2. 南方医科大学南海医院医学检验科,广东 佛山 528244;
3. 东 莞市第六人民医院医学检验科,广东 东莞 523008;
4. 广东医科大学东莞市医学活性分子开发与转化重点实验 室,广东 东莞 523808
2. Department of Laboratory Medicine, Nanhai Hospital, Southern Medical University, Foshan 528244, China;
3. Department of Laboratory Medicine, Dongguan Sixth People's Hospital, Dongguan 523008, China;
4. Dongguan Key Laboratory of Medical Bioactive Molecular Developmental and Translational Research, Guangdong Medical University, Dongguan 523808, China
近年来,结核耐药现象已变得日趋严重,尤以耐多药结核病(MDR-TB)和广泛耐药结核病(XDR-TB)的危害最大[1-3]。当前临床上采用的结核分枝杆菌药敏试验分为表型药敏试验和基因型药敏试验,表型药敏试验作为当前检测结核耐药性的主要方法,存在着检测周期长、成本高等缺点[4];而基因型药敏试验虽然在检测周期、灵敏度和特异度方面具有显著优势,但设备昂贵、操作流程复杂等问题也大大限制了其在MDR-TB检测中的临床应用[5-9]。
现有少数的结核耐药的代谢标志物研究报道中,基于代谢组学的生信分析起到了重要的作用,也取到了一系列的成果[10-12]。Huang等[13]发现黄嘌呤、4-吡哆酸盐、D-谷氨酸3种代谢物质可作为肺结核潜在的生物学标志物。Lau等[14]发现4种代谢物被鉴定为1-结核菌素腺苷衍生物,可能是结核杆菌代谢的新型标志物。Collins等[15]研究发现脂质相关代谢物磷脂酰甘油(PG(16:0_18: 1))和溶血磷脂酸(Lyso-PI(18:0))在血清中的含量升高,可作为活动性肺结核潜在的代谢标志物。然而,现阶段代谢组学技术更多的应用在肺结核生物学标志物中,针对不同结核耐药类型的代谢标志物研究较少,及其对临床应用价值的研究未见报道。本研究拟采用LC-MS/ MS技术检测中国南方广东地区人群中结核药敏、单耐药异烟肼、单耐药利福平、耐多药和多耐药患者的血清代谢物,筛选差异代谢物来寻找结核分枝杆菌不同耐药的潜在血清代谢诊断标志物,为结核杆菌耐药的筛选、诊断评估,提高其检测的灵敏度和特异性并提供一定的临床指导意义。
1 资料和方法 1.1 研究对象和样本采集选取佛山市第四人民医院、东莞市第六人民医院2017年10月~2018年3月做结核筛查的患者作为研究对象。受试者纳入标准:(1)痰涂片、痰培养结果阳性;(2)HIV阴性;(3)未经抗结核药物治疗;(4)同意加入本次研究并签署知情同意书。受试者排除标准:PNB检测阳性。基于痰涂片、痰培养和药敏实验结果,我们将结核药敏(DS)患者30例、单耐药异烟肼(MR-INH)患者8例、单耐药利福平(MR-RFP)患者2例、耐多药(MDR)患者14例、多耐药(PR)患者24例纳入研究。收集受试者血液样本,离心收集血清于-20 ℃保存用于LC-MS/MS检测。
1.2 血浆预处理取100 µL血浆样品,加入600 µL甲醇(含有1 mmol/L丁基羟基甲苯),超声5 min。加入1.8 mL甲基叔丁基醚,室温振荡1 h。加入500 µL水,室温孵育10 min,不间断振荡。静置2 min后12 000 r/min离心10 min。吸取600 µL上层脂质和300 µL下层(2:1)水相层混合转移到新EP管,真空干燥,用200 µL的乙腈和水(1:1)混合液溶解,离心取上清上机。
1.3 相液质联用LC-MS/MS检测代谢谱使用ACE(Aberdeen,Scotland)Excel2C-18PFP(100×2.1 mm,2 μm)色谱柱和C18保护柱。流动相A含有0.1%甲酸的水,B含有0.1%甲酸的乙腈。色谱梯度从2% B开始1 min,在10 min内进入98% B,保持2 min,并且在平衡3 min之前在30 s内降低至2%。进样量为2 μL,柱子保持在35 ℃。所有样品注射两次。质谱仪在加热电喷雾电离(HESI)模式下运行,喷雾电压为3.5 kV,毛细管温度为300 ℃,鞘气流量为50,辅助气体为10.S透镜RF水平设置为40.S透镜电压设置为25 V。全扫描采集分辨率为70 000,MS/MS采集列表为17 500。
