2. 暨南大学第二临床医学院深圳市人民医院泌尿外科,广东 深圳 518020
2. Department of Urology, Shenzhen People's Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, China
前列腺癌是常见的男性泌尿生殖系统恶性肿瘤, 其发生率在男性恶性肿瘤中排第二位[1]。前列腺癌根治术(RP)是局限性前列腺癌治疗的首选方案[2]。但是由于前列腺与阴茎海绵体神经血管束在解剖学上精密相连, 术中可直接或间接损伤前列腺包膜周围的海绵体神经, 进而导致神经传导障碍及后续继发阴茎海绵体血管、内皮和平滑肌功能障碍, 由此引发的术后勃起功能障碍(ED)发病率仍然居高不下[3]。临床治疗ED常用的药物PDE5抑制剂口服药疗效不佳; 而真空负压吸引、阴茎海绵体内血管活性药物注射等, 因其有创性及操作难度大, 导致应用受限[4-5]。因此亟需探索一种有效的RP后ED的治疗方法。双侧海绵体神经损伤(BCNI)模型是模拟RP后ED的经典模型[6]。海绵体神经损伤后, 阴茎缺氧、阴茎海绵体平滑肌细胞(CCSMC)表型转化、凋亡、海绵体组织纤维化等一系列病理生理反应是导致ED的原因[7-8]。阴茎海绵体是一种特殊的血管组织, 正常人阴茎海绵体组织中平滑肌细胞占40%~52%[9]。CCSMC在正常的勃起中处于核心地位[10]。CCSMC凋亡在BCNI大鼠ED扮演着非常重要的作用[11-12]。因此, 寻找有效方法的减少海绵体神经损伤后CCSMC的凋亡对勃起功能的恢复显得尤为重要。
H2S气体是近年来发现的第3种气体信号分子, 在心血管、神经、呼吸和泌尿等多个系统中发挥广泛的生物学效应, H2S可以通过抗细胞凋亡、抗氧化应激等机制对血管平滑肌发挥保护作用[13-15]。H2S存在于阴茎海绵体组织中, 具有促进阴茎勃起的作用[16]。既往研究表明, 补充外源性H2S能显著改善糖尿病大鼠及老年大鼠勃起功能障碍[17-18]。然而, 补充外源性H2S对BCNI大鼠勃起功能障碍的作用还未见报道。H2S能否在BCNI大鼠中发挥抗CCSMC凋亡的作用仍有待探索。因此, 本研究分析H2S对BCNI大鼠CCSMC凋亡及勃起功能障碍的影响。
1 材料和方法 1.1 实验材料 1.1.1 主要实验器材与试剂Biopac MP150生理仪套装(操作软件:Biopac System AcqKnowledge 4.4); Masson's Trichrome染色试剂盒(迈新试剂); TUNEL凋亡试剂盒(Roche, Basel, Switzerland); α-SMA一抗(Santa Cruz); Collagen-Ⅰ(Abcam); CBS、CSE、α-SMA、Cleaved caspase-3、Bax、Bcl-2一抗(proteintech); NaHS (H2S供体)(Sigma)。
1.1.2 实验动物8周龄的经交配实验证实具有正常勃起功能的SD雄性大鼠24只(由南方医科大学实验动物中心提供), 在南方医院实验动物中心SPF级环境饲养。
1.2 方法 1.2.1 实验动物分组将SD雄性大鼠随机分为3组, 每组8只, 分别为:Sham组、BCNI组、BCNI+NaHS组。BCNI+NaHS组腹腔注射100 μmol/(kg·d)的NaHS溶液; BCNI组每日腹腔注射等量生理盐水。
1.2.2 BCNI大鼠模型的建立在无菌环境下, SD大鼠用45 mg/kg戊巴比妥钠腹腔麻醉后固定, 下腹部备皮, 安尔碘消毒, 取下腹正中切口, 充分暴露前列腺, 沿着大鼠右侧前列腺背侧叶外下方, 找到盆腔神经节(呈星形, 又称盆腔星状神经节), 其内下方走行的分支即为主海绵体神经(性神经), 然后参照Fandel等[19]钳夹损伤模型制作方法, 即充分暴露双侧海绵体神经, 靠近盆神经节, 用直径5 mm止血钳钳夹双侧海绵体神经各2 min, 7号丝线关闭腹腔, 百多邦莫匹罗星软膏涂抹切口预防切口感染。Sham组则打开腹腔后找出海绵体神经但不做处理, 关闭腹腔。
1.2.3 阴茎勃起功能测定术后4周后各组大鼠均通过电刺激海绵体神经使阴茎海绵体充血勃起, 刺激参数:amplitude(5 mA), frequency(50 Hz), pulse width (61.66 ms), duration(60 s)。使用Biopac MP150生理仪套装连续监测阴茎海绵体内压(ICP)及平均动脉压(MAP)。
