2. 中南大学湘雅医院 麻醉科,湖南 长沙 410008
2. Department of Anesthesiology, Xiangya Hospital of Central South University, Changsha 410008, China
微小RNA(miRNA或miR)是生物体内一类由20~ 23个核苷酸组成的非编码单链小分子RNA,通过与靶标mRNA的3'-UTR区结合,导致mRNA的降解或翻译抑制,对基因表达发挥转录后调控作用[1]。在神经损伤、炎症等病理状态下,许多miRNAs的表达发生显著变化,通过靶向下游与离子通道、受体、神经炎症、胞内信号通路等相关的分子,诱发神经元和胶质细胞的表型和功能转换,介导慢性病理性疼痛的发生[2-3]。尽管越来越多的miRNAs被发现参与神经病理性疼痛的发生机制,但鲜有研究者关注miRNAs表达或功能变化的驱动机制。作为细胞基因组的转录产物,miRNA的表达和功能受到包括长链非编码RNA、环状RNA、RNA甲基化、组蛋白乙酰化等机制的调控[4-7]。本小组前期研究显示HDACs抑制剂曲古霉素A(TSA)通过抑制组蛋白去乙酰化酶2(HDAC2)的表达和功能,而HDAC2可能通过调控KCC2和Gad65的表达参与CCI大鼠神经病理性疼痛的发生机制[8]。作为一种广谱HDACs抑制剂,TSA是否还改变了CCI大鼠脊髓其他亚型的HDACs的表达,CCI大鼠脊髓miRNAs是否也受到组蛋白乙酰化的调控,成为TSA发挥镇痛作用的下游靶点,还有待进一步探索。本研究检测了TSA对CCI大鼠脊髓另一种重要的组蛋白去乙酰化酶——组蛋白去乙酰化酶4(HDAC4)表达的调控,筛选了CCI大鼠脊髓受TSA调控的差异表达miRNAs,并对其中两种miRNA的表达变化加以验证。探讨了TSA可能通过HDACs调控miRNAs的表达发挥镇痛作用的表观遗传学机制,为神经病理性疼痛的治疗和研究提供一条新思路。
1 材料和方法 1.1 实验动物分组及处理健康成年雄性清洁级SD大鼠,体质量230~270 g,由中南大学湘雅医院动物实验中心提供。随机分为3组:sham+DMSO组(S组)、CCI+DMSO组(C组)和CCI+TSA组(T组)。建立大鼠坐骨神经慢性缩窄性损伤(CCI)模型,sham组只暴露坐骨神经而不结扎。于术后第7天痛阈测定后,参照Mestre法[9]以鼠尾甩动为穿刺成功标志,分别鞘内注射5% DMSO液(生理盐水配制)、5% DMSO液、TSA 10 µg(曲古菌素TSA购于美国CST公司,溶解于5% DMSO中)各10 µL,连续3 d。于术前1 d(基础值)、术后3、7、10 d测定大鼠术侧后足机械性刺激缩足反应阈值(PWMT)。大鼠于术后第10天行为学测量完成后(鞘内注射完成1 d后)处死,冰上迅速取出腰段脊髓。
1.2 免疫荧光法检测HDAC4在CCI大鼠腰段脊髓的表达取脊髓冰冻切片,羊血清封闭1 h,以HDAC4(1: 150)抗体4 ℃孵育过夜,Dylight549标记驴抗小鼠IgG(1:200, Jackson)室温避光孵育2 h,PBS洗涤后甘油封片,DM5000型荧光显微镜(Leica)观察拍片。阴性对照用PBS代替一抗。
1.3 Western blot法测定HDAC4蛋白在CCI大鼠腰段脊髓的表达水平提取总蛋白在8%的SDS-PAGE胶中加样电泳,溴酚蓝电泳至分离胶底部时结束电泳。转膜,5%脱脂牛奶封闭3 h,用稀释的小鼠抗HDAC4单克隆抗体(1:600,Sigma公司,美国)于4 ℃孵育过夜,TBST洗涤后再加入HRP酶标记的二抗(1:5000,Jackson公司,美国)室温孵育1.5 h,洗涤后采用Gel Doc凝胶成像分析系统(Bio-Rad公司,美国)进行ECL显色,Gel-Pro Analyzer软件分析蛋白条带,以HDAC4与GAPDH的灰度值比值反映HDAC4的蛋白表达水平。
1.4 采用荧光实时定量PCR检测各组大鼠腰段脊髓HDAC4 mRNA水平采用All-in-One cDNA Synthesis Kit逆转录试剂盒(GeneCopoeia),逆转录反应合成cDNA,采用All-inOne qPCR Mix试剂盒(GeneCopoeia),按说明书操作,配制20 µL反应体系,使用ViiA 7实时荧光定量PCR系统(Life Tech)进行反应。反应终止后,分别得到每组样品的目的基因及内参基因的Ct值,根据2-△△CT定量方法计算mRNA的表达水平。
1.5 miRNA芯片检测及PCR验证微阵列实验委托联川生物公司进行,采用Sanger miRBase V19.0芯片。提取腰段脊髓组织总RNA(Trizol法),挑选两种miRNA采用实时荧光定量PCR进行验证。