1.4 代谢物的鉴定和筛选采用Pareto-scaling方法进行归一化,应用MetaboAnalyst 4.0软件进行差异分析和富集分析。本研究采用变异倍数分析(Fold Change Analysis,FC Analysis)、T检验对代谢物进行单变量统计分析,并绘制火山图(Volcano Plot),以P < 0.05、fold change>2倍或 < 0.5做为差异代谢指标的筛选标准。差异数据使用Metlin_AMRT_PCDL、Metlin_Lipids_AM_PCDL数据库比对,鉴定出相应代谢物。利用显著性差异的代谢物对各组样本进行层次聚类,通过等级聚类分析各组样本的聚类情况及差异代谢物在各组的表达模式,筛选出能预示不同结核耐药类型潜在的血清代谢标志物。
1.5 数据分析应用MetaboAnalyst-Enrichment Analysis方法对差异代谢物数据进行相关富集分析,MetaboAnalystPathway Analysis进行相关代谢通路分析。数据采用SPSS20.0软件分析,正态分布的计量资料结果以均数±标准差表示,两组间比较采用t检验,两组以上的比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 研究对象临床信息DS、DR患者各30(38.46%)、48(61.54%)例,DR患者中MR-INH患者8(16.67%)例、MR-RFP患者2(4.17%)例、MDR患者14(29.17%)例、PR组患者24(50.00%)例。有所有患者痰培养、痰涂片和MTB-Ag检测阳性,HIV检测阴性,所有样本未经抗结核治疗(表 1)。
| 表 1 研究对象基本信息 Tab.1 Demographic and clinical data of the patients |
通过液质联用技术我们考查了结核药敏和结核不同耐药类型患者血清代谢的功能研究。在SMICAP13.0软件正式分析前,对数据组进行归一化处理,以获得更加直观且可靠的结果。为了判别五组之间是否具有差异(结核药敏患者、单耐药异烟肼患者、单耐药利福平患者、耐多药患者、多耐药患者),我们采用PCA及OPLS-DA建模方法对样本进行分析。PCA主成分分析显示,DS组和DR组样本能明显区分开,其他组样本未能明显的区分开来,可能由于血清样本的多样性和复杂性使得潜在的差异未能体现。5组的OPLS-DA主成分分析显示,MDR、MR-INH、PR、DS组能很好的区分开来,可见组内关联性很高,组间差异很大,MR-RFP由于样本量太少不能很明显的看出聚类趋势(图 1)。
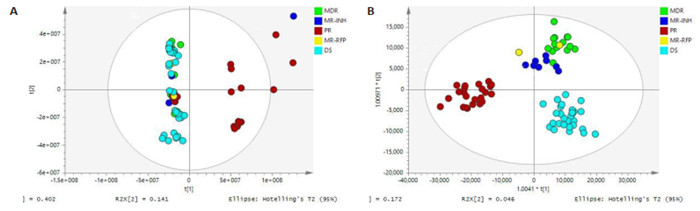
|
图 1 各组患者血清代谢物质的PCA分析及OPLS-DA分析 Fig.1 Analysis of serum metabolites in each group by PCA and OPLS-DA. A: PCA analysis of DS, MR-INH, MR-RFP, MDR, and PR group showed that the samples of DS group and MDR group could be distinguished obviously, but the samples of other groups could not; B: OPLS-DA analysis showed that MDR, MR-INH, PR, and DS groups can be well distinguished; MRRPF clustering trend is not obvious. |
为了进一步筛选不同组分之间的差异性物质,采用差异倍数分析(FC Analysis)、T检验对代谢物进行单变量统计分析,并绘制火山图(Volcano Plot),以P < 0.05、fold change>2倍或 < 0.5做为差异代谢指标的筛选标准。通过筛选我们发现,与DS相比MR-INH组有286种差异代谢物,其中显著上调的有171种,明显下调的115种;MR-RFP组有362种差异代谢物,其中显著上调的有202种,明显下调的160种;MDR组,有277种代谢差异物质,其中120种明显上调,157种明显下调;PR组,有1208种代谢差异物质,其中有643种明显上调,565种明显下调(图 2)。
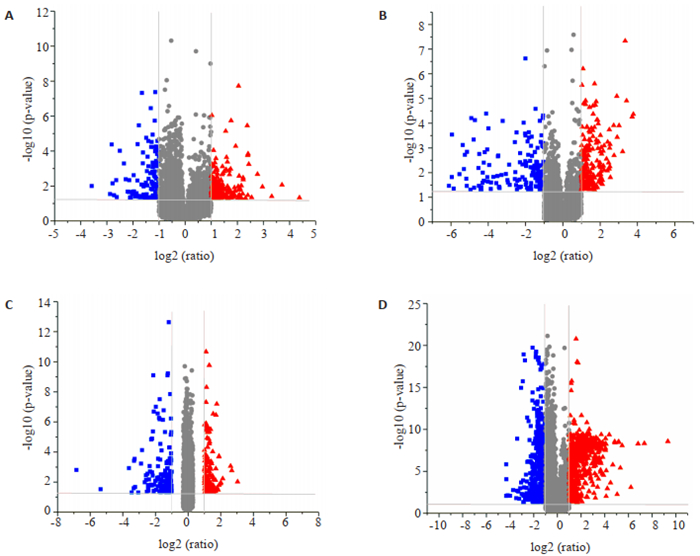
|
图 2 各组患者血清代谢物火山图(Volcano Plot) Fig.2 Volcano plots of serum metabolites in each group. A: Volcano Plot of serum metabolites in patients with MR-INH and DS; B: Volcano plot of serum metabolites in patients with MR-RFP and DS; C: Volcano plot of serum metabolites in patients with MDR and DS; D: Volcano plot of serum metabolites in patients with PR and DS. The red dot represent the significantly upregulated metabolite (FC>2, P < 0.05), the blue dot represent the significantly down-regulated metabolite (FC < 0.5, P < 0.05). |
