2. Department of Clinical Medicine, Xiangnan University, Chenzhou 423000, China;
3. Department of Histology and Embryology, School of Basic Medical Sciences, Central South University, Changsha 410013, China;
4. Department of Pharmacy, Xiangnan University, Chenzhou 423000, China;
5. Department of Anatomy, Histology and Embryology, Kunming Medical University, Yunnan 650500, China
LUO Mingying, E-mail: luomingying0403@163.com
2. 湘南学院 临床学院,湖南 郴州 423000;
3. 中南大学基础医学院组胚学系,湖南 长沙 410013;
4. 湘南学院药学院,湖南 郴州 423000;
5. 昆明医科大学人体解剖与组织胚胎学系,云南 昆明 650500
Hypercholesterolemia is an important controllable risk factor for cardiovascular and peripheral arterial disease, but whether it affects the outcome of vascular intervention has not been fully elucidated [1]. Acute occlusion of a major artery often leads to tissue ischemia that may result in tissue necrosis to necessitate limb amputation. Each year, millions of patients undergo coronary bypass surgery or endarterectomies, and these procedures have become very sophisticated and less invasive. However, there are still cases of global coronary and peripheral vascular diseases that are not amenable by these therapies[2].
Anecdotal evidence suggests that collateral vessels are able to compensate for complete occlusion of a major artery. Collateral vessel growth, or arteriogenesis, is the formation of collateral arteries from a pre-existing arteriolar network following the occlusion of a major artery, and serves as a natural defense mechanism to protect tissues from ischemia when the vascular endothelium and endothelial function remain intact[3]. As hypercholesterolemia contributes to vascular dysfunction, it should also, at least in theory, lead to poor collateral vessel growth. Intact endothelial function, expression of endothelial nitric oxide (NO), and optimal concentrations of reactive oxygen species (ROS) are the other prerequisites for arteriogenesis [4]. Reduced endothelial nitric oxide synthase (eNOS) activity and NO bioavailability are the central features of endothelial dysfunction that occurs in the presence of hypercholesterolemia.
Increased plasma levels of low-density lipoprotein (LDL) is an established risk factor for endothelial dysfunction and atherosclerosis[5]. The proatherosclerotic potential of LDL may further increase after its oxidative modification into oxidized LDL (oxLDL)[6]. Lectin-like oxLDL receptor 1 (LOX-1) in endothelial cells is the primary receptor that ligates oxLDL, which was found to down-regulate eNOS expression in human liver sinusoidal endothelial cells[6].
As hydroxymethylglutaryl coenzyme A reductase inhibitors, statins are widely used to reduce the risk of atherosclerosis and stroke among patients with hypercholesterolemia [7]. Statins have been shown to decrease the levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and oxLDL[8, 9], improve vascular endothelial function and blood flow in ischemic hind limb, and improve endothelium-dependent eNOSmediated vasomotor tone [10]. But so far consistent evidences have not been available to support the role of statins in promoting arteriogenesis and accelerating coronary collateral development in advanced coronary artery disease[11, 12].
In the present study, we aimed to test the hypothesis that LOX-1 is an essential regulator of eNOS activity in collateral vessels under hypercholesterolemia, and determine whether lowering plasma cholesterol levels promotes collateral vessel growth.
MATERIALS AND METHODS AnimalsForty male Sprague-Dawley (SD) rats weighing 200-250 g were purchased from Experimental Animal Center, Xiangya School of Medicine, Central South University. All the animal procedures were carried out in compliance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China. The rats were housed in a vivarium with 5 rats per cage under a controlled temperature (20-25 ℃) and were allowed free access to normal rat chow and drinking water. After 1 week of acclimation, the rats were randomly divided into 4 groups, namely, femoral artery ligation (L) group; hypercholesterolemia + femoral ligation (HL) group; hypercholesterolemia + atorvastatin + femoral ligation (AL) group; and hypercholesterolemia + normal saline + femoral ligation (NL) group (10 rats in each group). The rats in HL, AL and NL groups were intraperitoneally injected with a single dose of vitamin D3 (600 000 IU/kg) and fed with an atherogenic diet [13] containing 5% cholesterol, 0.5% sodium cholate, 0.2% propyl-thyracil, 5% refined sugar, 14.3% lard and 75% base feed (15 g/day). The rats in L group received an intraperitoneal injection of isovolumetric saline and were maintained on normal chow. After 8 weeks of feeding, the rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg), and the right femoral artery was ligated. The rats in AL and NL groups were subjected to intraperitoneal injection of 10 mg/kg atorvastatin (AL group) or normal saline (NL group) on a daily basis for 2 weeks after the operation.
Determination of blood lipid levelsTwo weeks after the operation, the rats were anesthetized with 3% sodium pentobarbital (10 mL/kg) and blood samples were collected from the femoral arteries. The plasma levels of TC, triglyceride (TG), high-density lipoprotein (HDL) and LDL in were determined by blood biochemical detection.
Tissue preparationThe rats were sacrificed under anesthesia with sodium pentobarbital two weeks after femoral ligation, and the gracilis muscles were collected for experiments. All the tissue samples were immediately embedded in tissue processing medium (O.C.T), frozen in liquid nitrogen and stored at -80 ℃.
