2. 广东医科大学附属医院 临床研究中心,广东 湛江 524001
2. Clinical Research Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China
高尔基体磷蛋白3(Golph3)参与调控高尔基体蛋白分选和运输,维护高尔基体结构稳态[1-3]。同时它还是一个癌基因,在多种肿瘤组织中表达异常升高[4-7]。Golph3的高表达与肿瘤进展程度、预后不良密切相关[8-11]。最近的研究表明Golph3影响肿瘤细胞对化疗药物的敏感性[12-14],但具体机制仍不完全清楚。我们的前期研究表明,Golph3在宫颈癌组织中高表达,Golph3可以促进宫颈癌细胞的增殖、迁移和侵袭。另外,Golph3的表达水平与紫杉醇处理后宫颈癌细胞的增殖密切相关。上调Golph3的表达,紫杉醇对宫颈癌细胞杀伤作用降低。但是,具体的作用机制仍不清楚。
肿瘤的化疗和放疗过程中会诱导自噬的发生[17-19]。多数研究证实自噬在化疗过程中发挥保护作,自噬能通过降解大分子废物、错误折叠的蛋白和损伤的细胞器来维护细胞内部稳态,为肿瘤细胞提供能量和营养元素[15-16]。这提示,自噬可能是紫杉醇处理条件下Golph3调控宫颈癌细胞增殖的一种作用机制。因此,本研究主要探讨Golph3是否通过影响细胞自噬调控紫杉醇引起的肿瘤细胞凋亡。
1 材料和方法 1.1 常用试剂Hela细胞(ATCC);胰酶、胎牛血清和MEM培养基(Gibico);一抗稀释液和二抗稀释液(碧云天生物技术有限公司);细胞培养耗材、细胞凋亡检测试剂盒(C1062M)、PVDF膜化学发光试剂盒和其他常规生化试剂(东莞科贸生物科技有限公司);自噬抑制剂3-MA(IM0190)(北京索莱宝科技有限公司);带有V5标签的Golph3过表达慢病毒由本课题组构建并保存;靶向Golph3的siRNA(上海吉玛制药技术有限公司)。siRNA序列:GOLPH3-si635 sense 5'CAAGAAAGGUAAUC UGUAATT3';anti-sense 5'UUACAGAUUACCUUUC UUGTT3'。V5标签抗体、GAPDH基因抗体和HRP标记二抗(CST公司);Golph3基因抗体(Abcam)。
1.2 稳定细胞株的构建通过慢病毒感染的方法构建过表达Golph3蛋白的hela稳定细胞株。病毒感染前1 d,按5×103/孔的密度将hela细胞接种至24孔板。第2天,使用MOI=20慢病毒感染hela细胞。慢病毒感染细胞时培养基加入终浓度为5 μg/mL的polybrene。感染12 h后吸出含有病毒液的培养基,换上完全培养基培养。24 h后,加入含有嘌呤霉素的培养基进行筛选。嘌呤霉素筛选2~3周后选出阳性克隆,通过免疫印记检测阳性克隆中Golph3蛋白表达水平。
1.3 透射电镜样品处理透射电镜样品由广东医科大学附属医院电镜室制备。胰酶消化法收集细胞,去除上清,在细胞沉淀中缓缓加入戊二醛固定液,固定过夜。第2天去除固定液。PBS洗3次,然后吸干上清。依次用50%乙醇、70%乙醇、90%乙醇、90%乙醇、90%丙酮、90%丙酮和100%丙酮进行脱水。树脂包埋和固化。超薄切片机切片,厚度约50~60 nm。3%醋酸铀-枸橼酸铅双染色。透射电镜(JEM1400,日本电子)观察、拍片。
1.4 免疫印记通过SDS-PAGE分离蛋白,蛋白电泳上样量为30 μg,100 V稳压电泳2 h,300 V稳压电泳0.5 h,300 mA稳流转膜1 h,5%脱脂奶粉封闭1 h,一抗4 ℃孵育过夜,TBST洗膜4次,5 min/次,HRP标记二抗室温孵育1 h,TBST洗膜4次,5 min/次,化学发光成像。
1.5 siRNA转染将5×104细胞种植于6孔板,培养过夜。转染前将六孔板中的细胞培养基吸干,加入1 mL OPTI-MEM培养基,放入二氧化碳培养箱内培养。按照如下体系配置转染液:(1)1.5 mL EP管内加入6 µL siRNA或对照siRNA(NC)和100 µL OPTI-MEM培养基,混匀后静置5 min;(2)另取新的1.5 mL EP管内加入3 µL转染试剂LIPOFACTAMINE2000和100 µL OPTI-MEM培养基,混匀后静置5 min;(3)将(2)管中溶液加入(1)管,混匀后静置20 min,此为siRNA转染液。将转染液加入六孔板中,摇匀后放入二氧化碳培养箱内培养。6 h后取出六孔板,吸干板中液体,加入含有10%胎牛血清的1640培养基,放入二氧化碳培养箱内培养。