通过数据库比对我们分别列出单耐药异烟肼、单耐药利福平、耐多药、多耐药与药敏患者比较差异的代谢物(表 2~5)。
| 表 2 与药敏患者相比单耐药异烟肼患者血清代谢组学差异指标筛选结果 Tab.2 Screening results of serum metabonomics difference indexes in patients with single drug resistance to INH compared with patients with drug sensitivity |
| 表 3 与药敏患者相比单耐药利福平患者血清代谢组学差异指标筛选结果 Tab.3 Screening results of serum metabonomics difference indexes in patients with single drug resistance to RFP compared with patients with drug sensitivity |
| 表 4 与药敏患者相比耐多药患者血清代谢组学差异指标筛选结果 Tab.4 Screening results of serum metabonomics difference indexes in patients with single drug resistance to MDR compared with patients with drug sensitivity |
| 表 5 与药敏患者相比多耐药患者血清代谢组学差异指标筛选结果 Tab.5 Screening results of serum metabonomics difference indexes in patients with single drug resistance to PR compared with patients with drug sensitivity |
各组间差异物质比较发现,Acetylagmatine、Aminopentol、(Z)-22-Hentriacontene-2, 4-dione、3-Methylbutanamine、DL-Cerebronic acid、2, 3-DINOR-THROMBOXANE B2、2, 3-dinor, 6-keto-PGF1&alpha、(3R)-3- isopropenyl-6-oxoheptanoic acid、isopropyl ester、Linolenyl oleate、Kamahine C、DG(18:2(9Z, 12Z)/0:0/ 18:2(9Z, 12Z)) (d5)、Heneicosanedioic acid、(3R)-3-isopropenyl-6-oxoheptanoic acid、Tetracosanyl oleate可作为区别单耐异烟肼患者的潜在标志物。1-Hexadecylamine、1-Octene、Tetrabutylammonium、Arginyl-Glutamine、3E, 5E-tridecadienoic acid、Phthioceranic acid (C45)、BUFEXAMAC、2-Hydroxy-24-ketooctacosanolide、2-Imino-4-methylpiperidine、Terephthalic acid可作为区别单耐利福平患者的潜在标志物。Trimethylamine、AV-Ceramide、N-Cyclohexanecarbonylpentadecylamine、Artomunoxanthentrione epoxide、13, 14-dihydro-16, 16-difluoro Prostaglandin D2、Magnoshinin、Quinapril hydrochloride、Tolvaptan、Soyasapogenol B 3-O-D-glucuronide、Voacamine、D-Glucosyldihydrosphingosine、Verazine可作为区别耐多药患者的潜在标志物。PIP(18:1(11Z)/18:3(6Z, 9Z, 12Z))、Oleoyl glycine、(7R, 8R, E)-6-((2R, E)-6, 7-dihydroxy-2, 5-dimethyloct-4- en-1-ylidene)-8-methyloctahydroindolizine-7, 8-dio、dimethyl amine、Longamide、10Z, 13Z-nonadecadienoic acid、Asteltoxin、isopropyl ester、3E, 9Z, 12Z, 15Z-octadecatetraenoic acid、Fasciculic acid C、Tetranactin、Phytolaccoside E可作为区别多耐药患者的潜在标志物。所有的差异数据详见表格文件。