1.6 数据处理及统计学分析采用统计软件SPSS 23.0对实验数据进行分析。计量资料以均数±标准误的形式表示。组间比较采用完全随机设计的单因素方差分析(ANOVA)方法,采用Bonferroni法进行post-hoc多重比较,Ρ < 0.05认为差异有统计学意义。
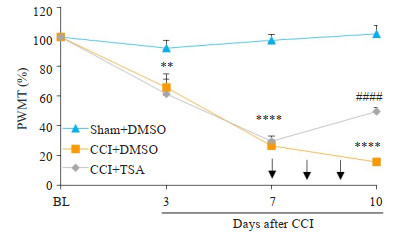
2 结果 2.1 机械痛阈的变化各组大鼠机械痛阈结果:C组与S组比较,大鼠机械痛阈于术后第3天开始显著下降,持续至第10天。鞘内注射TSA后,T组与C组比较,机械痛阈在第10天显著升高(图 1)。
|
图 1 各组大鼠术侧后肢机械痛阈的测定 Fig.1 Mechanical threshold of rats in different groups after operation. Intrathecal injection of TSA partially reversed the decrease in the mechanical threshold of CCI rats. **P < 0.01, ****P < 0.0001 CCI+DMSO vs Sham+DMSO group; ####P < 0.0001 CCI+TSA vs CCI+DMSO group. BL: Baseline; PWMT: Paw withdrawal mechanical threshold. Arrows indicate the time of intrathecal injection. CCI: Chronic constrictive injury. |
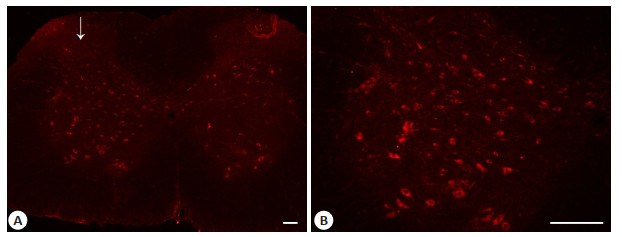
HDAC4蛋白主要表达于大鼠腰段脊髓灰质,阳性染色主要集中于细胞质,且术侧脊髓HDAC4阳性细胞数明显较对照侧多(图 2)。
|
图 2 CCI术后第10天大鼠腰段脊髓HDAC4免疫荧光染色 Fig.2 HDAC4 expression in the lumbar spinal cord of CCI rats on day 10 after the operation. A: Immunofluorescent signal of HDAC4 (red) detected in the lumbar spinal cord section; B: Immunofluorescent signal of HDAC4 (red) in the dorsal horn. Arrows indicate the ipsilateral side of CCI operation. Scale bar=100 μm. |
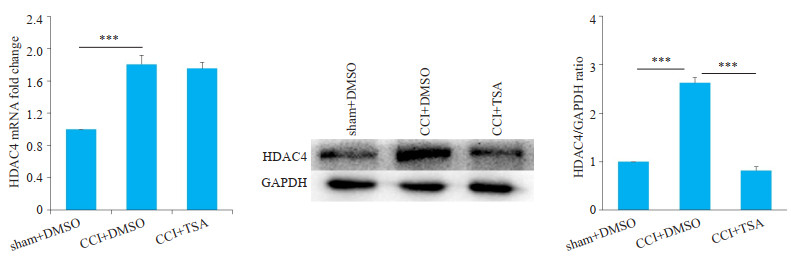
CCI术后第10天大鼠腰段脊髓中HDAC4蛋白水平及mRNA水平均明显升高,鞘注TSA后仅HDAC4蛋白水平下降而mRNA水平无明显降低(图 3)。
|
图 3 各组大鼠腰段脊髓HDAC4蛋白及mRNA水平 Fig.3 HDAC4 protein and mRNA expression levels in the lumbar spinal cord of the rats. Intrathecal injection of TSA suppresses HDAC4 protein expression but not its mRNA expression in the lumbar spinal cord of CCI rats (n=3, ***P < 0.001). |
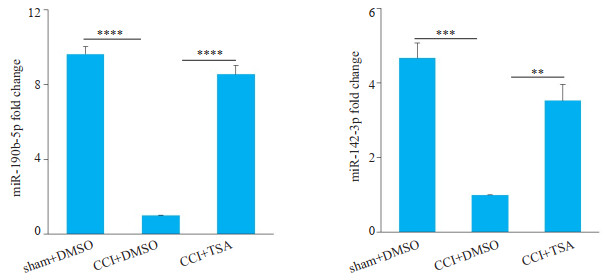
各组大鼠腰段脊髓miRNAs芯片结果分析:C组与S组比较,差异表达(P < 0.05)的miRNAs共有83种,鞘内注射TSA后逆转了CCI术后差异表达的miRNA共有58种(差异表达倍数fold change≥2或≤0.5),其中CCI术后表达下调,在鞘注TSA后表达部分恢复的miRNA有41种。查阅文献后,在最后41种miRNA中选择差异表达倍数较高,且在腰段脊髓中表达丰度高的两种miRNA(miR-190b-5p差异表达倍数约13倍,miR-142-3p差异表达倍数约21倍)进行real-time PCR验证(图 4)。
|
图 4 术后10 d各组大鼠腰段脊髓两种miRNA的表达 Fig.4 Expression of miRNAs in the spinal cord of rats in each group on day 10 after the operation. Real-time PCR reveals that intrathecal injection of TSA reverses the expression of miR-190b-5p and miR-142-3p in the lumbar spinal cord of CCI rats (n=4, **P < 0.01, ***P < 0.001, ****P < 0.0001. |
