2. Laboratory of Molecular Medicine, Hubei University of Arts and Science, Xiangyang 441053, China
2. 湖北文理学院分子医学实验室,湖北 襄阳 441053
Ulcerative colitis (UC) is an inflammatory disease of the colon characterized by mucosal erosion and lymphoid infiltration, and is associated with a high risk of colon cancer development[1, 2]. Dextran sodium sulphate (DSS)-induced colitis is a common experimental model of UC, which presents with symptoms and histological changes in the colon similar to those of human UC[3]. So far there is still no cure for UC, and current studies are focused on the pathogenic mechanisms to identify potential therapeutic targets for relieving the symptoms.
The pathogenesis of UC is poorly understood due to the involvement of the immunological, host and environmental factors. Immune function abnormalities and their interactions with the commensal microbiota in the digestive tract are the key factors contributing to the development of UC[4], which is also a typical disease involving CD4+ T helper cell responses [5, 6]and CD4+ Th1/Th17-mediated immune responses [7]. Th1 cells secrete pro-inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in the intestinal mucosa. Th17 cells infiltrate the inflamed intestine and produce IL-17A to mediate the pro-inflammatory responses such as recruitment of neutrophils and upregulation of TNF-α [8]. Inhibition of Th1/Th17-mediated pro-inflammatory responses and controlling T cell-mediated immune response can thus be a therapeutic strategy for UC[9-11].
Kirenol, the main diterpenoid component isolated from the Chinese medicinal herb Herba Siegesbeckiae[12-14], possesses anti-inflammatory and immunosuppressive activities and has long been used as a traditional medicine for treatment of arthritis [15]. Our previous studies suggested that kirenol could inhibit proliferation and induce apoptosis of inflammatory lymphocytes in a rat model of collagen-induced arthritis [16] and in a mouse model of MOG-induced experimental autoimmune encephalomyelitis [17]. However, the role of kirenol in gut damage and inflammatory response remains unclear.
In the present study, we further examined the effect of kirenol on inflammatory response in DSS-induced colitis and explored the mechanisms in light of T helper cell response, colon inflammation, and lymphocyte apoptosis.
MATERIALS AND METHODS DSS-induced colitis in rats and drug administrationSixteen female C57BL/6 mice (6 to 8 weeks old, weighing approximately 20 g) were bred at the Second Laboratory of Animal Biosafety, Xiangyang First People's Hospital Affiliated to Hubei University of Medicine. All the experimental procedures and protocols were approved by the Hubei University of Medicine Animal Ethics Committee and were performed in accordance with the institutional guidelines and regulations. The mice were randomized equally into UC model and kirenol groups, and all the mice were given 30 g/L DSS (relative molecular mass of 36 000-50 000; MP Biomedicals) in drinking water for 7 days to induce acute colitis. In kirenol group, the mice were treated daily with oral gavage of 2 mg/kg kirenol from day 0 to day 7, and those in the UC model group received equal volumes of distilled water in the same manner. All the mice were examined daily for the development of colitis, and the disease activity index (DAI) score was assessed using Cooper's method [18] following the scoring criteria listed in Tab. 1.
| Tab.1 Criteria for disease activity index (DAI) scoring of the mice with DSS-induced colitis |
After completion of the treatment, the mice were euthanized and the colon was harvested and weighed. The length from the anus to the cecum was measured and the length/body weight ratio (cm/g) of the colon was calculated. The solon segment (1 cm) near the anus was dissected and fixed in 4% polyformaldehyde, embedded in paraffin and sectioned at 5 μm for HE staining and observation under light microscopy to assess the severity of inflammation, extent of injury and crypt damage. The histopathological scores of the colon were determined based on the criteria listed in Tab. 2.
| Tab.2 Criteria for histopathological scoring of the colon tissue of the mice |
Mesenteric lymph nodes (MLNs) were harvested from the mice on day 7 after DSS treatment and pushed through a mesh to prepare the single cell suspension. The cells were washed, centrifuged at 1200 g/min for 5 min, and then counted.
Detection of cytokinesActivated lymphocytes can secrete cytokines. The lymphocytes from the MLNs in each group were plated (5 × 105 cells/well) in 96-well plates and stimulated in triplicate wells with anti-CD3 (1 μg/mL)+antiCD-28 CD3 (2 μg/mL) (BD Biosciences, USA). After 48 h, IFN-γ, IL-17A, IL-6 and TNF-α levels in the supernatants were detected using ELISA kits (eBioscience, USA).