1.6 细胞凋亡检测siRNA转染后,自噬抑制剂3-MA预处理转染后的hela细胞3 h,然后再使用紫杉醇处理细胞24 h。胰酶消化细胞,将细胞收集到15 mL离心管(前面提到的收集培养上清的离心管)。1000 r/min离心5 min。去掉上清,加入195 µL Binding buffer重悬细胞,将细胞悬液转移至1.5 mL EP管。加入5 µLAnexin V染色液,混匀,避光孵育5 min。加入5 µL PI染色液,混匀,避光孵育15 min。加入195 µL Binding buffer,混匀后使用流式细胞仪进行检测。
1.7 统计方法选用Graphpad Prism6.0统计软件,采用非配对t检验对实验组和对照组的数值进行统计分析,P<0.05为差异具有统计学意义。
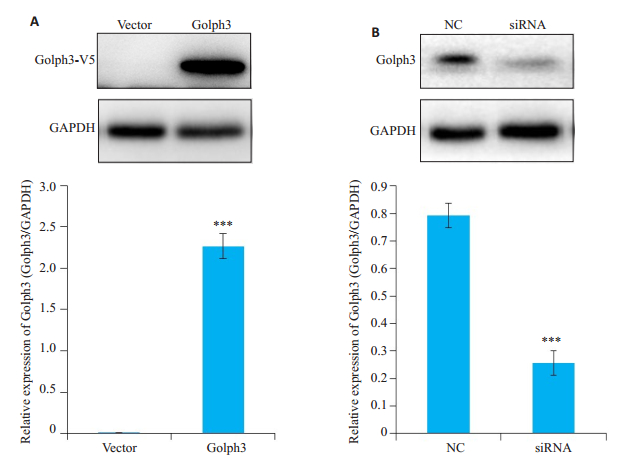
2 结果 2.1 hela细胞Golph3表达检测免疫印迹结果显示,在37 000~40 000左右的位置,Golph3稳定细胞株组出现一条特异性条带,而对照组(vector)没有(图 1A)。与NC组相比siRNA组Golph3蛋白的内源表达显著降低(P<0.05,图 1B)。
|
图 1 Hela细胞Golph3表达检测 Fig.1 Expression of Golph3 in HeLa cells. A: HeLa cells were infected by the lentivirus expressing Golph3 or the control lentivirus and treated with puromycin to screen the positive cells expressing Golph3. The over-expression of Golph3 in stable cell line was verified by Western blot analysis (GAPDH was used as loading control); B: HeLa cells were transfected with siRNA targeting Golph3 or NC, and the expression of Golph3 in HeLa cells was determined by Western blotting. ***P < 0.05 vs NC or vector. |
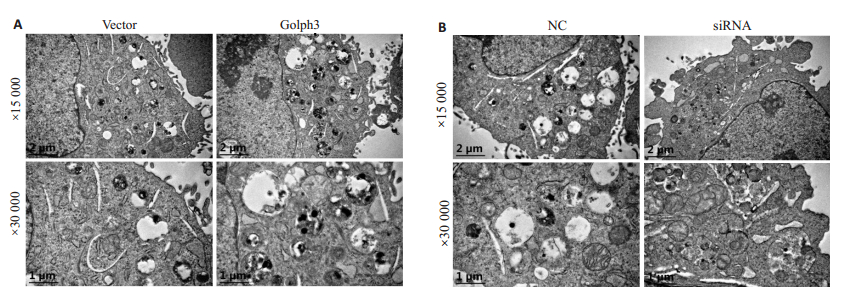
透射电镜观察结果显示,紫杉醇处理后hela细胞内可观察到自噬小体;自噬小体内含有未消化的线粒体。与对照组(vector)相比,Golph3组细胞中自噬小体的数量增多(图 2A)。与NC组相比,siRNA组细胞中自噬小体的数量减少(图 2B)。
|
图 2 透射电镜观察自噬小体 Fig.2 Effect of modulation of Golph3 expression on autophagy in HeLa cells. A: HeLa cells were infected by the lentivirus expressing Golph3 or the control lentivirus and treated with puromycin to screen the positive cells expressing Golph3; B: HeLa cells were transfected with siRNA targeting Golph3 or NC and treated with 1 μmol/L paclitaxel for 24 h. The autophagic bodies were examined using transmission electron microscope. |
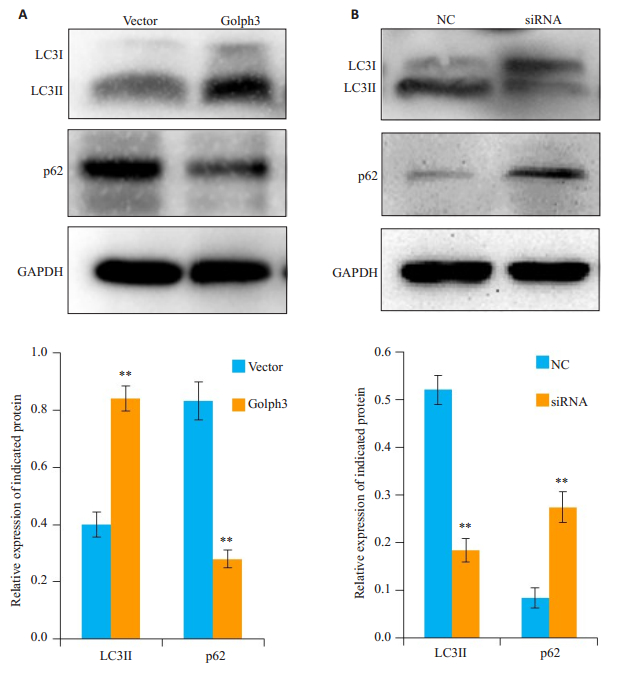
