肿瘤微环境(TME)是肿瘤细胞发生发展、侵袭转移的特殊环境,包括多种免疫细胞、免疫分子、细胞外基质、间质细胞、邻近组织细胞、微血管等成分[1]。由于肿瘤组织供氧不足,肿瘤细胞代谢方式转变为以糖酵解为主,产生大量乳酸[2];癌细胞特殊的代谢特征(Warburg effect)使肿瘤组织即使在有氧情况下也保持较高的糖酵解水平[3],胞外酸、胞内碱的现象成为肿瘤细胞的共同特征。肿瘤微环境失调pH、总体上表现出低pH值的理化特点[4-7]。近期研究表明,酸性肿瘤微环境也会对肿瘤相关免疫细胞产生不同影响。活化的CD4+ T细胞及CD8+ CTL存活及功能被抑制,而Treg能够适应缺血、缺氧、酸性的肿瘤微环境并发挥免疫抑制功能[8-9]。在酸性肿瘤微环境中促进CD4+ T细胞及CD8+ CTL存活或抑制Treg细胞存活及功能,相关问题的研究引起广泛关注。
二甲双胍作为二型糖尿病的一线用药,其降糖机制为减少糖原分解、抑制糖异生、抑制葡萄糖吸收并增加细胞胰岛素敏感性[10]。近年回顾性研究发现,二甲双胍可以可以降低二型糖尿病患者恶性肿瘤的发病率和死亡率,发挥抗肿瘤的作用,但其机制尚不明确[11-16]。二甲双胍抗肿瘤作用依赖于完整的抗肿瘤免疫反应,表现为可使免疫功能正常的小鼠肿瘤缩小,但对免疫缺陷的小鼠缺乏疗效[17]。已有研究表明,二甲双胍能降低肿瘤浸润Treg,还可抑制Naïve T细胞向Treg分化[18]。但二甲双胍抗肿瘤、抑制Treg细胞分化的作用机制有待进一步研究。
本研究以酸性环境中Treg细胞为研究对象,旨在进一步探究二甲双胍对酸性环境下的调节性T细胞的调控及二甲双胍的抗肿瘤作用。
1 材料和方法 1.1 实验动物和细胞系小鼠前列腺癌细胞RM-1细胞株,购于ATCC细胞库。
C57BL/6小鼠(4~8周)购于南方医科大学实验动物中心,并在南方医科大学动物中心的SPF条件下饲养。
1.2 细胞因子SA-mGM-CSF受温州医科大学高基民教授馈赠,储存于-20 ℃冰箱。
1.3 主要试剂及仪器二甲双胍冻干粉(Selleckchem,S102606),CD4+CD25+分选试剂盒(美天旎,130-091-041),BCEFE-AM pH荧光探针(碧云天,S1006),逆转录试剂盒(TAKARA,RR036A),QPCR试剂盒(TAKARA,RR420A),FITC标记的抗小鼠FOXP3流式抗体(eBioscience,11-5773-80),PE标记的抗小鼠mGM-CSF流式抗体(eBioscience,MC25736),FITC标记的抗小鼠CD8流式抗体(Biolegend,B217242),APC- Cy7标记的抗小鼠CD4流式抗体(Biolegend,B224685),抗小鼠CD3培养型抗体(eBioscience,4305129),抗小鼠CD28培养型抗体(eBioscience,4295656),PI-ANNEXIN-V凋亡试剂盒(联科生物,A80090544),CFSE增殖试剂盒(Biolegend,422701),CCK8试剂盒(日本同仁,CK04 500T),红细胞裂解液(索莱宝,No.20180823),胶原蛋白酶(I SIGMA,C1889)、DNAse I(SIGMA,D4513),Foxp3破膜固定试剂盒(eBioscience,2010080),IL-2(Proteintech,HZ-1015),流式稀释液(eBioscience,00-4222-26)。
流式细胞仪(BD公司,LSRFortessa),酶标仪(BioTek,epoch2),pH计(上海雷磁,PHS-3E型),定量PCR仪(赛默飞,ABI7500)。
1.4 试验方法 1.4.1 体外酸性环境下,二甲双胍作用下Treg细胞、Tcon细胞的凋亡、增殖的影响断颈处死6~8周C57BL/c小鼠,75%酒精浸泡5 min,灭菌器械取脾采用200目筛网研磨,获取细胞悬液。细胞悬液洗涤离心后,获取沉淀,加入2 mL红细胞裂解液液4 ℃黑暗环境裂解15 min,洗涤离心后,以1 mL PBS重悬,进行细胞计数。
采用CD4+CD25+磁珠分选试剂盒分选CD4+CD25+ Treg细胞、CD4+CD25- Tcon细胞:首先进行CD4阴性选择,细胞悬液离心取沉淀后,每107细胞加入10 μL CD8/ CD11b等鸡尾酒抗体及90 μL缓冲液,冰上孵育10 min,洗一遍,过磁柱,留取流下细胞悬液A,留取磁柱上结合的细胞液B;再进行CD25阳性选择,细胞悬液A离心取沉淀后,每107细胞加入10 μL CD25磁珠及90 μL缓冲液冰上孵育15 min,洗一遍,过磁柱,留取磁柱上结合的细胞(即为CD4+CD25+ Treg细胞),留取流下细胞悬液C。细胞悬液B加细胞悬液C即为CD4+CD25- Tcon细胞。细胞离心后取沉淀,以105细胞/孔加入96孔板,加入细胞因子1 μg/mL anti-CD3,1 μg/mL anti-CD28(Treg细胞孔再加入50 U/mL IL-2),于37 ℃含5% CO2孵箱内培养。
检测细胞增殖:磁珠分选获取Treg细胞、Tcon细胞,立即以无血清PBS洗两遍,106细胞/mL of PBS的细胞数重悬;每mL加入0.2 μL CFSE储液(终浓度1 μmol/L),混匀后室温黑暗条件孵育10 min;加入4~5倍体积的冷全培(或40% FBS in PBS)冰上孵育5 min以终止反应;以完全培养基洗三遍后,在pH 7.4或pH 6.7不加药或pH 6.7加入1 mmol/L二甲双胍刺激,培养72 h后,进行流式细胞术检测。