2.4 结核不同耐药类型血清代谢标志物聚类分析及鉴定对结核药敏、单耐药异烟肼、单耐药利福平、耐多药、多耐药结核患者的差异代谢物检测数据进行聚类分析(图 3)。相对于DS组,Acetylagmatine、Aminopentol、(Z)-22-Hentriacontene-2, 4-dione、3-Methylbutanamine、DL-Cerebronic acid在MR-INH组中上调,2, 3-DINOR-THROMBOXANE B2、2, 3-dinor, 6-keto-PGF1&alpha、(3R)-3-isopropenyl-6-oxoheptanoic acid、isopropyl ester、Linolenyl oleate、Kamahine C、DG (18:2(9Z, 12Z)/0:0/18:2(9Z, 12Z)) (d5)、Heneicosanedioic acid、(3R)-3-isopropenyl-6-oxoheptanoic acid、Tetracosanyl oleate在MDR-INH组中下调;在MR-RFP组中1-Hexadecylamine、1-Octene、Tetrabutylammonium、Arginyl-Glutamine上调,3E, 5E-tridecadienoic acid、Phthioceranic acid (C45)、BUFEXAMAC、2-Hydroxy- 24-keto-octacosanolide、2-Imino-4-methylpiperidine、Terephthalic acid下调;MDR组中Trimethylamine、AV-Ceramide、N-Cyclohexanecarbonylpentadecylamine、Artomunoxanthentrione epoxide、13, 14-dihydro-16, 16-difluoro Prostaglandin D2、Magnoshinin、Quinapril hydrochloride、Tolvaptan上调,Soyasapogenol B 3-O-D-glucuronide、Voacamine、D-Glucosyldihydrosphingosine、Verazine组下调;PIP(18:1(11Z)/18:3(6Z, 9Z, 12Z))、Oleoyl glycine、(7R, 8R, E)-6-((2R, E)-6, 7-dihydroxy-2, 5-dimethyloct-4-en-1-ylidene)-8-methy-loctahydroindolizine-7, 8-dio、dimethyl amine、Longam-ide在PR组中上调、10Z, 13Z-nonadecadienoic acid、Asteltoxin、isopropyl ester、3E, 9Z, 12Z, 15Z-octadecate-traenoic acid、Fasciculic acid C、Tetranactin、Phytolacco-side E在PR组中下调。上述在各组间差异表达的代谢物可作为区分结核不同耐药类型的潜在标志物。
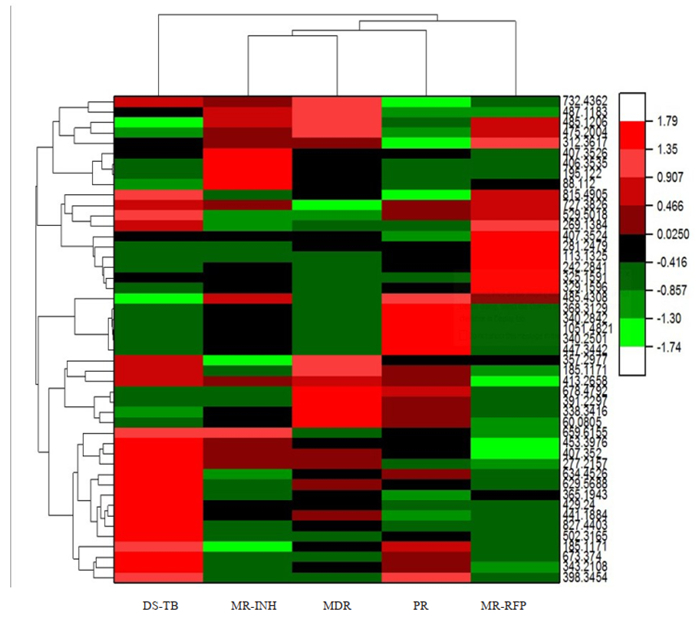
|
图 3 结核药敏、单耐药异烟肼、单耐药利福平、耐多药、多耐药结核患者潜血清代谢标志物的热图聚类分析 Fig.3 Thermographic cluster analysis of latent serum metabolic markers in tuberculosis patients with drug sensitivity, single drug resistance to isoniazid, single drug resistance to rifampicin, multi-drug resistance and poly-drug resistance. The transverse coordinate stands for sample groups and the longitudinal coordinate stands for m≤z of metabolites. |
采用MetaboAnalyst 4.0中的Pathway Analysis对上述差异代谢物质进行通路富集分析,结果显示Phosphatidylcholine(磷脂酰胆碱)、LysoPC(18:1(9Z))、Acetone(丙酮)、Trimethylamine(三甲胺)、(3R)-3-Isopropenyl-6-oxoheptanoate((3R)-3-异丙烯-6-氧庚酸酯)5种代谢物主要参与了Ketone Body Metabolism、Phospholipid Biosynthesis代谢。