免疫印迹结果显示,Golph3组细胞中自噬标记物LC3II表达减弱,p62表达升高(图 3A);与NC组相比,siRNA组细胞中LC3II升高,p62表达减弱(图 3B)。
|
图 3 免疫印记检测自噬标记物表达 Fig.3 Effect of Golph3 overexpression and knockdown on expressions of LC3II and p62 in HeLa cells. A: HeLa cells were infected by the lentivirus expressing Golph3 or the control lentivirus and treated with puromycin to screen the positive cells expressing Golph3; B: HeLa cells were transfected with the siRNAtargeting Golph3 or NC and treated with 1 μmol/L paclitaxel for 24 h. The expressions of autophagy marker LC3II and p62 were examined by Western blotting (GAPDH was used as the loading control). **P < 0.05 vs NC or vector. |
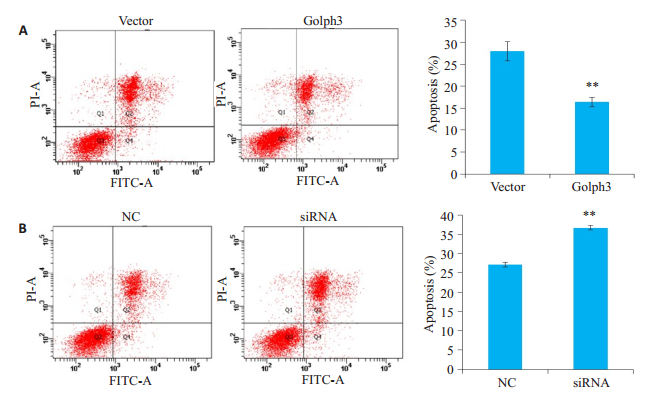
流式细胞仪检测结果显示,与vector组相比,Golph3组细胞凋亡率显著降低(P<0.01,图 4A);与NC组相比,siRNA组细胞凋亡率升高(P<0.01,图 4B)。
|
图 4 过表达或干扰Golph3对细胞凋亡的影响 Fig.4 Paclitaxel-induced apoptosis in HeLa cells with Golph3 overexpression (A) and knockdown (B). A: HeLa cells were infected by the lentivirus expressing Golph3 or the control lentivirus and treated with puromycin to screen the positive cells; B: HeLa cells were transfected with the siRNA targeting Golph3 or NC and treated with 1 μmol/L paclitaxel for 24 h. The total cell apoptosis were examined using PI/Anexin V-FITC double staining and flow cytometry. **P < 0.01 vs NC or vector. |
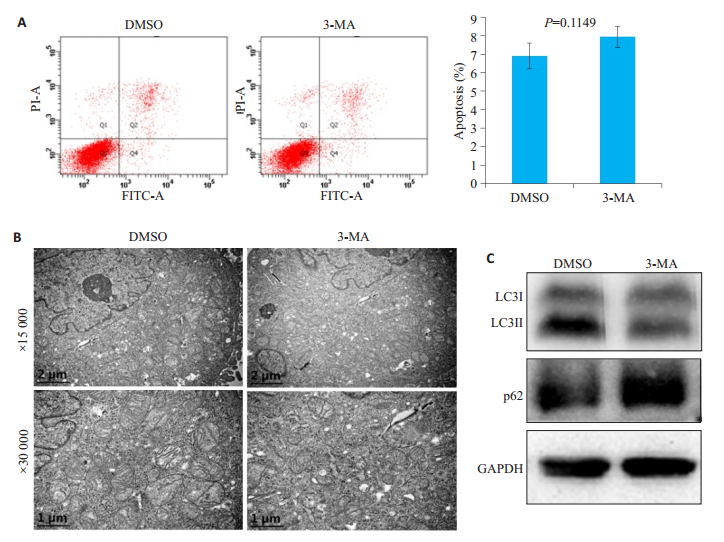
流式细胞仪检测结果显示,与对照组DMSO相比,3-MA处理组细胞凋亡率无显著变化(P=0.1149,图 5A)。透射电镜结果显示,与对照组DMSO相比,3-MA处理组细胞自噬小体数量无显著变化(图 5B)。免疫印记检测结果显示,与对照组DMSO相比,3-MA处理组LC3II表达降低,而p62表达升高(图 5C)。
|
图 5 自噬抑制剂3-MA对hela细胞自噬和凋亡的影响 Fig.5 Effects of the autophagy inhibitor 3-MA on apoptosis and autophagy of HeLa cells. Hela cells were pretreated with autophagy inhibitor 3-MA for 3 h. A: Total cell apoptosis examined using PI/Anexin V-FITC double staining and flow cytometry; B: Autophagic bodies examined using transmission electron microscope; C: Expressions of autophagy marker LC3II and p62 examined by Western blotting (GAPDH as the loading control). |