检测细胞凋亡:磁珠分选获取Treg细胞、Tcon细胞,并在pH 7.4或pH 6.7不加药或pH 6.7加入1 mmol/L二甲双胍刺激,培养72 h后,获取各样本沉淀以500 μL流式稀释液重悬并加入5 μL Annexin V(稀释比1:100)、10 μL PI(稀释比1:50),进行流式细胞术检测。
| 表 1 引物序列 Tab.1 Primer sequences |
磁珠分选获取Treg细胞、Tcon细胞,并在pH 7.4或pH 6.7不加药或pH 6.7加入1 mmol/L二甲双胍刺激,培养72 h后,获取各样本沉淀以500 μL流式稀释液重悬,并同上述胞核染色步骤进行核内Foxp3染色后,进行流式细胞术检测。
1.4.3 使用胞内pH敏感的荧光染料BCECF-AM染色用HEPES缓冲液制备Treg细胞、Tcon细胞悬浮液,细胞浓度为4×107个/mL。将1 mmol/L的BCECF-AM溶液加入细胞悬液中,使BCECF-AM终浓度为3 μmol/L。37 ℃培养30 min。用HEPES缓冲液清洗细胞3次,制成3×106个/mL的细胞悬液。使用荧光显微镜检测细胞的荧光强度。
1.4.4 qRT-PCR检测glycolysis/OXPHOS相关基因的转录水平以Trizol法提取在pH 6.7或pH 7.4培养的Treg细胞、Tcon细胞的总RNA,测量其浓度和纯度,进行逆转录得到cDNA。按照试剂商提供的说明书加样扩增反应体系。其中,引物序列:
以β-actin作为内参,由Ct值计算出2-△△Ct来评价mRNA相对表达量。
1.4.5 实验分组及动物模型建立将32只雄性C57BL/c小鼠随机分为4组(各组n=8):对照组(PBS)、二甲双胍治疗组(MET)、疫苗治疗租(Vaccine)、联合治疗组(Vaccine+ MET)。在小鼠右侧后腿腹股沟区单次皮下注射状态良好的RM-1前列腺癌细胞100 μL(浓度为1×106/mL),建立小鼠前列腺癌模型[19]。
于肿瘤细胞接种后第4天开始,按体质量分别计算每只小鼠的给药量后,按100 mg·kg-1·d-1给予MET治疗组及联合治疗组小鼠腹腔注射二甲双胍,每隔3 d 1次。于肿瘤细胞接种后第4天开始,给予疫苗治疗组及联合治疗组肌注疫苗,每隔4 d 1次,每次疫苗接种量为1×106/100 μL。对照组在相同时间点和同等处理方式对小鼠注射PBS。每隔3 d记录1次肿瘤大小,计算方式为V=(length*width2)/2,直至接种后第25日。
接种后第25日,用1%戊巴比妥钠腹腔注射,进行深度麻醉后处死,获取肿瘤及血液样本。
1.4.6 采用流式细胞术检测瘤内及外周血内CD4+、CD8+、CD4+Foxp3+细胞亚群的变化在接种肿瘤细胞后的第25日,通过眼球摘除法获取200 μL全血,各样本加入2 mL红细胞裂解液,于4 ℃黑暗环境反应15 min,以PBS离心洗涤3次后,用100 μL流式稀释液重悬细胞,获得细胞悬浮液。
处死小鼠后剥离并获取肿瘤组织,将肿瘤剪切成1 mm3的碎块后,加入300 μL胶原蛋白酶I(1 mg/mL)、300 μL DNAse I(50 U/mL)、2.4 mL PBS混合成乳糜状后,37 ℃温箱孵育30 min后,若仍有未充分消化组织使用220 μm孔径滤网研磨过滤。消化后悬液以IMDM培养液洗涤3次,以1 mL IMDM重悬,进行下一步流式染色。
包膜染色:吸取各样本细胞悬液,离心获取细胞沉淀,以100 μL流式稀释液重悬。加入APC-Cy7-CD4(稀释比例1:100)、FITC-CD8(稀释比例1:100),混匀4 ℃避光孵育20 min。离心洗涤后留取细胞沉淀。
胞核染色:向已经包膜染色的细胞沉淀中加入1 mL Foxp3/Fixation/Permeabilization buffer,混匀避光室温孵育30 min;孵育结束后,加入2 mL 1 × Permeabilization Buffer(胞核),离心1200 r/min, 5 min,尽量移除上清;加入2 mL 1×Permeabilization Buffer(胞核),离心1200 r/min 5 min,尽量移除上清, 移除保留100 μL上清;加入PerCP-Cy5.5标记的抗小鼠Foxp3流式抗体(1:100稀释),混匀后避光室温孵育30 min;加入2 mL 1×Permeabilization Buffer(胞核),离心1200 r/min 5 min,尽量移除上清;加入1 mL染色缓冲液(Flow Cytometry Staining Buffer),洗涤后以400 μL重悬于提前标记的流式管;4 h内上机检测。
1.4.7 统计学处理计量资料以均数±标准差表示,采用SPSS(版本20.0)进行单因素方差分析,两两间多重比较采用LSD法(方差齐时)或Dunnett's T3检验(方差不齐时)。P < 0.05为差异有统计学意义。
