaging, 2016, 97(10): 1003-17. DOI:10.1016/j.diii.2016.06.020 |
| [13] |
Hou S, Lin X, Wang S, et al. Combination of positron emission tomography/computed tomography and chest thin-layer highresolution computed tomography for evaluation of pulmonary nodules[J]. Medicine, 2018, 97(31): e11640-52. DOI:10.1097/MD.0000000000011640 |
| [14] |
Goo JM, Im JG, Do KH, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases[J]. Radiology, 2000, 216(1): 117-21. DOI:10.1148/radiology.216.1.r00jl19117 |
| [15] |
Sathekge MM, Maes A, Pottel H, et al. Dual time-point FDG PET/ CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area[J]. South Afr Med J, 2010, 100(9): 598-601. DOI:10.7196/SAMJ.4082 |
| [16] |
Veronesi G, Travaini LL, Maisonneuve P, et al. Positron emission tomography in the diagnostic work-up of screening-detected lung nodules[J]. Eur Respir J, 2015, 45(2): 501-10. DOI:10.1183/09031936.00066514 |
| [17] |
吕平欣, 周新华, 骆宝建, 等. 肺结核球18F-脱氧葡萄糖符合线路断层的显像特点观察[J]. 中华结核和呼吸杂志, 2010, 33(8): 597-600. DOI:10.3760/cma.j.issn.1001-0939.2010.08.015 |
| [18] |
王全师, 吴湖炳, 王明芳, 等. 19例良性病变患者18F-FDG PET显像特点分析[J]. 中华核医学杂志, 2003, 23(4): 210-1. DOI:10.3760/cma.j.issn.2095-2848.2003.04.006 |
| [19] |
Totanarungroj K, Chaopotong S, Tongdee T. Distinguishing small primary lung cancer from pulmonary tuberculoma using 64-slices multidetector CT[J]. J Med Assoc Thai, 2012, 95(4): 574-82. |
| [20] |
Macmahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017[J]. Radiology, 2017, 84(1): 161659-71. |
| [21] |
Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines[J]. Chest, 2013, 143(5 Suppl): e93-120. |
| [22] |
Watkins RE, Plant AJ. Does smoking explain sex differences in the global tuberculosis epidemic[J]. Epidemiol Infect, 2006, 134(2): 333-9. DOI:10.1017/S0950268805005042 |
| [23] |
Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis[J]. J Thorac Oncol, 2010, 5(1): 23-8. DOI:10.1097/JTO.0b013e3181c41e8d |
| [24] |
胡成平, 李敏. 女性非小细胞肺癌纵览[J]. 中华结核和呼吸杂志, 2012, 35(2): 89-90. DOI:10.3760/cma.j.issn.1001-0939.2012.02.004 |
| [25] |
Al- Ameri A, Malhotra P, Thygesen H, et al. Risk of malignancy in pulmonary nodules: a validation study of four prediction models[J]. Lung Cancer, 2015, 89(1): 27-30. DOI:10.1016/j.lungcan.2015.03.018 |
| [26] |
程远, 王振光, 杨光杰, 等. 18F-FDG PET/CT孤立性肺结节恶性风险预测模型的建立及效能评价[J]. 中华核医学与分子影像杂志, 2019, 39(3): 129-32. |
 2020, Vol. 40
2020, Vol. 40