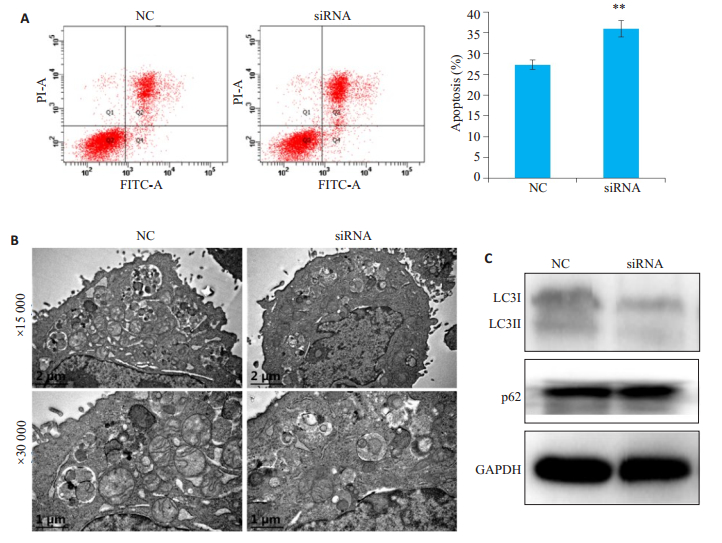
凋亡的影响流式细胞仪检测结果显示,siRNA组细胞凋亡率高于NC组(P<0.01,图 6A);透射电镜结果显示,与NC组相比,siRNA组细胞中自噬小体的数量减少(图 6B);免疫印记检测结果显示,与对照组NC组相比,siRNA组LC3II表达降低,而p62表达升高(图 6C)。
|
图 6 自噬抑制剂3-MA处理后下调Golph3表达对细胞凋亡的影响 Fig.6 Autophagy inhibition enhances the effect of siRNA on apoptosis. Hela cells were transfected with the siRNA targeting Golph3 or the control siRNA (NC). The transfected cells were pretreated with autophagy inhibitor 3-MA for 3h, and then treated with 1 μmol/L paclitaxel for 24 h. A: Total cell apoptosis examined using PI/Anexin V-FITC double staining and flow cytometry, **P < 0.01 vs NC; B: Autophagic bodies examined using transmission electron microscope; C: Expressions of LC3II and p62 examined by Western blotting. |
研究表明,自噬是调控肿瘤细胞对化疗药物敏感性的一种重要机制[16-20]。对肿瘤细胞而言自噬是一把双刃剑,一方面过度的自噬可诱导自噬性细胞死亡,另一方面自噬对肿瘤细胞有保护作用,通过将自身坏死的细胞器和错误折叠的蛋白质降解,重新利用养分维持癌细胞生存,造成了对化疗药物的耐受[21-23]。Golph3是高尔基体基质蛋白,主要定位于反式高尔基体膜上[24]。它参与调控高尔基体蛋白运输和分选,维护高尔基体结构和内部稳态[1-2, 25]。最近的研究发现,下调Golph3表达可增强肿瘤细胞对化疗药物的敏感性[13, 26]。抑制Golph3表达可增强结肠癌细胞HT29对化疗药物5-FU和顺铂的敏感性[13, 26]。本研究首次报道Golph3通过调控自噬影响宫颈癌细胞的紫杉醇敏感性。本研究结果显示上调宫颈癌hela细胞中Golph3的表达,可以促进细胞自噬;而下调Golph3的表达则抑制hela细胞自噬。本研究还发现,使用抑制剂3-MA预处理hela细胞,并同时下调Golph3的表达,可以提高紫杉醇引起的hela细胞的细胞凋亡率。这提示,Golph3可能调控细胞自噬影响紫杉醇引起的细胞凋亡。Golph3可成为紫杉醇化疗的分子靶点。
目前自噬的研究主要集中在3个领域:自噬体膜的来源问题;细胞器自噬,特别是线粒体自噬;beclin1复合物的形成和调控蛋白以及信号通路在自噬中的作用。我们目前的研究结果仅仅提示Golph3对细胞自噬有促进作用,其具体机制仍有待进一步研究。国外的研究表明Golph3在线粒体也有表达并且执行一定的功能。有研究报道,线粒体中富含Golph3,线粒体DNA消耗之后将引起Golph3表达增加[27]。本研究通过透射电镜的观察发现,紫杉醇处理宫颈癌细胞后,自噬小体里面含有未被完全消化的线粒体。而上调Golph3表达后,hela细胞自噬小体数量增多。因此本研究推测Golph3可能对线粒体自噬有调控作用。但是,目前的结果仍不能解释Golph3通过什么机制影响线粒体自噬。我们将在后续研究中探讨Golph3对线粒体自噬的影响和分子机制。