2 结果 2.1 Treg细胞、Tcon细胞在正常/酸性环境下的增殖情况体外模拟酸性的肿瘤微环境,将细胞培养基调至pH 6.7,培养72 h后检测Treg细胞、Tcon细胞增殖变化。结果发现,较之pH 7.4的对照组,Treg细胞在酸性培养条件下增殖(P < 0.05,图 1A,B),而Tcon细胞在酸性培养条件下增殖被抑制(P < 0.001,图 1C,D)。
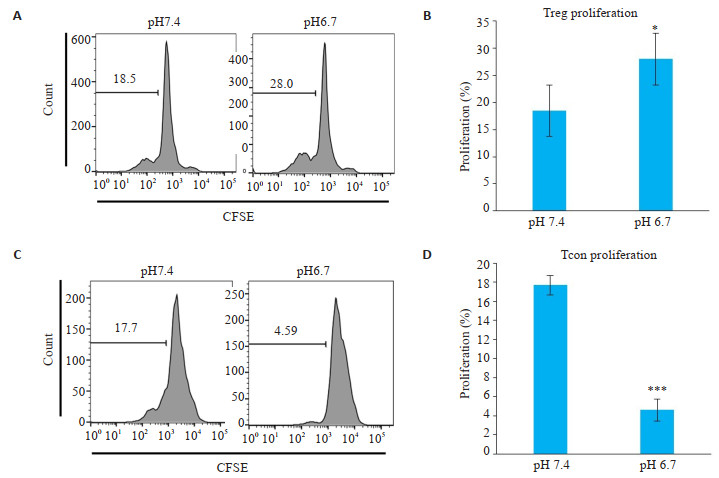
|
图 1 Treg细胞、Tcon细胞在正常/酸性环境下增殖情况 Fig.1 Proliferation of Treg cells or Tcon cells in normal/acidic environment. A, B: Flow cytometry results and corresponding statistical analysis of Treg cells in pH 7.4 and pH 6.7 with anti-CD3, anti-CD28 and anti-IL-2 stimulation (P < 0.05); C, D: Flow cytometry of Tcon cells in pH 7.4 and pH 6.7 with anti-CD3 and anti-CD28 stimulation (P < 0.001). Data are presented as Mean±SD (n=3). *P < 0.05 vs control group; ***P < 0.001 vs control group. |
将细胞培养基调至pH 6.7,体外模拟酸的肿瘤微环境,培养24 h后检测相关基因表达。结果显示,酸性条件下Treg细胞的OXPHOS相关基因pgc1a(P < 0.001,图 2A)、cox5b(P < 0.01,图 2A)表达显著上调,糖酵解相关基因glut1、pkm2表达差异不明显。而Tcon细胞在酸性环境下,OXPHOS相关基因表达差异不显著(图 2B)。
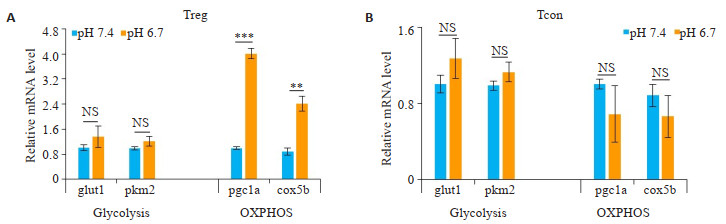
|
图 2 胞外为酸性时,Treg细胞OXPHOS途径增强 Fig.2 An acidic extracellular pH enhances OXPHOS pathway in Treg cells. A: Glycolytic-related genes (glut1, pkm2) have no significant difference in Treg cells under pH 7.4 or pH 6.7 culture medium with anti-CD3, antiCD28 and anti-IL-2 stimulation, and oxidative phosphorylation OXPHOS-related genes (pgc1a and cox5b) are up-regulated (P < 0.001 or 0.01). B: No significant changes are seen in glycolytic-related genes (glut1, pkm2) and oxidative phosphorylation OXPHOS in Tcon cells cultured at pH 7.4 or pH 6.7 with anti-CD3 and anti-CD28 stimulation. Data are presented as Mean±SD (n=3). **P < 0.01 vs control group; ***P < 0.001 vs control group; NS not significant vs control group. |
细胞的代谢途径及胞内酸碱度与细胞的增殖密切相关,为了明确OXPHOS对酸性环境下Treg细胞存活及增殖的作用,我们首先检测了酸性环境下细胞胞内pH(pHi)的变化。结果发现,Treg细胞在酸性培养条件下pHi仍保持碱性(P < 0.01,图 3A,B),而Tcon细胞在酸性培养条件下pHi显著降低,呈酸性(P < 0.001,图 3A,B)。加入OXPHOS抑制剂二甲双胍后,可显著降低酸性环境下Treg细胞的pHi(P < 0.001,图 3A,B)。
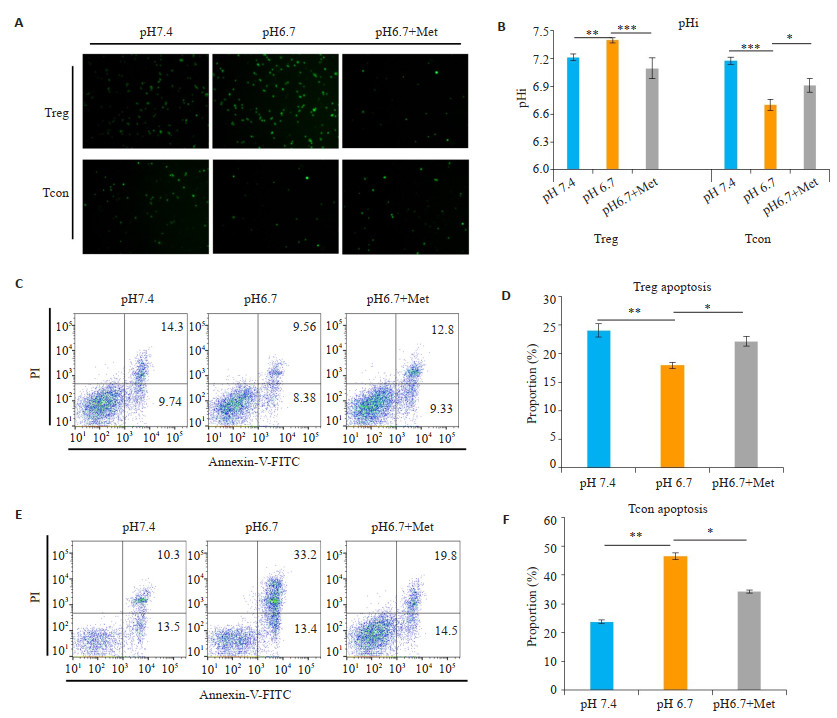
|
图 3 酸性环境下Treg胞内偏碱,二甲双胍逆转Treg细胞pHi并诱导凋亡 Fig.3 Treg cells show an alkaline intracellular pH in acidic medium, which is reversed by metformin. A: Treg cells and Tcon cells was stained by intracellular pH-sensitive fluorescent dye BCECF-AM after culturing in pH 7.4 or pH 6.7 or pH 6.7 with anti-CD3, anti-CD28 (Treg cells cultured with anti-IL-2 additionally) under metformin intervention (pH 6.7+Met), and