组蛋白乙酰化及去乙酰化修饰是基因表达调控主要表观遗传学机制之一,该过程由组蛋白乙酰基转移酶(HATs)和组蛋白去乙酰化酶(HDACs)共同催化。HATs促使染色质的解聚,激活转录;HDACs通过移除组蛋白N端赖氨酸ε-氨基上的乙酰基团,恢复组蛋白与DNA链的结合,促进染色质浓聚,阻碍转录元件与DNA链结合,从而使靶基因保持沉默状态[10-11]。我们的前期研究已经发现,脊髓HDAC2表达上调介导了CCI大鼠的机械和热痛敏感,TSA能抑制HDAC2的活性以及蛋白表达,发挥镇痛作用。HDAC4是Ⅱ类HDACs的一种亚型,在神经系统中稳定表达,研究证明HDAC4组蛋白去乙酰化催化域缺失时,大鼠对热痛的敏感性降低[12],亦有研究发现Ⅱa类HDACs(HDAC4, 5, 7, 9)亚型抑制剂的镇痛作用比广谱型抑制剂更强,其中HDAC4可能发挥主要作用[13]。Lin等发现脊髓神经结扎模型中脊髓背角神经元HDAC4磷酸化,使其从胞核向胞质内转移,从而削弱了HDAC4对hmgb1基因表达的抑制作用,介导神经病理性疼痛的发生[14-15]。也有研究者发现,CCI大鼠DRG内HDAC4表达上调,如果使用miR-206-3p模拟物抑制其表达,能有效缓解大鼠的疼痛行为,如过表达HDAC4则削弱miR-206-3p模拟物的镇痛作用[16]。本研究结果发现HDAC4表达于腰段脊髓灰质,主要位于细胞质内;CCI术后HDAC4总蛋白及mRNA水平均表达升高。TSA是一种研究最为广泛、结构较简单、抑制HDAC活性高的广谱HDAC抑制剂,对Ⅰ类和Ⅱ类HDACs的多种亚型具有抑制作用。本研究中鞘内注射TSA可有效缓解CCI大鼠的机械痛敏,逆转HDAC4蛋白表达水平的增高,却不改变其mRNA水平。这提示与其对HDAC2的作用类似,TSA不影响HDAC4基因的转录,而可能通过促进泛素化介导的HDAC4蛋白降解,影响其蛋白表达水平[17-18]。尽管明确了HDAC4的表达上调受到了TSA的抑制,但这是否参与介导了TSA的镇痛作用,还需要进一步的动物行为学试验明确。
HDAC抑制剂能够改变众多基因的表达,如:高通量分析显示MS-275能显著改变横核内435种基因的表达[19]。除了对靶基因启动子组蛋白乙酰化水平的直接调节作用,HDACs还能以各种转录因子以及其他表观遗传学调控因子为节点,间接对下游靶基因进行转录和转录后调控[20-22]。考虑到使用有效剂量的HDAC抑制剂后,靶基因变化并不一致,上调和下调的基因数目大致相等,这种复杂的间接机制可能更为重要[23]。本研究使用miRNA芯片进行高通量分析,发现大鼠CCI术后第10天腰段脊髓有83种miRNAs发生显著表达变化,而鞘注TSA能完全或部分逆转其中58种miRNAs的表达变化。与我们的发现一致,高通量分析显示神经病理性疼痛模型动物的脑、脊髓和背根神经节内有大量miRNAs表达发生显著变化[24-27]。运用基因编辑技术或者化学合成的miRNA模拟物或抑制物,现已明确miR-183簇、miR-17-92簇、miR-21-5p和miR-7a等多种miRNAs在神经病理性疼痛的发生和维持中发挥重要作用[28-31]。这些miRNAs可能通过作用于神经炎症因子、离子通道、细胞内信号通路或者其他表观遗传学调控子等多种下游靶点参与疼痛通路的调节[16, 29, 32-33]。除了动物实验,一些临床研究也发现某些miRNAs可能成为帮助神经病理性疼痛诊断和分型的标志物[34-35]。
HDACs对miRNAs的表达存在显著的调节作用:HEK细胞中过表达HDAC1能下调9%和上调46.1%的成熟miRNAs表达[36]。使用HDACs抑制剂LAQ824能使SKBR3乳腺癌细胞约40%的miRNAs表达发生迅速而显著的变化,其中22种miRNAs表达上调,5种miRNAs表达下调[37]。与这些研究结果一致,我们的高通量芯片结果显示鞘注TSA能完全或部分逆转CCI大鼠脊髓58种miRNAs的表达变化,其中41种miRNAs在CCI术后表达下调,并在鞘注TSA后表达部分恢复。我们选取了表达丰度较高、组内差异较小、组件差异大的miR- 190b-5p、miR-142-3p进行real-time PCR验证,其表达变化与芯片检测结果一致。miR-190是参与神经炎症调控的一种微小RNA,对帕金森病的研究发现脂多糖可抑制体外培养的BV2细胞中miR-190的水平,而过表达miR-190能抑制促炎介质iNOS,IL-6、TNF-α和TGF-β1的表达,增加抗炎介质IL-10的表达,并且在MPTP诱导的帕金森小鼠模型中发挥抑制小胶质细胞活化、抗炎和缓解神经损伤的作用[38]。miR-142-3p在神经元、小胶质细胞和星形胶质细胞内均有分布,在脊神经结扎(SNL)小鼠模型的DRG内miR-142-3p表达显著下调,过表达miR-142-3p能通过抑制HMGB1有效缓解小鼠的机械和热痛敏感[39]。在纤维肌痛女性患者的血液中,miR-142-3p的表达水平也显著降低,但其意义尚不明确[40]。脊髓小胶质细胞激活以及HMGB1介导的神经炎症是神经病理性疼痛的重要原因之一。因此,这两种miRNAs的变化很可能与坐骨神经损伤后大鼠的疼痛行为以及TSA的镇痛作用相关,我们将通过进一步研究予以明确。