3 讨论结核耐药是当今全球面临的重要公共卫生问题,结核侵入机体后会引起宿主一系列的免疫反应,具体体现在血清代谢物中,因此,血清代谢物中含有大量反应结核的信息[12, 16]。采用代谢组学方法筛查和检测样本血清中的代谢标志物,对早期发现、诊断,指导治疗和判断结核分枝杆菌耐药具有重要意义[17]。本研究选取DS、MR-INH、MR-RFP、MDR和PR患者的血清样本进行代谢组学分析。结果发现,与DS组相比,在MR-INH、MR-RFP、MDR和PR组中Eicosanoyl-EA含量均明显升高;Phthalic acid Mono-2-ethylhexyl Ester在MR-RFP组中含量降低,在其它三组中含量均升高。相较于DS组,N-stearoyl glutamic acid、25-dihydroxy-26, 27-dimethyl-20, 21-didehydro-23-oxavitamin D3、25-hydroxy-cholesterol(d3)、Penaresidin A 4种代谢物在MR-INH、MR-RFP、MDR组中含量具有明显差异,除25-dihydroxy-26,27-dimethyl-20,21-didehydro-23-oxavitamin D3在MR-RFP组中含量降低外,其余代谢物含量均明显升高。此外,结果分析还发现在MR-INH、MR-RFP和PR组种5-Pentacosyl-1, 3-benzenediol含量显著升高,值得注意的是,9, 15-dioxo-11R-hydroxy-2, 3, 4, 5-tetranor-prostan-1, 20-dioic acid在MR-INH、MDR和PR组中的含量均明显低于DS组。综上分析,此8种代谢物可能是指示结核杆菌耐药的潜在代谢标志物。本研究筛选出的邻苯二甲酸单-2-乙基己基酯(Phthalic acid Mono-2-ethylhexyl Ester, MEHP)是邻苯二甲酸二乙基己基酯(DEHP)在人体中的代谢物,其毒性是DEHP的10倍[18]。既往研究发现,MEHP升高可通过GFER蛋白激活Akt通路引起子宫颈癌细胞增殖[19]、抑制卵母细胞发育[20]。与之相似的是,本研究同样发现在MR-INH、MR-RFP、MDR和PR组中,MEHP、Eicosanoyl-EA的含量与DS组相比存在明显差异,我们推测MEHP和Eicosanoyl-EA有可能是结核杆菌产生耐药的关键调节物质。然而该两种物质是否与上述其他6种代谢物相互作用引起结核杆菌耐药,以及通过何种代谢通路影响结核耐药,需要进一步探究。
异烟肼、利福平是目前治疗结核病的一线药物,但随着耐药结核的愈演愈烈,快速诊断结核的耐药种类已成为临床急需解决的问题[21-23]。在国内外,通过构建代谢物模型来诊断结核耐药类型的研究还未见报道。本研究通过LC-MC技术检测5组患者血清样本中的代谢物发现,在MR-INH组中,Acetylagmatine、Aminopentol、PAF C-16、PE(18:0/0:0)含量明显升高,Tetracosanyl oleate含量显著降低,此5种代谢物有可能作为结核耐异烟肼的潜在代谢标志物。与DS组相比,Ala His Pro Thr的含量在MR-RPF组中明显升高,Glycinoprenol-9、Terephthalic acid、2-Imino-4-methylpiperidine、2-Hydroxy- 24-keto-octacosanolide、TG(12:0/12:0/20:1(11Z))[iso3]的含量显著降低,此6种代谢物可能是指示结核耐利福平的潜在代谢标志物。相较于DS组,在MDR组中,Trimethylamine含量升高,Verazine、Cer(d18:0/12:0)的含量明显下降,此3种代谢物可能是耐多药结核分支结核杆菌所特有的。与DS相比,PR组中PIP(18:1(11Z)/ 18:3(6Z, 9Z, 12Z))、Pro Arg Trp Tyr、N-Methyldioctylamine的含量高达20倍以上,His His Arg Arg、Oleoyl glycine、Cer(d18:0/20:0(2OH))、PG(14:0/14:0)的含量高达12倍以上,此7种代谢物可能是多耐药结核的特异代谢标志物。Loots du T发现在耐利福平结核杆菌中,氧化应激产物如烷烃、醇、脂肪酸等代谢物浓度升高,推测23种代谢物可作为代谢标志物[24-25]。通过上述结果分析,本研究发现不同耐药结核杆菌的代谢物种类与含量存在着差异性,为构建诊断不同种类耐药结核杆菌的代谢物模型提供了实验基础。