observed by fluorescence microscope. B: The statistical chart to quantify the experimental results. Treg cells remain alkaline at pHi under acidic culture conditions (P < 0.01). Addition of the OXPHOS inhibitor metformin significantly reduced the pHi of Treg cells in an acidic environment (P < 0.001). However, the pHi of the Tcon cells was significantly reduced under acidic culture conditions (P < 0.001). C, D: Apoptotic flow cytometry results of Treg cells at pH 7.4, pH 6.7 or pH 6.7 under the intervention of Metformin and corresponding statistical table. Decreased apoptosis (early apoptosis + late apoptosis) of Treg cells under acidic culture conditions (P < 0.01). Metformin significantly increased the apoptosis of Treg cells in acidic environment (P < 0.05). E, F: Apoptotic flow cytometry results and corresponding statistical table of Tcon cells in pH 7.4, pH 6.7 or pH 6.7 under the intervention of Metformin. Increased apoptosis of Tcon cells under acidic culture conditions (P < 0.01). Metformin reduced the apoptosis of Tcon cells in acidic environment (P < 0.05) (Mean±SD, n=3). *P < 0.05 vs corresponding control group; **P < 0.05 vs corresponding control group; ***P < 0.001 vs corresponding control group. |
酸性环境下,将Treg细胞、Tcon细胞培养72 h后流式细胞术检测其凋亡变化。结果显示,与pH 7.4对照组相比,Treg细胞在酸性培养条件下凋亡(早期凋亡+晚期凋亡)减少(P < 0.01,图 3C,D),而Tcon细胞在酸性培养条件下凋亡增多(P < 0.01,图 3E,F)。二甲双胍可显著增加酸性环境下Treg细胞凋亡(P < 0.05,图 3C,D)。此外,二甲双胍可降低Tcon细胞在酸性环境下的凋亡(P < 0.05,图 3E,F)。
2.4 二甲双胍显著降低酸性环境下Foxp3+ Treg比例为了进一步明确二甲双胍对酸性环境下Treg的作用,我们检测了Foxp3的表达(Treg细胞重要转录因子)。分选CD4+CD25+ Treg细胞后,将Treg细胞在pH 7.4、pH 6.7及pH 6.7环境下加入二甲双胍培养72 h。在酸性环境下,Treg细胞中Foxp3表达显著增多(P < 0.001,图 4),而二甲双胍能减少Treg细胞中Foxp3+细胞比例(P < 0.001,图 4)。
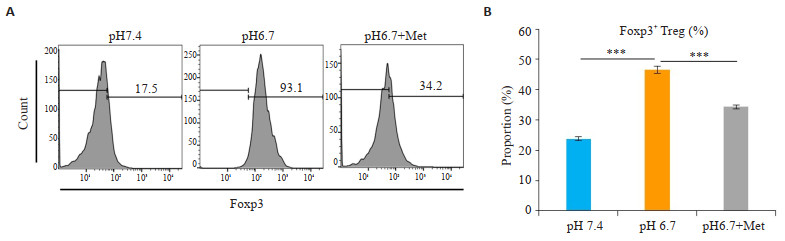
|
图 4 二甲双胍显著降低酸性环境下Foxp3+ Treg比例 Fig.4 Metformin significantly reduces the proportion of Foxp3+ Treg in an acidic environment. A: Flow cytometry results of Foxp3+ Treg cells cultured in pH 7.4, pH 6.7 or pH 6.7 with anti-CD3, anti-CD28 and anti-IL-2 after intervention of metformin. B: Correspondence table. In acidic environments, Foxp3 expression was significantly increased in Treg cells (P < 0.001), while metformin reduces the proportion of Foxp3+ cells in Treg cells (P < 0.001) (Mean±SD, n=3). ***P < 0.001 vs corresponding control group. |