Apoptosis assayThe MLNs from UC mice were harvested on day 7, and single cell suspension was prepared. Then cells were stained with Annexin V-FITC-PI (Antgene, Wuhan) or anti-CD4-PE/Annexin V-FITC (BD Biosciences, USA) for analysis of the percentages of Annexin V+ PI+ cells or CD4+ Annexin V+ cells using flow cytometry (BD Biosciences, USA), respectively.
Statistical analysisThe results are presented as Mean±SD. The difference in DAI scores between the distilled water- and kirenol-treated groups was tested using one-way ANOVA. The differences in colon length, histological score, cytokine production and cell frequency between distilled water- and kirenol-treated mice were evaluated with two-tailed unpaired Student's t-test. All the statistical tests were performed using Prism 5, and a P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS Kirenol ameliorates DSS-induced UCThe mice were treated with a daily oral gavage of kirenol or distilled water for 8 days, starting on day 0 of DSS treatment. Daily evaluation of the DAI showed that kirenol treatment significantly ameliorated the severity of DSS-induced UC in the mice, and the DAI scores began to decrease significantly since day 4 as compared with those in the UC model group (Fig. 1).
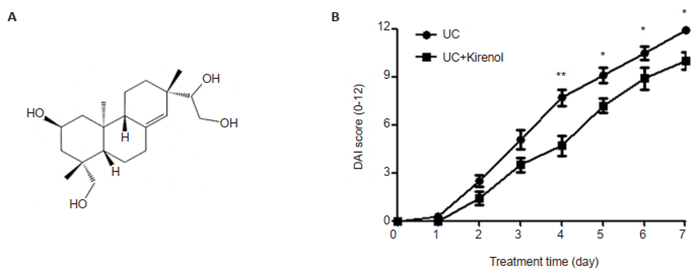
|
Fig.1 Kirenol relieves DSS-induced ulcerative colitis in mice. A: Chemical structure of kirenol; B: DAI scores of the UC model mice and kirenol-treated mice (*P < 0.05, **P < 0.005, n=8). |
We used colon shortening as a marker of colon inflammation. We collected the colons from the mice on day 7, and as shown in Fig. 2, the colon of kirenoltreated mice was significantly longer than that of UC model mice with on average length of 6.838±0.8467 cm. In addition, the colon length/body weight ratio was significantly greater in kirenol-treated mice than distilled water-treated UC mice. Histological examinations of the colon tissues revealed massive inflammatory infiltration in the colon of UC mice (Fig. 3A), whereas only minimal infiltration by inflammatory lymphocytes was found in kirenol-treated UC mice (Fig. 3B), and the histopathological scores differed significantly between the two groups (Fig. 3C).
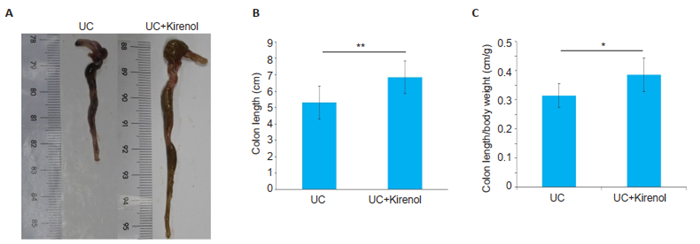
|
Fig.2 Gross observation of the colon samples from UC model and kirenol-treated mice. A: A representative image of colons in the two groups; B, C: Statistical analysis of the colon length and colon length/body weight ratio, respectively (*P < 0.05, **P < 0.005, n=8). |
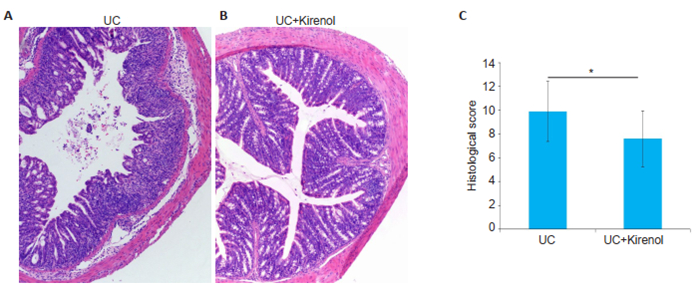
|
Fig.3 Kirenol attenuates the severity of colon injury in DSS-treated mice. A, B: Representative image of the colons in UC model and kirenol-treated groups, respectively (HE staining, × 100); C: Comparison of histopathological scores between the two groups (*P < 0.05, n=8). |
To investigate the immunological mechanisms through which kirenol alleviates UC in mice, we examined the levels of IFN-γ, IL-17A, IL-6 and TNF-α using ELISA in the supernatants of single cell suspensions from the MLNs of UC mice after incubation in the presence of anti-CD3+ anti-CD28 for 48 h. The results showed that compared with the control treatment, kirenol treatment of UC mice significantly reduced the levels of IFN-γ, IL-17A, IL-6 and TNF-α in the activated MLN lymphocytes (Fig. 4), suggesting that kirenol downregulated Th1/Th17-mediated inflammatory response.