通过该项研究,我们发现鞘内注射TSA可抑制CCI大鼠脊髓HDAC4表达上调,并首次发现TSA能逆转神经病理性疼痛大鼠脊髓多种miRNAs的表达变化,结合我们的前期研究以及其他学者的发现,我们推测TSA可能通过抑制HDACs表达与功能,调控多种miRNAs的表达,从而靶向更多的下游相关基因,发挥对神经病理性疼痛的镇痛作用。尽管我们发现HDAC2与HDAC4的表达与miRNAs的变化相关,但其对miRNAs的调控作用有待通过进一步特异性干预实验明确。此外,参与调控miRNA的HDACs亚型可能有多种,这些HDACs在不同种类miRNA转录调控中的具体作用和机制也可能不尽相同,值得进一步探索。
| [1] |
Lundstrom K. Micro-RNA in disease and gene therapy[J]. Curr Drug Discov Technol, 2011, 8(2): 76-86. DOI:10.2174/157016311795563857 |
| [2] |
Mj Lopez-Gonzalez ML, Favereaux A. MicroRNA and chronic pain:From mechanisms to therapeutic potential[J]. Pharmacol Ther, 2017, 180: 1-15. DOI:10.1016/j.pharmthera.2017.06.001 |
| [3] |
Descalzi G, Ikegami D, Ushijima T, et al. Epigenetic mechanisms of chronic pain[J]. Trends Neurosci, 2015, 38(4): 237-46. DOI:10.1016/j.tins.2015.02.001 |
| [4] |
Militello G, Weirick T, John D, et al. Screening and validation of lncRNAs and circRNAs as miRNA sponges[J]. Brief Bioinform, 2017, 18(5): 780-8. |
| [5] |
Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency[J]. Nature, 2013, 495(7441): 333-8. DOI:10.1038/nature11928 |
| [6] |
Alarcon CR, Lee H, Goodarzi H, et al. N6-methyladenosine marks primary microRNAs for processing[J]. Nature, 2015, 519(7544): 482-5. DOI:10.1038/nature14281 |
| [7] |
Sampath D, Liu C, Vasan K, et al. Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia[J]. Blood, 2012, 119(5): 1162-72. DOI:10.1182/blood-2011-05-351510 |
| [8] |
Ouyang B, Chen D, Hou X, et al. Normalizing HDAC2 levels in the spinal cord alleviates thermal and mechanical hyperalgesia after peripheral nerve injury and promotes GAD65 and KCC2 expression[J]. Front Neurosci, 2019, 13: 346. DOI:10.3389/fnins.2019.00346 |
| [9] |
Mestre C, Pelissier T, Fialip J, et al. A method to perform direct transcutaneous intrathecal injection in rats[J]. J Pharmacol Toxicol Methods, 1994, 32(4): 197-200. DOI:10.1016/1056-8719(94)90087-6 |