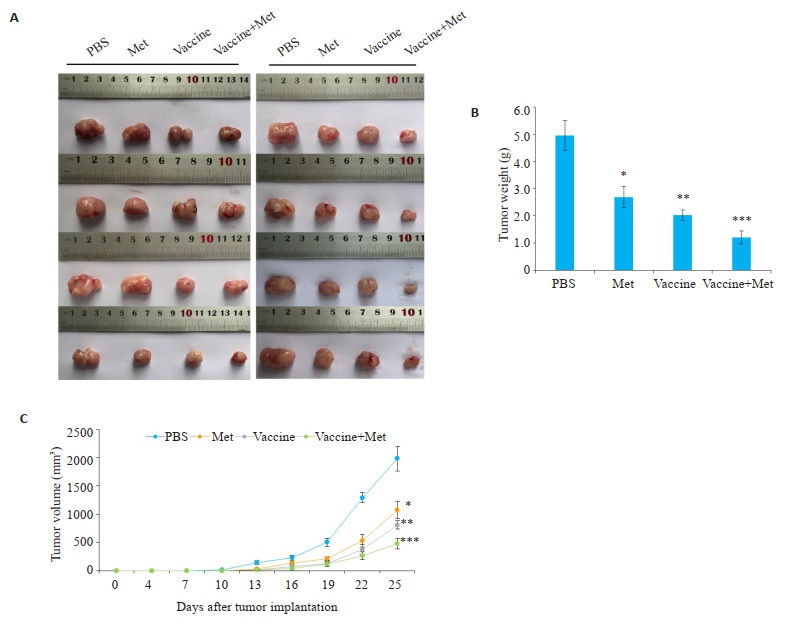
将32只C57BL/6雄性小鼠随机分为PBS组(PBS)、二甲双胍治疗组(Met)、疫苗治疗组(Vaccine)及联合治疗组(Vaccine+Met),建立小鼠前列腺癌模型。成功植瘤后连续监测肿瘤大小,并于植瘤后第25天获取肿瘤。
较之PBS组(PBS),疫苗治疗组(Vaccine,P < 0.01,图 5)或二甲双胍治疗组(Met,P < s0.05,图 5)单独治疗均能有效抑制肿瘤生长,差异具有统计学意义;联合治疗后(Vaccine+Met,P < 0.001,图 5),肿瘤体积生长抑制更多,具有显著差异。
|
图 5 二甲双胍、肿瘤疫苗对小鼠前列腺癌的治疗效果 Fig.5 The therapeutic effect of metformin and tumor vaccine on mouse prostate cancer A: Comparison of tumor volume after exfoliation B: Tumor volume size after exfoliation. Compared with PBS group (PBS), vaccine- treatment group (Vaccine, P < 0.01) or metformin treatment group (Met, P < 0.05) alone could effectively inhibit tumor growth, combined therapy (Vaccine+Met) had the strongest effect on inhibiting tumor volume growth (P < 0.001) (Mean±SD, n=3). *P < 0.05 vs PBS group, **P < 0.01 vs PBS group; ***P < 0.001 vs PBS group. |
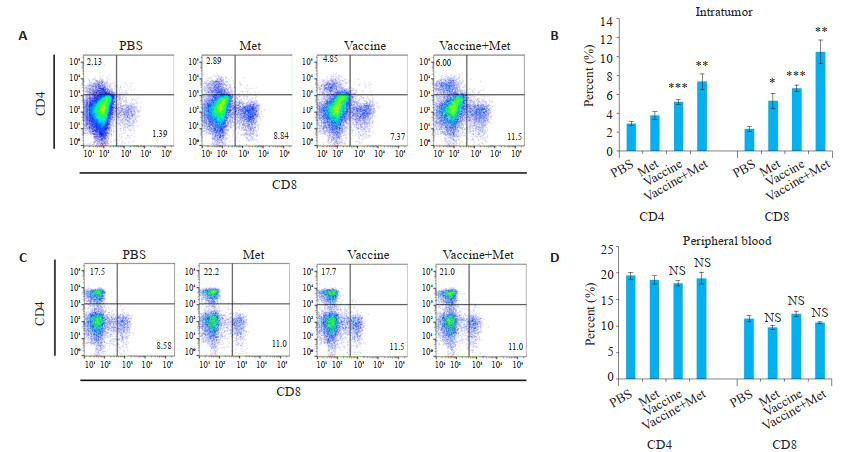
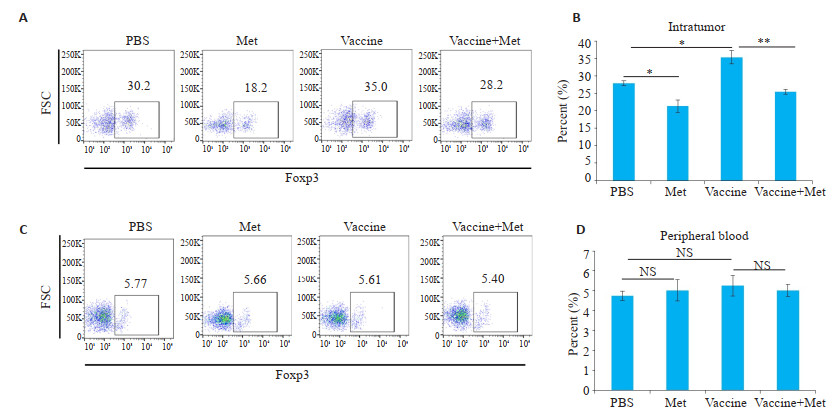
疫苗治疗组(Vaccine)小鼠血液和肿瘤组织中的CD4+、CD8+ T淋巴细胞明显增多(P < 0.001,图 6A,B),但肿瘤组织中Foxp3+ Treg细胞也明显增多(P < 0.05,图 7A,B)。联合治疗后,Foxp3+ Treg细胞明显减少(P < 0.01,图 7A,B),血液和肿瘤组织中效应T细胞增加更显著(P < 0.01,图 6A,B)。
|
图 6 二甲双胍、肿瘤疫苗对瘤内及外周血内CD4+、CD8+细胞亚群的影响 Fig.6 Effect of metformin and tumor vaccine on CD4+, CD8+ cell subsets in intratumoral and peripheral blood. A, B: Flow cytometry results of intratumoral CD4+, CD8+ cell subsets and corresponding statistical table. The CD4+ and CD8+ T lymphocytes in the blood and tumor tissues of the vaccine-treated group (Vaccine) were significantly increased (P < 0.001). C, D: Flow cytometry results of CD4+, CD8+ cell subsets in blood and corresponding statistical tables (Mean±SD, n=3). *P < 0.05 vs PBS group, **P < 0.01 vs PBS group; ***P < 0.001 vs PBS group. |
|
图 7 二甲双胍、肿瘤疫苗对瘤内及外周血内CD4+Foxp3+细胞亚群的影响 Fig.7 Effect of metformin and tumor vaccine on CD4+Foxp3+ cell subsets in intratumoral and peripheral blood. A, B: Flow cytometry results of intratumoral CD4+Foxp3+ cell subsets and corresponding statistical table. The Foxp3+ Treg cells in the tumor tissues increased (P < 0.001) C, D: Flow cytometry results of CD4+Foxp3+ cell subsets in blood and corresponding statistical tables (Mean±SD, n=3). *P < 0.05 vs corresponding group; **P < 0.01 vs corresponding group. |