1.2.4 组织取材SD大鼠阴茎取材:将阴茎海绵体内测压后的各组大鼠, 小心去除包皮、筋膜组织后剪下阴茎组织并弃龟头, PBS液反复清洗; 选取阴茎中部作为石蜡切片的组织, 放入4%多聚甲醛溶液固定后石蜡包埋用于后续Masson's Trichrome染色和免疫组化染色。其余阴茎组织均放置液氮速冻, 随后可长期放置-80℃冰箱或者立即进行Western blot检测相关指标。
1.2.5 Masson's Trichrome染色常规石蜡切片脱蜡后, 采用Masson's Trichrome染色试剂盒说明书进行, 光镜观察, 镜下平滑肌细胞为红色, 胞核为黑色, 胶原组织为蓝色。图像分析软件Image-pro Plus6.0计算平滑肌和胶原的平均光密度, 计算smooth muscle/collagen的比值评估阴茎海绵体平滑肌的含量。
1.2.6 免疫组织化学染色常规脱蜡, PBS冲洗3次, 3 min/次; 3%H2O2室温孵育10 min, PBS冲洗; 微波温和修复15 min; PBS冲洗3次; 5%山羊血清室温封闭30 min; 按1:200稀释比例抗CBS、CSE一抗, 4℃过夜; PBS冲洗3次; 滴加二抗, 室温1 h, PBS冲洗3次, 3 min/次; DAB显色, PBS冲洗; 苏木素复染3 min, 双蒸水冲洗; 1%盐酸酒精分化3 s, 双蒸水冲洗; 常规脱水透明, 中性树胶封片。图像分析软件Image-pro Plus6.0计算阳性细胞平均光密度。
1.2.7 Western blot取出组织后称质量, 液氮下充分研磨组织, 按每10 μL裂解液/mg加入裂解液于冰上继续研磨, 离心, 取上清以BCA法测量蛋白浓度, 加入缓冲液后煮沸, 以30 μg总蛋白进行SDS-PAGE电泳, 电转至PVDF膜。5%脱脂奶粉封闭1 h, 加入抗α-SMA(1:200)、CBS(1:200)、CSE(1:200)、Caspase 3(1:1000)、Bax (1:1000)、Bcl-2(1:1000)一抗4℃孵育过夜, TBST缓冲液漂洗3次后用相应的二抗于室温孵育1 h, TBST缓冲液漂洗3次后用ECL法显色, 使用ImageJ图像分析软件分析条带灰度值, 用目的蛋白灰度值/β-actin灰度值代表目的蛋白的相对表达量。
1.2.8 TUNEL+α-SMA组织免疫荧光双染石蜡切片常规脱蜡至水, PBS冲洗3次, 3 min/次; 3%H2O2室温孵育10 min, PBS冲洗; 微波温和修复15 min; PBS冲洗3次; 5%山羊血清室温封闭30 min; 按1:200稀释比例抗α-SMA一抗, 4℃过夜; FITC-标记的荧光二抗避光孵育1 h, TUNEL染色根据试剂盒说明书(Roche, Basel, Switzerland)进行。抗荧光淬灭剂封片, 正置显微镜观察并拍照, 计算海绵窦内平滑肌细胞(呈红色荧光)中凋亡阳性细胞数(呈绿色荧光)。
1.3 统计学处理采用SPSS 22.0软件进行统计学分析, 数据以均数±标准差表示, one-way ANOVA分析结果, 根据方差齐性分析结果, 使用LSD法或Dunnett's法进行两组间比较, P < 0.05为差异有统计学意义。
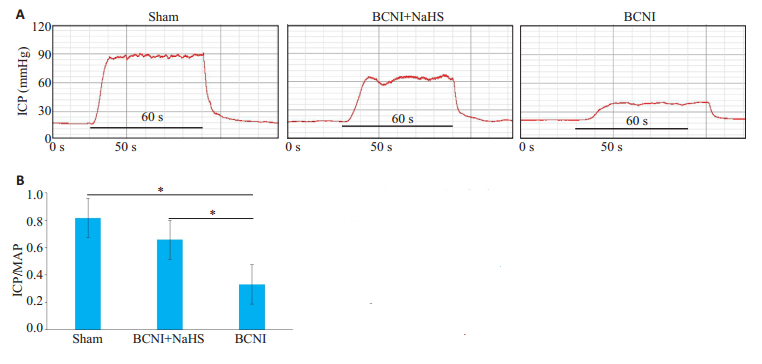
2 结果 2.1 外源性H2S可部分改善BCNI大鼠勃起功能电刺激大鼠阴茎海绵体神经后显示, Sham、BCNI+ NaHS、BCNI组ICP/MAP比值分别为:0.81 ± 0.038、0.65±0.049、0.33±0.046, 两两比较LSD检验显示, Sham组和BCNI+NaHS组ICP/MAP比值高于BCNI组(P < 0.05);且Sham组ICP/MAP比值高于BCNI+NaHS组(P < 0.05, 图 1)
|
图 1 各组大鼠的勃起功能 Fig.1 Erectile function of rats in each group. A:Representative ICP recordings (at 5 V) in each group. The stimulus interval indicated by the solid bar is 60 s. The erectile function is determined in vivo by electrical stimulation of the cavernous nerve of the rats in the Sham group, BCNI+NaHS group and BCNI group; B:Erectile function of each group presented as the maximal ICP/MAP ratio in the bar graph (n=6; *P < 0.05). |