在功能富集分析中,我们发现磷脂酰胆碱代谢物质参与了Ketone Body Metabolism、Phospholipid Biosynthesis代谢。磷脂酰胆碱是一种两性分子,作为二棕榈酰磷脂酰胆碱重要组成成分,参与了肺泡表面活性物质的生成。Wood等通过血浆脂质组学分析发现,在结核患者血浆中,甘油酰胆碱骨架经sn-2去酰化重塑生成溶血磷脂酰胆碱,导致血浆中包括磷脂酰胆碱在内的多种脂质物质发生改变[26]。López-Hernández等发现在2型糖尿病结核易感性的患者中,脂质代谢的变化主要体现在甘油磷脂上,而2型糖尿病合并结核病的患者血清中,均出现磷脂酰胆碱水平的下降[27]。同样的,我们在上述4组结核耐药患者的血清中检测出磷脂酰胆碱含量降低,推测结核耐药与磷脂酰胆碱代谢通路异常调节有关。
由于耐药类型的不同使得血清代谢标志物的表达存在着差异,本文通过LC-MS/MS和代谢组学分析首次报道了不同结核耐药类型患者的血清代谢物,为构建诊断不同结核杆菌耐药类型的代谢物模型,提供了理论基础。代谢物诊断模型具有检测周期短、特异度和准确度高、高通量等优点,解决了现有结核耐药诊断技术检测周期短、工作量大、成本高等问题,具有较大的临床应用前景。我们将在上述实验的基础上增加样本量对潜在代谢标记物在各组的含量进行验证实验,对明确差异代谢物的功能做进一步研究并深入探讨其参与结核耐药的作用机制。
| [1] |
Seaworth BJ, Griffith DE. Therapy of Multidrug-resistant and extensively drug-resistant tuberculosis[J]. Microbiol Spectr, 2017, 5(2): 1-23. |
| [2] |
van den Hof S. Hotspots for transmission of extensively drugresistant tuberculosis[J]. Int J Tuberc Lung Dis, 2019, 23(6): 643-4. DOI:10.5588/ijtld.19.0272 |
| [3] |
Nelson KN, Jenness SM, Mathema B, et al. Social mixing and clinical features linked with transmission in a network of extensively drug-resistant (XDR) tuberculosis cases in KwaZulu-Natal, South Africa[J]. Clin Infect Dis, 2019, ciz636. DOI:10.1093/cid/ciz636 |
| [4] |
Cui ZL, Wang J, Zhu CT, et al. Evaluation of a novel biphasic culture medium for recovery of mycobacteria:a multi-center study[J]. PLoS One, 2012, 7(4): e36331. DOI:10.1371/journal.pone.0036331 |
| [5] |
Chakravorty S, Simmons AM, Rowneki MA, et al. The new xpert MTB/RIF ultra:improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing[J]. MBio, 2017, 8(4): e00812-7. |
| [6] |
Yf L, Beena PJ, Kls D, et al. Primary multidrug resistant tuberculosis and utility of line probe assay for its detection in smear-positive sputum samples in a tertiary care hospital in south india[J]. J Pathog, 2016, 2016: 6235618. |
| [7] |
多丽娜, 王婷婷, 宋兴勃, 等. 分子线性探针技术分析四川地区结核分枝杆菌耐药情况[J]. 南方医科大学学报, 2011, 31(5): 822-4. |
| [8] |
Lu W, Chen C, Shao Y, et al. Evaluation of biochip system in determining isoniazid and rifampicin resistances of mycobacterium tuberculosis in sputum samples[J]. PLoS One, 2012, 7(12): e52953. DOI:10.1371/journal.pone.0052953 |
| [9] |