研究报道Golph3可激活哺乳动物雷帕霉素靶蛋白mTOR信号通路,并调节癌细胞对mTOR抑制剂雷帕霉素的敏感性[12, 28]。mTOR信号通路是调控细胞自噬的一个重要通路。mTOR可磷酸化ULK1的Ser757位点并抑制ULK1的活化,最终抑制细胞自噬[29-30]。这提示,Golph3可通过mTOR信号通路调控紫杉醇诱导的细胞自噬
| [1] |
Dippold HC, Ng MM, Farber-Katz SE, et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding[J]. Cell, 2009, 139(2): 337-51. DOI:10.1016/j.cell.2009.07.052 |
| [2] |
Wood CS, Schmitz KR, Bessman NJ, et al. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking[J]. J Cell Biol, 2009, 187(7): 967-75. DOI:10.1083/jcb.200909063 |
| [3] |
Kuna RS, Field SJ. GOLPH3: a golgi phosphatidylinositol(4) phosphate effector that directs vesicle trafficking and drives cancer[J]. J Lipid Res, 2019, 60(2): 269-75. DOI:10.1194/jlr.R088328 |
| [4] |
Rizzo R, Parashuraman S, D'angelo G, et al. GOLPH3 and oncogenesis: what is the molecular link[J]. Tissue Cell, 2017, 49(2 PtA): 170-4. |
| [5] |
Wen Y, Tan X, Wu X, et al. Golgi phosphoprotein 3 (GOLPH3) promotes endometrial carcinoma cell invasion and migration by regulating the epithelial-mesenchymal transition[J]. Cancer Biomark, 2019, 26(1): 21-30. DOI:10.3233/CBM-190096 |
| [6] |
Peñalver-González B, Vallejo-Rodríguez J, Mentxaka G, et al. Golgi oncoprotein GOLPH3 gene expression is regulated by functional E2F and CREB/ATF promoter elements[J]. Genes (Basel), 2019, 10(3): 247-58. DOI:10.3390/genes10030247 |
| [7] |
Tang W, Han M, Ruan B, et al. Overexpression of GOLPH3 is associated with poor survival in Non-small-cell lung cancer[J]. Am J Transl Res, 2016, 8(4): 1756-62. |
| [8] |
Wang R, Ke ZF, Wang F, et al. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer andmediates itsmetastasis through upregulating MMP-2 and MMP-9[J]. Cell Physiol Biochem, 2015, 35(3): 969-82. DOI:10.1159/000369753 |
| [9] |
Wang Z, Jiang B, Chen L, et al. GOLPH3 predicts survival of colorectal cancer patients treated with 5-fluorouracil-based adjuvant chemotherapy[J]. J Transl Med, 2014, 12(1): 15-26. DOI:10.1186/1479-5876-12-15 |