Treg细胞通过抑制机体免疫反应,促使肿瘤细胞逃脱机体的免疫监视[20-21]。靶向Treg细胞、提高肿瘤免疫应答的肿瘤免疫治疗策略已获得学者共识。目前研究表明,Treg清除治疗肿瘤的手段主要分为,阻断Treg迁移至肿瘤部位、阻断免疫检查点(Treg重要表面标志)、使用化疗药物降低Treg扩增[22-23]。但由于相关治疗手段缺乏特异性,导致多毒副作用、抗肿瘤效果低下。因此,寻找一个安全有效的Treg清除治疗肿瘤的手段十分重要。
在正常组织中,细胞外pH(pHe)数值波动于7.4左右,而细胞内pH(pHi)波动于7.2左右;然而肿瘤细胞pHe多为6.5~7.0,呈酸性,pHi为碱性或接近中性[24]。肿瘤微环境酸性成为肿瘤的共同特征。但是酸性肿瘤微环境对Treg细胞的调控鲜有报道,pH对肿瘤相关免疫细胞的调控有重要研究价值。经研究证实,Treg细胞在培养环境为酸性时,细胞内pH(pHi)呈碱性,而Tcon细胞pHi呈酸性。同时,细胞外pH(pHe)为酸性时,Treg细胞偏向通过OXPHOS途径产生能量,而Tcon细胞代谢途径改变不显著。相关研究表明,活化的CD4+ T细胞及CD8+ CTL主要通过有氧糖酵解(Warburg effect)获取能量,这与肿瘤细胞的糖代谢方式相同[25-26]。因此,效应性T细胞与肿瘤细胞在肿瘤微环境存在着能量竞争,当肿瘤细胞获得数量优势时势必会竞争性地抑制效应性T细胞能量代谢。然而,Treg细胞具有糖酵解和氧化磷酸化(OXPHOS)两种代谢途径提供能量,其中OXPHOS是Treg维持功能的主要代谢方式[27],OXPHOS途径的活化使Treg能够适应缺血、缺氧、酸性的肿瘤微环境并发挥免疫抑制功能。二甲双胍可以抑制线粒体电子传递链复合物,有效抑制OXPHOS并且激活糖酵解[28-29]。本实验中,二甲双胍能够有效抑制酸性培养条件下Treg细胞的增殖、促进其凋亡,并且能够减少Foxp3+ Treg细胞的比例。研究结果提示二甲双胍可能通过调节细胞OXPHOS活性影响Treg细胞的活性及功能。当使用二甲双胍刺激酸性环境下的Treg细胞时,Treg细胞pHi呈酸性,提示Treg细胞pHi很可能受OXPHOS调控。有趣的是,酸性条件并不影响Tcon细胞OXPHOS活性,代谢途径的差异可能与Treg细胞和Tcon细胞对酸性环境的不同适应性相关,具体机制有待进一步研究。
随着研究和临床的发展,免疫治疗已成为手术、放化疗之外的有力治疗肿瘤的手段,特别是术后易复发和转移的癌症治疗[19, 30]。然而,肿瘤疫苗治疗后不仅提高了效应性T细胞比例、增强抗肿瘤免疫,同时也显著提高了肿瘤微环境中的调节性T细胞(Treg)的细胞比例。因此,清除或抑制肿瘤疫苗治疗后Treg细胞的功能将会有利于肿瘤免疫治疗。在前期研究的基础上,我们利用了锚定GM-CSF细胞因子的前列腺癌全肿瘤细胞疫苗联合二甲双胍治疗实验性前列腺癌。结果表明,较之对照组和单独治疗组,二甲双胍联合疫苗延缓了肿瘤增长、促进了肿瘤内CD4+T细胞及CD8+T细胞的比例提高、下调肿瘤内Treg细胞比例。由实验结果推断,二甲双胍联合GM-CSF肿瘤疫苗后,可能通过减少肿瘤微环境中Treg细胞比例,从而增强了疫苗的疗效。
本研究证实二甲双胍可诱导肿瘤酸性环境下Treg细胞比例下降,从而增强SA-GM-CSF修饰的前列腺癌疫苗对实验性前列腺癌的抗肿瘤作用。二甲双胍诱导Treg细胞比例下调的机制可能是通过抑制Treg细胞的OXPHOS代谢渠道,从而逆转Treg细胞耐酸效应而实现的。由研究结果可推测,二甲双胍可作为未来临床抗肿瘤药物或辅助药物用于癌症治疗。
| [1] |
Hui L, Chen Y. Tumor microenvironment:Sanctuary of the devil[J]. Cancer Lett, 2015, 368(1): 7-13. |
| [2] |
Gillies RJ, Robey I, Gatenby RA. Causes and Consequences of increased glucose metabolism of cancers[J]. J Nucl Med, 2008, 49(Suppl 2): 24S-42S. |
| [3] |
Icard P, Shulman S, Farhat D, et al. How the warburg effect supports aggressiveness and drug resistance of Cancer cells[J]. Drug Resist Updat, 2018, 38: 1-11. DOI:10.1016/j.drup.2018.03.001 |
| [4] |
Lin B, Chen H, Liang D, et al. Acidic pH and high-HO dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition[J]. ACS Appl Mater Interfaces, 2019, 11(12): 11157-66. DOI:10.1021/acsami.8b22487 |
| [5] |