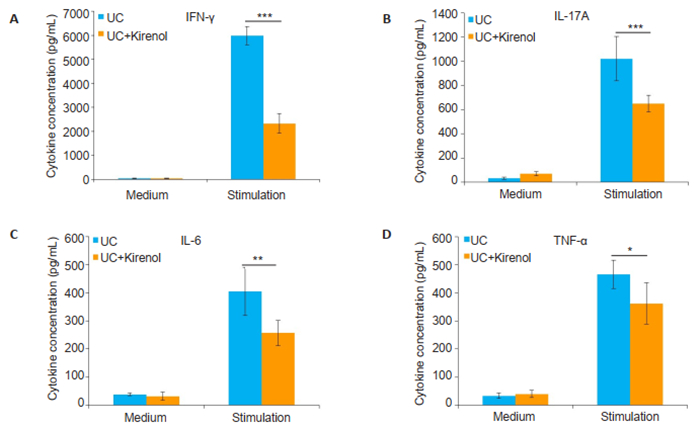
|
Fig.4 Kirenol inhibits secretion of inflammatory cytokines by activated MLNs lymphocytes. The lymphocytes were obtained from the MLNs of UC mice on day 7 and cultured in the presence of anti-CD3 +anti-CD28 for 48 h. IFN-γ (A), IL-17A (B) IL-6 (C) and TNF-α (D) secretion by activated lymphocytes in the supernatants was determined by ELISA assay. The results are presented as Means±SD (*P < 0.05, **P < 0.005, ***P < 0.005, n=8). |
The reduced inflammation in T cell-driven colitis could be a result of impaired T cell survival in kirenol-treated mice. We then assessed the apoptosis of lymphocytes and CD4+ T cells from the MLNs of UC mice treated with kirenol. We observed higher percentages of apoptotic lymphocytes and CD4+ T cells in the MLNs of kirenol-treated mice as compared with those of UC mice (Fig. 5), indicating that kirenol promoted apoptosis of the lymphocytes especially CD4+ T cells.
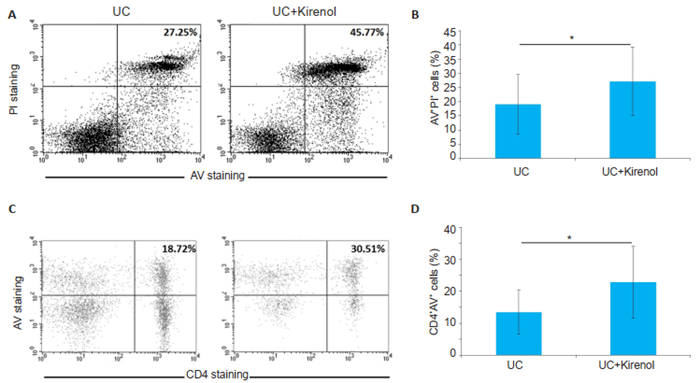
|
Fig.5 Kirenol induces apoptosis of lymphocytes and CD4+T cells from the MLNs of UC mice. A: Representative data of AV+ PI+ cells analyzed by flow cytometry; B: Quantification of the proportion of AV+PI+ cells (*P < 0.05, n=8); C: Representative data of CD4+AV+ cells analyzed by flow cytometry; D: Quantification of the proportion of CD4+AV+ cells (*P < 0.05, n=8). |
Our results show that in mice with DSS-induced UC, kirenol reduces intestinal inflammation and the DAI scores by ameliorating histological inflammation, inhibiting inflammatory cytokine production, and promoting apoptosis of lymphocytes especially CD4+ T cells, which further confirms the immunomodulatory activities of kirenol as have been validated in different models of immunopathology[16, 17, 19].