| [10] |
M Haberland RM, Olson EN. The many roles of histone deacetylases in development and physiology:implications for disease and therapy[J]. Nat Rev Genet, 2009, 10(1): 32-42. DOI:10.1038/nrg2485 |
| [11] |
Peserico A, Simone C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance[J]. J Biomed Biotechnol, 2011, 2011: 371832. DOI:10.1155/2011/371832 |
| [12] |
Rajan I, Savelieva KV, Ye GL, et al. Loss of the putative catalytic domain of HDAC4 leads to reduced thermal nociception and seizures while allowing normal bone development[J]. PLoS One, 2009, 4(8): e6612. DOI:10.1371/journal.pone.0006612 |
| [13] |
Bai G, Wei D, Zou S, et al. Inhibition of class Ⅱ histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia[J]. Mol Pain, 2010, 6: 51. DOI:10.1186/1744-8069-6-51 |
| [14] |
Lin TB, Hsieh MC, Lai CY, et al. Modulation of nerve injury-induced HDAC4 cytoplasmic retention contributes to neuropathic pain in rats[J]. Anesthesiology, 2015, 123(1): 199-212. DOI:10.1097/ALN.0000000000000663 |
| [15] |
Lin TB, Hsieh MC, Lai CY, et al. Melatonin relieves neuropathic allodynia through spinal MT2-enhanced PP2Ac and downstream HDAC4 shuttling-dependent epigenetic modification of hmgb1 transcription[J]. J Pineal Res, 2016, 60(3): 263-76. DOI:10.1111/jpi.12307 |
| [16] |
Wen J, He T, Qi F, et al. MiR-206-3p alleviates chronic constriction injury-induced neuropathic pain through targeting HDAC4[J]. Exp Anim, 2019, 68(2): 213-20. DOI:10.1538/expanim.18-0091 |
| [17] |
Wu D, Huang Q, Zhang Y, et al. Screening of selective histone deacetylase inhibitors by proteochemometric modeling[J]. BMC Bioinformatics, 2012, 13: 212. DOI:10.1186/1471-2105-13-212 |
| [18] |
Chang J, Varghese DS, Gillam MC, et al. Differential response of cancer cells to HDAC inhibitors trichostatin A and depsipeptide[J]. Br J Cancer, 2012, 106(1): 116-25. DOI:10.1038/bjc.2011.532 |
| [19] |
Covington HE 3rd, Maze I, LaPlant QC, et al. Antidepressant actions of histone deacetylase inhibitors[J]. J Neurosci, 2009, 29(37): 11451-60. DOI:10.1523/JNEUROSCI.1758-09.2009 |