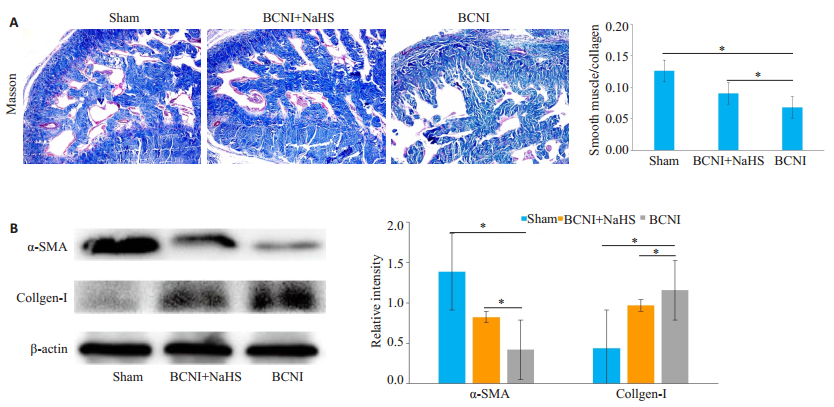
阴茎组织Masson's Trichrome染色显示, Sham组的阴茎海绵体内皮及平滑肌(红色)排列分布规则、完整及延续性好, BCNI+NaHS稍次之, 而BCNI组内皮及平滑肌排列紊乱、稀少及出现断裂; Sham、BCNI+NaHS、BCNI组平滑肌/胶原的比值分别为:0.13±0.10, 0.09± 0.01, 0.07±0.01;Sham组和BCNI+NaHS组平滑肌/胶原比值均高于BCNI组(P < 0.05, 图 2)。Western blot检测结果表明, 与BCNI组比较, BCNI+NaHS组α-SMA蛋白表达升高, Collagen-Ⅰ蛋白表达降低。
|
图 2 各组大鼠阴茎海绵体中α-SMA和Collagen-Ⅰ的表达情况 Fig.2 Expression of α-SMA and collagen-I in the corpus cavernosum of the rats in each group. A:Masson's Trichrome staining of penile cavernous tissue with the smooth muscle stained red and the collagen tissue stained blue (Original magnification:×100). The ratio of smooth muscle to collagen was defined as the percentage of smooth muscle area in a given field; B:Western blot analysis of the protein levels of α-SMA and collagen-I with β-actin as the loading control. The bar graph shows the relative protein levels of α-SMA and collagen-I (n=6, *P < 0.05). |
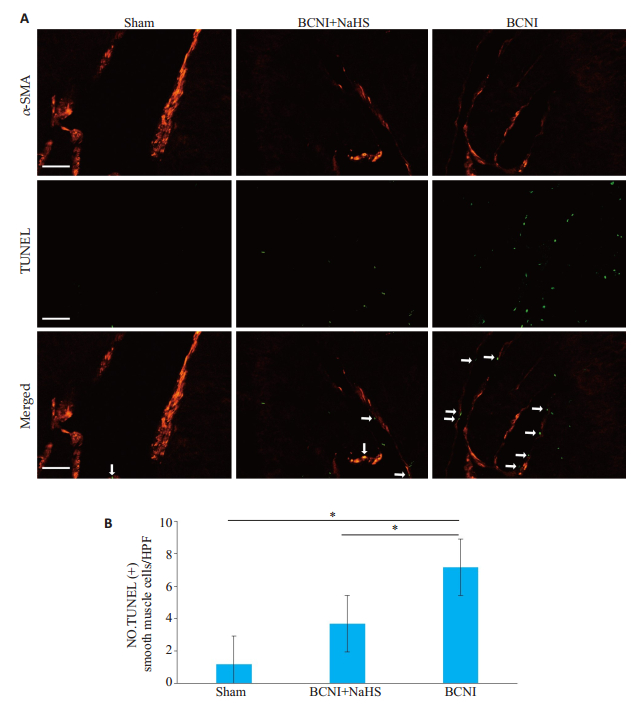
通过TUNEL+α-SMA组织免疫荧光双染实验结果发现, Sham、BCNI+NaHS、BCNI组CCSMC凋亡细胞数分别为:1.17±0.75、3.67±0.82、0.7.12±0.75。两两比较LSD检验显示, 与Sham组相比, BCNI组α-SMA阳性的平滑肌细胞凋亡数显著显著升高; 而BCNI+NaHS组α-SMA阳性的平滑肌细胞凋亡数显著低于BCNI组(P < 0.05, 图 3)。
|
图 3 各组大鼠阴茎海绵体平滑肌细胞凋亡水平的比较 Fig.3 Cell apoptosis in the cavernous tissue of the rats in each group. A:TUNEL + α-SMA immunofluorescence double staining of the penile cavernous tissue in each group (×400); B:Bar graph showing the number of apoptotic cells in the smooth muscle cells per high-power field (n=6, *P < 0.05). |
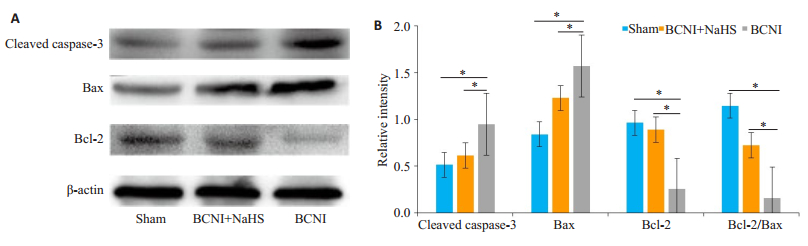
Western blot检测3组大鼠阴茎组织中凋亡相关蛋白的表达变化, 结果表明:与Sham组相比, BCNI组Cleaved caspase-3、Bax蛋白表达显著升高(P < 0.05), Bcl-2蛋白表达及Bcl-2/Bax比值降低(P < 0.05), 而BCNI+NaHS组较BCNI组Cleaved caspase-3、Bax蛋白表达显著降低(P < 0.05), Bcl-2蛋白表达及Bcl-2/Bax比值升高(P < 0.05, 图 4)。
|
图 4 各组大鼠凋亡相关蛋白的表达情况 Fig.4 Expressions of caspase-3, Bax and Bcl-2 in the corpus cavernosum of the rats in each group. A, B:Western blot analysis of the protein levels of caspase-3, Bax and Bcl-2 with β-actin as the loading control. The bar graph shows the relative protein level of caspase-3, Bax, and Bcl-2 and the Bcl-2/Bax ratio (n=6, *P < 0.05). |