The pathogenesis of UC has not been fully understood but it is generally recognized that UC arises from the abnormal activation of the mucosal immune system, which results in chronic inflammation in association with the dysregulation of the cytokine network. Th1/Th17 are associated with autoimmune diseases and inflammatory reactions[20, 21], and their activation plays a pivotal role in the pathogenesis of UC[22]. Studies have shown that CD4+ T cells deficient in Th1-related transcription factors are unable to induce colitis after their transfer in recipient mice [23]. IFN-γ level is found to increase significantly in patients with UC [24]. The IL-17A-producing CD4+ Th17 cells also play a critical role in UC [25], and IL-17A cooperates with other Th17 cytokines or Th1 cytokines to expand UC-associated inflammatory response.
Although kirenol has been used in several models of inflammatory diseases, we show here for the first time, to our knowledge, that kirenol is effective for treatment of chronic colitis in mice. We have shown previously that kirenol potently inhibits experimental autoimmune encephalomyelitis by inhibiting the differentiation of Th1 and Th17 cells and inducing apoptosis of effector T cells[17]. Consistently, in this study we observed that kirenol treatment significantly lowered IFN-γ and IL-17A secretion by Th1/Th17 cells and promoted apoptosis of CD4+ T lymphocytes in UC mice. We found that kirenol treatment also led to reduced colon inflammation in UC mice as shown by a reduced production of IL-6 and TNF-α, both identified as the key inflammatory cytokines in UC.
Our results strongly suggest that the activation of Th1/Th17 cells plays essential roles in chronic inflammation and is the main immune response in UC, and promoting CD4+ Th1/Th17 apoptosis can inhibit autoimmune inflammation. Kirenol treatment of UC mice not only reduced the production of the proinflammatory cytokines IFN-γ, IL-17A, IL-6 and TNF-α, indicating the controlled Th1 and Th17 responses, but also induced apoptosis of the lymphocytes, especially CD4+ T cells. As the inflammatory mediators are mainly secreted by CD4+ Th1 and Th17, the beneficial effect of kirenol is very likely associated with the suppressed secretion of inflammatory mediators as a result of enhanced apoptosis of inflammatory CD4+ T cells. However, further studies are still needed to fully elucidate the immunosuppressant mechanisms of kirenol.
Taken together, our findings demonstrate the therapeutic potential of kirenol for T cell-driven colitis. Kirenol reduces the severity of UC by inhibiting IFN-γ, IL-17A, IL-6 and TNF-α secretion and inducing apoptosis of lymphocytes, especially CD4+ T cells. Promoting apoptosis of CD4+ T cells is a likely explanation for the downregulated secretion of inflammatory cytokines in kirenoltreated UC mice.
| [1] |
Jawad N, Direkze N, Leedham SJ. Inflammatory bowel disease and colon cancer[J]. Recent Results Cancer Res, 2011, 185: 99-115. |
| [2] |
Wechsler JB, Szabo A, Hsu CL, et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis[J]. Mucosal Immunol, 2018, 11(3): 861-70. DOI:10.1038/mi.2017.121 |
| [3] |
Hino K, Saito A, Asada R, et al. Increased susceptibility to dextran sulfate sodium-induced colitis in the endoplasmic reticulum stress transducer OASIS deficient mice[J]. PLoS One, 2014, 9(2): e88048. DOI:10.1371/journal.pone.0088048 |
| [4] |
Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases[J]. Proc Natl Acad Sci USA, 2007, 104(34): 13780-5. DOI:10.1073/pnas.0706625104 |
| [5] |
Boirivant M, Fuss IJ, Chu A, et al. Oxazolone colitis:A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4[J]. J Exp Med, 1998, 188(10): 1929-39. DOI:10.1084/jem.188.10.1929 |
| [6] |
Loo TT, Gao Y, Lazarevic V. Transcriptional regulation of CD4(+) TH cells that mediate tissue inflammation[J]. J Leukoc Biol, 2018, 104(6): 1069-85. DOI:10.1002/JLB.1RI0418-152RR |
| [7] |
Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease[J]. Gut, 2003, 52(1): 65-70. DOI:10.1136/gut.52.1.65 |