| [20] |
Wu Y, Lyu H, Liu H, et al. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung Cancer[J]. Sci Rep, 2016, 6: 31093. DOI:10.1038/srep31093 |
| [21] |
Conte M, Dell'aversana C, Sgueglia G, et al. HDAC2-dependent miRNA signature in acute myeloid leukemia[J]. FEBS Lett, 2019, 593(18): 2574-84. DOI:10.1002/1873-3468.13521 |
| [22] |
Chen S, Ye J, Chen X, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NFkappaB pathway dependent of HDAC3[J]. J Neuroinflammation, 2018, 15(1): 150. DOI:10.1186/s12974-018-1193-6 |
| [23] |
Mv Iorio CP, Croce CM. Interplay between microRNAs and the epigenetic machinery:an intricate network[J]. Biochim Biophys Acta, 2010, 1799(10/12): 694-701. DOI:10.1016/j.bbagrm.2010.05.005 |
| [24] |
Liu Y, Wang L, Lao J, et al. Changes in microRNA expression in the brachial plexus avulsion model of neuropathic pain[J]. Int J Mol Med, 2018, 41(3): 1509-17. |
| [25] |
Genda Y, Arai M, Ishikawa M, et al. microRNA changes in the dorsal Horn of the spinal cord of rats with chronic constriction injury:a TaqMan (R) low density array study[J]. Int J Mol Med, 2013, 31(1): 129-37. DOI:10.3892/ijmm.2012.1163 |
| [26] |
Arai M, Genda Y, Ishikawa M, et al. The miRNA and mRNA changes in rat hippocampi after chronic constriction injury[J]. Pain Med, 2013, 14(5): 720-9. DOI:10.1111/pme.12066 |
| [27] |
Chang HL, Wang HC, Chunag YT, et al. miRNA expression change in dorsal root ganglia after peripheral nerve injury[J]. J Mol Neurosci, 2017, 61(2): 169-77. |
| [28] |
Peng C, Li L, Zhang M D, et al. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes[J]. Science, 2017, 356(6343): 1168-71. DOI:10.1126/science.aam7671 |
| [29] |
Sakai A, Saitow F, Maruyama M, et al. MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated Potassium channels in chronic neuropathic pain[J]. Nat Commun, 2017, 8: 16079. DOI:10.1038/ncomms16079 |
| [30] |
Zhong L, Xiao W M, Wang F, et al. miR-21-5p inhibits neuropathic pain development via directly targeting C-C motif ligand 1 and tissue inhibitor of metalloproteinase-3[J]. J CELL BIOCHEM, 2019, 120(10): 16614-23. |