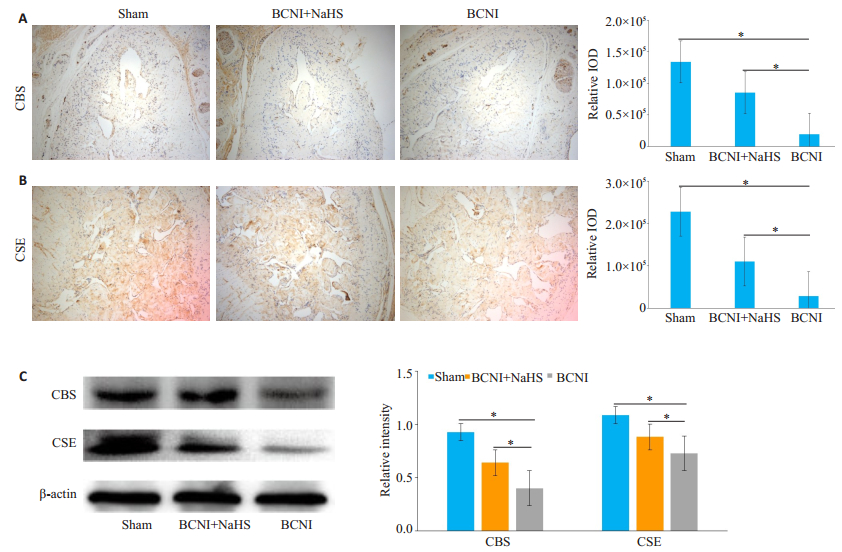
源性H2S生成酶CBS、CSE的表达免疫组化染色和Western blot检测阴茎组织中内源性H2S生成酶CBS、CSE的表达, 结果显示:两者主要分布在海绵窦内平滑肌上; 与Sham组相比, BCNI组CBS、CSE的表达量降低(P < 0.05);而BCNI+NaHS组较BCNI组CBS、CSE的表达量提高(P < 0.05, 图 5)。
|
图 5 各组大鼠阴茎内源性H2S生成酶CBS、CSE表达情况 Fig.5 CSE and CBS expressions in the cavernous tissue in each group. A, B:Immunohistochemical staining of the cavernous tissue using anti-CSE or anti-CBS (×100). The staining intensity was quantified as the integrated optical density (IOD). C:Western blot analysis of the protein levels of CSE and CBS with β-actin as the loading control. The bar graph shows the relative protein levels of CSE and CBS (n=6, *P < 0.05). |
本研究通过钳夹损伤SD大鼠双侧海绵体神经来构建ED大鼠模型(BCNI组), 通过每天腹腔注射NaHS (H2S供体)溶液来修复BCNI组大鼠的勃起功能, 持续4周后检测外源性H2S干预组ICP/MAP比值较BCNI组显著提高, 说明外源性H2S对海绵体神经损伤大鼠勃起功能具有一定的修复作用。进一步研究发现, 外源性H2S能显著降低BCNI组大鼠CCSMC的凋亡及Cleaved caspase-3、Bax蛋白的表达, 提高抗凋亡蛋白Bcl-2的表达及Bcl-2/Bax比值, 恢复内源性H2S合成酶CBS、CSE蛋白的表达。
尽管保留神经的根治性前列腺切除术已取得了显著进展, 但仍有许多患者在术后发生勃起功能的丧失[20]。海绵体神经损伤导致阴茎组织中的CCSMC和内皮细胞凋亡增加被认为是RP术后ED的重要机制之一[11, 21]。已有的研究认为CCSMC的凋亡增加是海绵体神经损伤导致的ED罪魁祸首而非内皮细胞。如有研究发现, 双侧海绵体神经钳夹损伤后4周, 阴茎勃起功能明显降低, 同时CCSMC发生大量凋亡, 抑制海绵体细胞凋亡可以改善勃起功能[12]。有报道运用间充质干细胞来源的外泌体改善CNI诱导的大鼠ED, 其机制与抑制CCSMCs凋亡有关[22]。由此可见, 发掘可干预CCSMC凋亡的手段将有助于改善海绵体神经损伤导致的ED。
凋亡是细胞正常的程序性死亡的方式, 受到一系列基因和蛋白的调控[23]。Caspase-3的激活是细胞凋亡信号通路中不可替代的环节, 被激活的caspase-3作用于下游特定的信号分子, 使细胞产生一系列的生物形态学改变, 最终可诱导细胞凋亡。Bcl-2可以抑制细胞色素C的释放而阻断caspase-9的激活, 从而抑制细胞凋亡; 相反, 促凋亡蛋白Bax作用于线粒体外膜, 导致细胞色素C的释放, 从而促进细胞凋亡[24]。
H2S是一种可存在于包括阴茎组织在内的气体信号分子, 近年来越来越多的研究证实其具有调控细胞凋亡的作用。如有研究表明H2S可抑制高糖诱导的心肌细胞的凋亡, 减弱Cleaved caspase-3表达的升高, 提高细胞活性[25]。据报道CSE基因缺失加重单侧输尿管梗阻后肾小管细胞凋亡, Bax表达的升高, Bcl-2表达降低[26]。H2S还可以降低氧化低密度脂蛋白刺激的血管平滑肌细胞caspase-3/9活性、Bax/Bcl-2比值, 起到稳定动脉粥样硬化斑块的作用[27]。尽管目前的研究已初步提示H2S可调控细胞凋亡, 然而其能否参与海绵体神经损伤导致的ED, 以及其中的潜在机制仍有待进一步阐明。基于此, 我们通过建立BCNI大鼠模型研究气体分子H2S的供体NaHS在大鼠阴茎勃起功能中的调控作用及机制。本研究发现, 对比Sham组和BCNI+NaHS组, BCNI组大鼠ICP/MAP比值显著降低, 即表现为ED。