Lee HC, Wei YH. Mitochondrial DNA instability and metabolic shift in human cancers[J]. Int J Mol Sci, 2009, 10(2): 674-701. DOI:10.3390/ijms10020674 |
| [6] |
Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer:relevance to Warburg hypothesis and beyond[J]. Pharmacol Ther, 2009, 121(1): 29-40. DOI:10.1016/j.pharmthera.2008.09.005 |
| [7] |
Fukumura D, Jain RK. Tumor microvasculature and microenvironment:targets for anti-angiogenesis and normalization[J]. Microvasc Res, 2007, 74(2/3): 72-84. DOI:10.1016/j.mvr.2007.05.003 |
| [8] |
Huber V, Camisaschi C, Berzi A, et al. Cancer acidity:An ultimate frontier of tumor immune escape and a novel target of immunomodulation[J]. Semin Cancer Biol, 2017, 43: 74-89. DOI:10.1016/j.semcancer.2017.03.001 |
| [9] |
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy[J]. Cancer Res, 2016, 76(6): 1381-90. DOI:10.1158/0008-5472.CAN-15-1743 |
| [10] |
Li M, Hu X, Xu Y, et al. A possible mechanism of metformin in improving insulin resistance in diabetic rat models[J]. Int J Endocrinol, 2019, 2019: 3248527. |
| [11] |
Patel S, Singh N, Kumar L. Evaluation of effects of metformin in primary ovarian cancer cells[J]. Asian Pac J Cancer Prev, 2015, 16(16): 6973-9. DOI:10.7314/APJCP.2015.16.16.6973 |
| [12] |
Chen G, Feng W, Zhang S, et al. Metformin inhibits gastric cancer via the inhibition of HIF1 alpha/PKM2 signaling[J]. Am J Cancer Res, 2015, 5(4): 1423-34. |
| [13] |
Mei ZB, Zhang ZJ, Liu CY, et al. Survival benefits of metformin for colorectal cancer patients with diabetes:a systematic review and meta-analysis[J]. PLoS One, 2014, 9(3): e91818. DOI:10.1371/journal.pone.0091818 |
| [14] |
来成军, 曲红华, 郭晓宇, 等. 二甲双胍对肾癌786-O细胞迁移和侵袭的影响[J]. 山东大学学报:医学版, 2014, 52(2): 29-32. |
| [15] |
Zhang ZJ, Zheng ZJ, Shi R, et al. Metformin for liver cancer prevention in patients with type 2 diabetes:a systematic review and Meta-Analysis[J]. J Clin Endocrinol Metab, 2012, 97(7): 2347-53. DOI:10.1210/jc.2012-1267 |
| [16] |
Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling[J]. Cell Death Dis, 2014, 5: e1088. DOI:10.1038/cddis.2014.59 |
| [17] |
Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin[J]. Proc Natl Acad Sci USA, 2015, 112(6): 1809-14. DOI:10.1073/pnas.1417636112 |
| [18] |