| [31] |
Sakai A, Saitow F, Miyake N, et al. miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability[J]. Brain, 2013, 136(9): 2738-50. DOI:10.1093/brain/awt191 |
| [32] |
Zhan LY, Lei SQ, Zhang BH, et al. Overexpression of miR-381 relieves neuropathic pain development via targeting HMGB1 and CXCR4[J]. Biomed Pharmacother, 2018, 107: 818-23. DOI:10.1016/j.biopha.2018.08.053 |
| [33] |
Zhang Y, Su Z, Liu HL, et al. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models[J]. Biomed Pharmacother, 2018, 107: 644-9. DOI:10.1016/j.biopha.2018.08.005 |
| [34] |
Liu JC, Xue DF, Wang XQ, et al. MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-kappaB signaling[J]. Kaohsiung J Med Sci, 2019, 35(3): 139-45. DOI:10.1002/kjm2.12025 |
| [35] |
Asahchop EL, Branton WG, Krishnan A, et al. HIV-associated sensory polyneuropathy and neuronal injury are associated with miRNA-455-3p induction[J]. JCI Insight, 2018, 3(23): e122450. DOI:10.1172/jci.insight.122450 |
| [36] |
T Wada JK, Furukawa Y. Histone deacetylase 1 enhances microRNA processing via deacetylation of DGCR8[J]. EMBO Rep, 2012, 13(2): 142-9. DOI:10.1038/embor.2011.247 |
| [37] |
Scott GK, Mattie MD, Berger CE, et al. Rapid alteration of microRNA levels by histone deacetylase inhibition[J]. Cancer Res, 2006, 66(3): 1277-81. DOI:10.1158/0008-5472.CAN-05-3632 |
| [38] |
Sun Q, Wang S, Chen J, et al. MicroRNA-190 alleviates neuronal damage and inhibits neuroinflammation via Nlrp3 in MPTPinduced Parkinson's disease mouse model[J]. J Cell Physiol, 2019, 234(12): 23379-87. DOI:10.1002/jcp.28907 |
| [39] |
Shi Y, Zhao CM, Zhang Y, et al. MicroRNA-142-3p relieves neuropathic pain by targeting high mobility group box 1[J]. J Cell Physiol, 2018, 41(1): 501-10. |
| [40] |
Jl Bjersing MB, Mannerkorpi K. Profile of circulating microRNAs in fibromyalgia and their relation to symptom severity:an exploratory study[J]. Rheumatol Int, 2015, 35(4): 635-42. DOI:10.1007/s00296-014-3139-3 |
 2019, Vol. 39
2019, Vol. 39