BCNI组大鼠阴茎组织平滑肌含量显著降低, 而胶原含量显著增加。TUNEL+α-SMA组织免疫荧光双染结果发现BCNI组CCSMC的凋亡细胞数目显著增加; BCNI组大鼠阴茎组织中促凋亡蛋白Cleaved caspase-3、Bax的表达明显上调, 抗凋亡蛋白Bcl-2的表达及Bcl-2/Bax比值明显下降。外源性H2S干预能显著降低CCSMC的凋亡及Cleaved caspase-3、Bax蛋白的表达, 提高抗凋亡蛋白Bcl-2的表达及Bcl-2/Bax比值, 这些结果提示外源性H2S供体NaHS可通过抑制CCSMC凋亡, 增加阴茎海绵窦内平滑肌的含量, 减少胶原沉积, 改善BCNI大鼠的勃起功能。说明H2S在海绵体神经损伤导致的ED的发生发展过程中起着重要作用。
作为一种气体分子, H2S的生成与其内源性合成酶密切相关, 其中CBS、CSE是广泛分布于哺乳动物的细胞内的内源性H2S的合成的关键酶[28]。人类和大鼠阴茎组织中也存在H2S的生成酶系统[29-30]。CBS、CSE同样表达于CCSMC中, 它们的表达降低常常引起H2S水平降低进而导致平滑肌细胞的功能障碍[16]。研究显示, 糖尿病ED大鼠阴茎组织的内源性H2S水平明显降低[18]; 老年ED大鼠的内源性H2S合成不足, 补充外源性H2S可以明显改善勃起功能障碍[31]。这些研究表明, H2S对阴茎勃起功能发挥重要作用, 但其能否通过调节组织内内源性合成酶改善海绵体神经损伤导致的ED仍不明确。本研究发现BCNI大鼠发生ED的同时内源性H2S生成酶CBS、CSE蛋白表达水平降低, 提示BCNI大鼠阴茎组织也存在内源性H2S生成不足的情况。我们进一步使用外源性H2S干预发现BCNI+NaHS组大鼠的阴茎组织内CBS、CSE蛋白表达水平较BCNI组大鼠得到恢复。该结果与国外的研究相符, 如研究报道外源性H2S干预可以减轻糖尿病大鼠心肌中CSE蛋白表达的降低[32]; 有文献报道称外源性H2S通过激活Nrf2提高CBS、CSE的转录和翻译[33]。但是外源性H2S是通过何种机制调节CCSMC中CBS、CSE蛋白的表达还有待进一步研究。
尽管本研究已初步证实H2S可通过抑制CCSMC凋亡改善BCNI大鼠的勃起功能, 然而H2S作为一种重要的气体效应分子, 其参与的信号通路众多。有研究报道, 抑制c-Jun氨基末端激酶可以通过恢复c-Jun氨基末端激酶相关途径来减轻海绵体细胞凋亡, 从而改善BCNI大鼠的勃起功能[34-35]。H2S可通过抑制c-Jun氨基末端激酶和p38 MAPK通路并激活PI3K/Akt信号通路, 在体外和体内均可减少高糖诱导的心肌细胞凋亡[36]。H2S能否通过c-Jun/Bcl-2/Bax调控BCNI大鼠CCSMC凋亡将是接下来深入研究和探讨的方向。
综上所述, 外源性H2S可以抑制BCNI大鼠阴茎组织Cleaved caspase-3、Bax上调和促进Bcl-2表达、减轻CCSMC凋亡并改善BCNI大鼠勃起功能。本研究为推进H2S气体信号分子作为海绵体神经损伤性ED的治疗提供理论依据。
| [1] |
Farhood B, Mortezaee K, Haghi-Aminjan H, et al. A systematic review of radiation-induced testicular toxicities following radiotherapy for prostate cancer[J]. J Cell Physiol, 2019, 234(9): 14828-37. DOI:10.1002/jcp.28283 |
| [2] |
Farhood B, Geraily G, Abtahi SMM. A systematic review of clinical applications of polymer gel dosimeters in radiotherapy[J]. Appl Radiat Isot, 2019, 143(2): 47-59. |
| [3] |
Wang X, Liu C, Xu Y, et al. Combination of mesenchymal stem cell injection with icariin for the treatment of diabetes-associated erectile dysfunction[J]. PLoS One, 2017, 12(3): e0174145-56. DOI:10.1371/journal.pone.0174145 |
| [4] |