Phelan J, O'sullivan DM, Machado D, et al. The variability and reproducibility of whole genome sequencing technology for detecting resistance to anti-tuberculous drugs[J]. Genome Med, 2016, 8(1): 132. DOI:10.1186/s13073-016-0385-x |
| [10] |
Silva CA, Graham B, Webb K, et al. A pilot metabolomics study of tuberculosis immune reconstitution inflammatory syndrome[J]. Int J Infect Dis, 2019, 84: 30-8. DOI:10.1016/j.ijid.2019.04.015 |
| [11] |
du Preez I, Luies L, Loots DT. The application of metabolomics toward pulmonary tuberculosis research[J]. Tuberculosis (Edinb), 2019, 115: 126-39. DOI:10.1016/j.tube.2019.03.003 |
| [12] |
Weiner J3, Maertzdorf J, Sutherland JS, et al. Metabolite changes in blood predict the onset of tuberculosis[J]. Nat Commun, 2018, 9(1): 5208. DOI:10.1038/s41467-018-07635-7 |
| [13] |
Huang H, Shi LY, Wei LL, et al. Plasma metabolites Xanthine, 4-Pyridoxate, and D-glutamic acid as novel potential biomarkers for pulmonary tuberculosis[J]. Clin Chim Acta, 2019, 498(11): 135-42. |
| [14] |
Lau SK, Lam CW, Curreem SO, et al. Identification of specific metabolites in culture supernatant of Mycobacterium tuberculosis using metabolomics:exploration of potential biomarkers[J]. Emerg Microbes Infect, 2015, 4(1): e6. DOI:10.1038/emi.2015.6 |
| [15] |
Collins JM, Walker ID, Jones DP, et al. High-resolution plasma metabolomics analysis to detect Mycobacterium tuberculosis-associciated metabolites that distinguish active pulmonary tuberculosis in humans[J]. PLoS One, 2018, 13(10): e0205398. DOI:10.1371/journal.pone.0205398 |
| [16] |
Zhou J, Yin Y. Use of liquid Chromatography-Mass SpectrometryBased metabolomics to identify biomarkers of tuberculosis[J]. Methods Mol Biol, 2019, 1859: 241-51. DOI:10.1007/978-1-4939-8757-3_13 |
| [17] |
Takenami I, de Oliveira CC, Petrilli JD, et al. Serum antiphospholipid antibody levels as biomarkers for diagnosis of pulmonary tuberculosispatients[J]. Int J Tuberc Lung Dis, 2018, 22(9): 1063-70. DOI:10.5588/ijtld.17.0874 |
| [18] |