| [10] |
Zhang L, Guo F, Gao X, et al. Golgi phosphoprotein 3 expression predicts poor prognosis in patients with prostate cancer undergoing radical prostatectomy[J]. Mol Med Rep, 2015, 12(1): 1298-304. |
| [11] |
Zhou B, Wang G, Gao S, et al. Expression of GOLPH3 protein in colon cancer tissues and its association with the prognosis of patients[J]. Oncol Lett, 2016, 12(5): 3936-40. DOI:10.3892/ol.2016.5215 |
| [12] |
Scott KL, Kabbarah O, Liang MC, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer[J]. Nature, 2009, 459(7250): 1085-90. DOI:10.1038/nature08109 |
| [13] |
Zhou ZP, Wang LP, Hong ZS, et al. Silencing GOLPH3 gene expression reverses resistance to cisplatin in HT29 colon cancer cells via multiple signaling pathways[J]. Int J Oncol, 2018, 53(3): 1183-92. |
| [14] |
Zhu K, Zhao Q, Yue J, et al. GOLPH3 overexpression correlates with poor response to neoadjuvant therapy and prognosis in locally advanced rectal cancer[J]. Oncotarget, 2016, 7(42): 68328-38. |
| [15] |
Kumar A, Singh UK, Chaudhary A. Targeting autophagy to overcome drug resistance in cancer therapy[J]. Future Med Chem, 2015, 7(12): 1535-42. DOI:10.4155/fmc.15.88 |
| [16] |
Kumar P, Zhang DM, Degenhardt K, et al. Autophagy and transporterbased multi-drug resistance[J]. Cells, 2012, 1(3): 558-75. DOI:10.3390/cells1030558 |
| [17] |
Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate[J]. Cell, 2014, 157(1): 65-75. DOI:10.1016/j.cell.2014.02.049 |
| [18] |
Levine B. Cell biology-autophagy and cancer[J]. Nature, 2007, 446(7137): 745-7. DOI:10.1038/446745a |
| [19] |
Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy[J]. Cell, 2017, 170(3): 548-63. DOI:10.1016/j.cell.2017.07.008 |
| [20] |
Chen S, Rehman SK, Zhang W, et al. Autophagy is a therapeutic target in anticancer drug resistance[J]. Biochim Biophys Acta, 2010, 1806(2): 220-9. |