Campbell JD, Burnett AL. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury[J]. Int J Mol Sci, 2017, 18(8): 1257-69. |
| [5] |
Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction[J]. Nat Rev Dis Primers, 2016, 2(7): 16003-15. |
| [6] |
Jung AR, Park YH, Jeon SH, et al. Therapeutic effect of controlled release of dual growth factor using heparin-pluronic hydrogel/ gelatin-Poly (ethylene glycol)-tyramine hydrogel system in a rat model of cavernous nerve injury[J]. Tissue Eng Part A, 2018, 24(23/ 24): 1705-14. |
| [7] |
Park J, Son H, Chai JS, et al. Chronic administration of LIMK2 inhibitors alleviates cavernosal veno-occlusive dysfunction through suppression of cavernosal fibrosis in a rat model of erectile dysfunction after cavernosal nerve injury[J]. PLoS One, 2019, 14(3): e0213586-97. DOI:10.1371/journal.pone.0213586 |
| [8] |
Zhang HB, Wang ZQ, Chen FZ, et al. Maintenance of the contractile phenotype in corpus cavernosum smooth muscle cells by Myocardin gene therapy ameliorates erectile dysfunction in bilateral cavernous nerve injury rats[J]. Andrology, 2017, 5(4): 798-806. DOI:10.1111/andr.12375 |
| [9] |
Moreland RB, Traish A, Mcmillin MA, et al. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle[J]. J Urol, 1995, 153(3 Pt 1): 826-34. |
| [10] |
Burchardt T, Burchardt M, Karden J, et al. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat[J]. J Urol, 2000, 164(5): 1807-11. DOI:10.1016/S0022-5347(05)67111-X |
| [11] |
User HM, Hairston JH, Zelner DJ, et al. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction[J]. J Urol, 2003, 169(3): 1175-9. DOI:10.1097/01.ju.0000048974.47461.50 |
| [12] |
Kim SJ, Choi SW, Hur KJ, et al. Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury[J]. Korean J Urol, 2012, 53(10): 726-32. DOI:10.4111/kju.2012.53.10.726 |
| [13] |
Liu S, Xin D, Wang L, et al. Therapeutic effects of L-Cysteine in newborn mice subjected to hypoxia-ischemia brain injury via the CBS/H2S system: role of oxidative stress and endoplasmic reticulum stress[J]. Redox Biol, 2017, 13(6): 528-40. |