Singh N, Dalal V, Mahto JK, et al. Biodegradation of phthalic acid esters (PAEs) and in silico structural characterization of mono-2-ethylhexyl phthalate (MEHP) hydrolase on the basis of close structural homolog[J]. J Hazard Mater, 2017, 338(15): 11-22. |
| [19] |
Yang WL, Tan W, Zheng JF, et al. MEHP promotes the proliferation of cervical cancer via GPER mediated activation of Akt[J]. Eur J Pharmacol, 2018, 824(5): 11-6. |
| [20] |
Kalo D, Carvalho AV, Archilla C, et al. Mono (2-ethylhexyl) phthalate (MEHP) induces transcriptomic alterations in oocytes and their derived blastocysts[J]. Toxicology, 2019, 421(1): 59-73. |
| [21] |
Mekonnen B, Mihret A, Getahun M, et al. Evaluation of the tuberculosis culture color plate test for rapid detection of drug susceptible and drug-resistant mycobacterium tuberculosis in a resource-limited setting, addis ababa, ethiopia[J]. PLoS One, 2019, 14(5): e0215679. DOI:10.1371/journal.pone.0215679 |
| [22] |
Mokry J, Porvaznik I, Kusnir P, et al. Detection of resistance to anti-tuberculosis drugs in the clinical isolates of mycobacterium tuberculosis from slovakia through comparison between phenotypic and genetic methods and evaluation of restistance levels with clinical paramerers[J]. J Physiol Pharmacol, 2019, 70(1): 105-14. |
| [23] |
范贵荣, 杨致邦, 黄进. 异烟肼对脓肿分枝杆菌L型的诱导作用[J]. 南方医科大学学报, 2013, 33(7): 1036-40. |
| [24] |
Loots DT. An altered Mycobacterium tuberculosis metabolome induced by katG mutations resulting in isoniazid resistance[J]. Antimicrob Agents Chemother, 2014, 58(4): 2144-9. DOI:10.1128/AAC.02344-13 |
| [25] |
Loots DT. New insights into the survival mechanisms of rifampicinresistant mycobacterium tuberculosis[J]. J Antimicrob Chemother, 2016, 71(3): 655-60. DOI:10.1093/jac/dkv406 |
| [26] |
Wood PL, Tippireddy S, Feriante J. Plasma lipidomics of tuberculosis patients:altered phosphatidylcholine remodeling[J]. Future Sci OA, 2018, 4(1): FSO255. DOI:10.4155/fsoa-2017-0011 |
| [27] |
Lopez-Hernandez Y, Lara-Ramirez EE, Adrian Lopez J, et al. Glycerophospholipid metabolism alterations in patients with type 2 diabetes mellitus and tuberculosis comorbidity[J]. Arch Med Res, 2019, 50(2): 71-8. DOI:10.1016/j.arcmed.2019.05.006 |
 2019, Vol. 39
2019, Vol. 39