| [21] |
Liu L, Yang M, Kang R, et al. DAMP-mediated autophagy contributes to drug resistance[J]. Autophagy, 2011, 7(1): 112-4. DOI:10.4161/auto.7.1.14005 |
| [22] |
Sun WL, Chen J, Wang YP, et al. Autophagy protects breast cancer cells from epirubicin-induced apoptosis and facilitates epirubicinresistance development[J]. Autophagy, 2011, 7(9): 1035-44. DOI:10.4161/auto.7.9.16521 |
| [23] |
Wei MF, Chen MW, Chen KC, et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells[J]. Autophagy, 2014, 10(7): 1179-92. DOI:10.4161/auto.28679 |
| [24] |
Buschman MD, Xing M, Field SJ. The GOLPH3 pathway regulates Golgi shape and function and is activated by DNA damage[J]. Front Neurosci, 2015, 9(12): 362-73. |
| [25] |
Starr T, Forsten-Williams K, Storrie B. Both post-Golgi and intraGolgi cycling affect the distribution of the Golgi phosphoprotein GPP130[J]. Traffic, 2007, 8(9): 1265-79. DOI:10.1111/j.1600-0854.2007.00607.x |
| [26] |
Wang MZ, Qiu CZ, Yu WS, et al. GOLPH3 expression promotes the resistance of HT29 cells to 5-fluorouracil by activating multiple signaling pathways[J]. Mol Med Rep, 2018, 17(1): 542-8. |
| [27] |
Nakashima-Kamimura N, Asoh S, Ishibashi Y, et al. MIDAS/GPP34, a nuclear gene product, regulates total mitochondrial mass in response to mitochondrial dysfunction[J]. J Cell Sci, 2005, 118(Pt 22): 5357-67. |
| [28] |
Buschman MD, Rahajeng J, Field SJ. GOLPH3 links the golgi, DNA damage, and cancer[J]. Cancer Res, 2015, 75(4): 624-7. |
| [29] |
Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nat Cell Biol, 2011, 13(2): 132-41. DOI:10.1038/ncb2152 |
| [30] |
Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation[J]. Autophagy, 2011, 7(8): 924-6. DOI:10.4161/auto.7.8.15860 |
 2019, Vol. 39
2019, Vol. 39