| [14] |
Meng G, Zhao S, Xie L, et al. Protein S-sulfhydration by Hydrogen sulfide in cardiovascular system[J]. Br J Pharmacol, 2018, 175(8): 1146-56. DOI:10.1111/bph.13825 |
| [15] |
Kimura H. Hydrogen sulfide and polysulfide signaling[J]. Antioxid Redox Signal, 2017, 27(10): 619-21. DOI:10.1089/ars.2017.7076 |
| [16] |
Aydinoglu F, Adıbelli EÖ, Yılmaz-Oral D, et al. Involvement of RhoA/Rho-kinase in l-cysteine/H2S pathway-induced inhibition of agonist-mediated corpus cavernosal smooth muscle contraction[J]. Nitric Oxide, 2019, 85(7): 54-60. |
| [17] |
毛玉山, 叶小磊, 麦一峰, 等. 硫化氢对糖尿病大鼠ED的影响和机制研究[J]. 中国病理生理杂志, 2014, 34(1): 139-43. DOI:10.3969/j.issn.1000-4718.2014.01.023 |
| [18] |
Zhang Y, Yang J, Wang T, et al. Decreased endogenous hydrogen sulfide generation in penile tissues of diabetic rats with erectile dysfunction[J]. J Sex Med, 2016, 13(3): 350-60. DOI:10.1016/j.jsxm.2016.01.002 |
| [19] |
Fandel TM, Albersen M, Lin G, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury[J]. Eur Urol, 2012, 61(1): 201-10. |
| [20] |
Song KM, Chung JS, Choi MJ, et al. Effectiveness of intracavernous delivery of adenovirus encoding Smad7 gene on erectile function in a mouse model of cavernous nerve injury[J]. J Sex Med, 2014, 11(1): 51-63. |
| [21] |
Klein LT, Miller MI, Buttyan R, et al. Apoptosis in the rat penis after penile denervation[J]. J Urol, 1997, 158(2): 626-30. DOI:10.1016/S0022-5347(01)64572-5 |
| [22] |
Ouyang X, Han X, Chen Z, et al. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury[J]. Stem Cell Res Ther, 2018, 9(1): 246-58. |
| [23] |
Fuchs Y, Steller H. Programmed cell death in animal development and disease[J]. Cell, 2011, 147(4): 742-58. DOI:10.1016/j.cell.2011.10.033 |
| [24] |
Gottlieb RA. Mitochondria and apoptosis[J]. Science, 2001, 10(3/4): 147-61. |
| [25] |
Liang W, Chen J, Mo L, et al. ATP-sensitive K+ channels contribute to the protective effects of exogenous Hydrogen sulfide against high glucose-induced injury in H9c2 cardiac cells[J]. Int J Mol Med, 2016, 37(3): 763-72. DOI:10.3892/ijmm.2016.2467 |