Kunisada Y, Eikawa S, Tomonobu N, et al. Attenuation of CD4+CD25+regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug[J]. EBioMedicine, 2017, 25: 154-64. DOI:10.1016/j.ebiom.2017.10.009 |
| [19] |
莫丽君.清除Treg细胞提高SA-mGM-CSF前列腺癌细胞疫苗的抗肿瘤效果[D].广州: 南方医科大学, 2018.
|
| [20] |
Ohue Y, Nishikawa HT. Cells in cancer:can Treg cells be a new therapeutic target?[J]. Cancer Sci, 2019, 110(7): 2080-9. DOI:10.1111/cas.14069 |
| [21] |
Munn DH, Sharma MD, Johnson TS. Treg destabilization and reprogramming:implications for cancer immunotherapy[J]. Cancer Res, 2018, 78(18): 5191-9. DOI:10.1158/0008-5472.CAN-18-1351 |
| [22] |
Chaudhary B, Abd Al Samid M, al-Ramadi BK, et al. Phenotypic alterations, clinical impact and therapeutic potential of regulatory T cells in cancer[J]. Expert Opin Biol Ther, 2014, 14(7): 931-45. DOI:10.1517/14712598.2014.900539 |
| [23] |
Sampson JH, Schmittling RJ, Archer GE, et al. A pilot study of IL-2R alpha blockade during lymphopenia depletes regulatory t-cells and correlates with enhanced immunity in patients with glioblastoma[J]. PLoS One, 2012, 7(2): e31046. DOI:10.1371/journal.pone.0031046 |
| [24] |
Persi E, Duran-Frigola M, Damaghi M, et al. Systems analysis of intracellular pH vulnerabilities for cancer therapy[J]. Nat Commun, 2018, 9(1): 2997. DOI:10.1038/s41467-018-05261-x |
| [25] |
Maciver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes[J]. Annu Rev Immunol, 2013, 31: 259-83. DOI:10.1146/annurev-immunol-032712-095956 |
| [26] |
Fernández-Ramos AA, Poindessous V, Marchetti-Laurent C, et al. The effect of immunosuppressive molecules on T-cell metabolic reprogramming[J]. Biochimie, 2016, 127: 23-36. DOI:10.1016/j.biochi.2016.04.016 |
| [27] |
Angelin A, Gil-de-Gomez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in Low-Glucose, High-Lactate environments[J]. Cell Metab, 2017, 25(6): 1282. DOI:10.1016/j.cmet.2016.12.018 |
| [28] |
Lei Y, Yi Y, Liu Y, et al. Metformin targets multiple signaling pathways in cancer[J]. Chin J Cancer, 2017, 36(7): 17. DOI:10.1186/s40880-017-0184-9 |
| [29] |
Thakur S, Daley B, Gaskins K, et al. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo[J]. Clin Cancer Res, 2018, 24(16): 4030-43. DOI:10.1158/1078-0432.CCR-17-3167 |
| [30] |
石小军.SA-GM-CSF、SA-IL-2膜修饰的MB49膀胱癌细胞疫苗序贯应用于治疗转移性膀胱癌的实验研究[D].广州: 南方医科大学, 2012.
|
 2019, Vol. 39
2019, Vol. 39