| [8] |
Wallace KL, Zheng LB, Kanazawa Y, et al. Immunopathology of inflammatory bowel disease[J]. World J Gastroenterol, 2014, 20(1): 6-21. DOI:10.3748/wjg.v20.i1.6 |
| [9] |
Dias AM, Correia A, Pereira MS, et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease[J]. Proc Natl Acad Sci USA, 2018, 115(20): E4651-E60. DOI:10.1073/pnas.1720409115 |
| [10] |
Zhang H, Wu H, Liu L, et al. 1, 25-dihydroxyvitamin D3 regulates the development of chronic colitis by modulating both T helper (Th)1 and Th17 activation[J]. APMIS, 2015, 123(6): 490-501. DOI:10.1111/apm.12378 |
| [11] |
Sarazin A, Dendooven A, Delbeke M, et al. Treatment with P28GST, a schistosome-derived enzyme, after acute colitis induction in mice:decrease of intestinal inflammation associated with a down regulation of Th1/Th17 responses[J]. PLoS One, 2018, 13(12): e0209681. DOI:10.1371/journal.pone.0209681 |
| [12] |
Xiang Y, Zhang H, Fan CQ, et al. Novel diterpenoids and diterpenoid glycosides from Siegesbeckia orientalis[J]. J Nat Prod, 2004, 67(9): 1517-21. DOI:10.1021/np0400407 |
| [13] |
Jiang Z, Yu QH, Cheng Y, et al. Simultaneous quantification of eight major constituents in Herba Siegesbeckiae by liquid chromatography coupled with electrospray ionization time-of-flight tandem mass spectrometry[J]. J Pharm Biomed Anal, 2011, 55(3): 452-7. DOI:10.1016/j.jpba.2011.02.023 |
| [14] |
Kim J, Kim MB, Yun JG, et al. Protective effects of standardized siegesbeckia glabrescens extract and its active compound kirenol against UVB-induced photoaging through inhibition of MAPK/NF-kappaB pathways[J]. J Microbiol Biotechnol, 2017, 27(2): 242-50. DOI:10.4014/jmb.1610.10050 |
| [15] |
Huo L, Jiang Z, Lei M, et al. Simultaneous quantification of kirenol and ent-16beta, 17-dihydroxy-kauran-19-oic acid from Herba Siegesbeckiae in rat plasma by liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic studies[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2013, 937: 18-24. DOI:10.1016/j.jchromb.2013.08.019 |
| [16] |
Lu Y, Xiao J, Wu ZW, et al. Kirenol exerts a potent anti-arthritic effect in collagen-induced arthritis by modifying the T cells balance[J]. Phytomedicine, 2012, 19(10): 882-9. DOI:10.1016/j.phymed.2012.04.010 |
| [17] |
Xiao J, Yang R, Yang L, et al. Kirenol attenuates experimental autoimmune encephalomyelitis by inhibiting differentiation of Th1 and th17 cells and inducing apoptosis of effector T cells[J]. Sci Rep, 2015, 5: 9022. DOI:10.1038/srep09022 |
| [18] |
Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis[J]. Lab Invest, 1993, 69(2): 238-49. |
| [19] |
Lu Y, Xiao J, Wu Z, et al. Effects of kirenol on bovine type Ⅱ collagen-induced rat lymphocytes in vivo and in vitro[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2012, 32(1): 1-6. |
| [20] |
Ma C, Wu W, Lin R, et al. Critical role of CD6highCD4+T cells in driving Th1/Th17 cell immune responses and mucosal inflammation in IBD[J]. Journal of Crohn's&colitis, 2019, 13(4): 510-24. |
| [21] |
Tortola L, Pawelski H, Sonar SS, et al. IL-21 promotes allergic airway inflammation by driving apoptosis of FoxP3(+) regulatory T cells[J]. J Allergy Clin Immunol, 2019, 143(6): 2178-89. DOI:10.1016/j.jaci.2018.11.047 |
| [22] |
Raza A, Yousaf W, Giannella R, et al. Th17 cells:interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease[J]. Expert Rev Clin Immunol, 2012, 8(2): 161-8. DOI:10.1586/eci.11.96 |
| [23] |
Feuerstein JD, Cheifetz AS. Ulcerative colitis:epidemiology, diagnosis, and management[J]. Mayo Clin Proc, 2014, 89(11): 1553-63. DOI:10.1016/j.mayocp.2014.07.002 |
| [24] |
Hisamatsu T, Kanai T, Mikami Y, et al. Immune aspects of the pathogenesis of inflammatory bowel disease[J]. Pharmacol Ther, 2013, 137(3): 283-97. DOI:10.1016/j.pharmthera.2012.10.008 |
| [25] |
Zhu Q, Zheng P, Zhou J, et al. Andrographolide affects Th1/Th2/Th17 responses of peripheral blood mononuclear cells from ulcerative colitis patients[J]. Mol Med Rep, 2018, 18(1): 622-6. |
 2019, Vol. 39
2019, Vol. 39