| [26] |
Han SJ, Noh MR, Jung JM, et al. Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis[J]. Free Radic Biol Med, 2017, 112(5): 423-32. |
| [27] |
Xiong Q, Wang Z, Yu Y, et al. Hydrogen sulfide stabilizes atherosclerotic plaques in apolipoprotein E knockout mice[J]. Pharmacol Res, 2019, 144(1): 90-8. |
| [28] |
Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter[J]. Nat Rev Drug Discov, 2015, 14(5): 329-45. DOI:10.1038/nrd4433 |
| [29] |
D'emmanuele DR, Sorrentino R, Maffia P, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation[J]. Proc NatlAcad Sci USA, 2009, 106(11): 4513-8. DOI:10.1073/pnas.0807974105 |
| [30] |
Srilatha B, Adaikan PG, Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction-- a pilot study[J]. Eur J Pharmacol, 2006, 535(1/3): 280-2. |
| [31] |
Srilatha B, Muthulakshmi P, Adaikan PG, et al. Endogenous hydrogen sulfide insufficiency as a predictor of sexual dysfunction in aging rats[J]. Aging Male, 2012, 15(3): 153-8. DOI:10.3109/13685538.2012.668722 |
| [32] |
Liu M, Li Y, Liang B, et al. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway[J]. Int J Mol Med, 2018, 41(4): 1867-76. |
| [33] |
Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613[J]. Antioxid Redox Signal, 2013, 19(5): 465-81. DOI:10.1089/ars.2012.4944 |
| [34] |
Kim SW, Lee J, Park J, et al. Combination of LIM-kinase 2 and Jun amino-terminal kinase inhibitors improves erectile function in a rat model of cavernous nerve injury[J]. Urology, 2019, 131(4): 136-43. |
| [35] |
Park J, Chai JS, Kim SW, et al. Inhibition of Jun N-terminal kinase improves erectile function by alleviation of cavernosal apoptosis in a ratmodel of cavernous nerveinjury[J]. Urology, 2018, 113(16): 253-9. |
| [36] |
Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy[J]. Clin Sci (Lond), 2015, 128(5): 325-35. DOI:10.1042/CS20140460 |
 2019, Vol. 39
2019, Vol. 39