ImmunohistochemistryThe cryosections (10 μm thick) were fixed in 4% paraformaldehyde. Following incubation in 0.5% BSA (Sigma, Saint Louis, MO, USA), the sections were incubated with the primary antibodies of anti-LOX-1 antibody (1:200, Abcam, Cambridge, England) or antieNOS antibody (1: 100, BD Biosciences, NJ, USA) overnight, followed by biotin-SP-conjugated affinipuredonkey-anti-rabbit or anti-mouse IgG (1:200, Dianova, Hamburg, Germany) and Cy2-conjugated streptavidin (1: 200, Biotrend, Cologne, Germany) for 2 h at room temperature. The mesenchymal cells and vascular smooth muscle cells were visualized by Cy3-conjugated vimentin (1:500, Sigma, SL, USA) and Cy3-conjugated α-SM-actin (1: 500, Sigma, Saint Louis, MO, USA) antibodies for 1 h at room temperature, and the cell nuclei were stained with DAPI. The immunofluorescence intensity of LOX-1 and eNOS was quantitatively analyzed using Image J v1.45 (NIH, MD, USA) and expressed in the arbitrary unit AU/μm2. In all the staining procedures, PBS instead of the first antibody was used as the negative control to exclude non-specific binding of the primary or secondary detection system.
Cell cultureHuman umbilical vein vascular endothelial cells (HUVECs) were purchased from ScienCell. The cells were cultured on gelatin-coated plastic dishes in M199 medium (Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, CA, USA), 100 U/mL penicillin-streptomycin and endothelial cell growth supplement (ScienCell, CA, USA) in a humidified incubator at 37 ℃ with 5% CO2. Only the cells within 8 passages were used for subsequent experiments. The identity of HUVECs was confirmed by their cobblestone morphology and strong positive immunoreactivity to von Willebrand factor.
Small interfering RNA transfectionLOX-1 was knocked down by RNA interference (RNAi) following the protocols for small interfering RNA (siRNA) transfection provided by Santa Cruz Biotechnology. Briefly, HUVECs at 75% confluence were transfected with siRNA against LOX-1 (5'-GAATCTGAATCTCCA AGAA-3', GeneChem, Shanghai, China) or a scrambled RNA (negative control, 5'-TTCTCCGAACGTGTCACG T-3', GeneChem, Shanghai of China) via Lipofectamine 2000 (Invitrogen, CA, USA). After 48 h, the medium was replaced with M199 medium, and the cells were treated with oxLDL (50 μg/mL, Peking Union-Biology, Beijing, China) for 24 h.
Reverse transcription-polymerase chain reaction (RT-PCR)The treated HUVECs in each group were harvested and the total RNA was extracted using Trizol reagent (Invitrogen, USA). The same amount of total RNA (1 μg) was reverse transcribed into cDNA using the RevertAidTM First Strand cDNA synthesis kit according to the manufacturer's instructions. qRT-PCR was performed using the Bio-Rad real-time PCR system and the SYBR Green PCR Master Mix with the following primers: LOX-1,
sense, 5'-AGCAAATGGAACTTCACCACCAG-3',
antisense, 5'-AGCTTCTTCTGCTTGTTGCC-3'; eNOS,
sense, 5'-AGGAACCTGTGTGACCCTCA-3',
antisense, 5'-CGAGGTGGTCCGGGTATCC-3'; GAPDH,
sense, 5'-CCACCCATGGCAAATTCCATGGCA-3',
antisense, 5'-TCTAGACGGCAGGTCAGGTCCAC C-3'.
Western blottingThe HUVECs were lysed in RIPA buffer (Cwbiotech, Beijing, China) on ice and the protein concentration was quantified using BCA method (Vector Labortatories, CA, USA). Equal amounts of the protein were loaded for SDS-PAGE and blotting on nitrocellulose membranes. The blots were incubated overnight with anti-LOX-1 antibody or anti-eNOS antibody (Cwbiotech, Beijing, China) with GAPDH as the loading control. The protein bands of LOX-1, eNOS and GAPDH were visualized using an ECL kit (Thermo, MA, USA).
NO measurementNitrite content in the cell culture supernatant was measured to indirectly reflect the content of NO in the HUVECs using Griess reagent kit (Promega, WI, USA). Briefly, the HUVECs were seeded in 24-well plates, and an equal volume of Griess reagent (1% sulfanilic acid, 0.1% N-napthylethylenediamine, and 5% phosphoric acid) was added to 50 μL culture supernatants. The plate was incubated at room temperature for 10 min, and the absorbance at 540 nm was measured using a multifunctional microplate reader (BioTek, VT, USA).
Statistical analysisThe data of the continuous variables are expressed as Mean ± SD. Comparisons of the means between two treatment groups were made using Mann-Whitney U test or Student's t test. The comparisons among multiple groups were done by ANOVA (post-hoc analysis). All statistical analyses were performed using Graphpad Prism version 5.0 (Graphpad Software, San Diego, CA). A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS Body weightThe body weight of the rats did not show significant differ- ences among the 4 groups at the end of study (data not shown).
Blood lipid analysisTab. 1 presents the changes of blood lipids in the 4 groups. In the rats with femoral artery ligation, high-cholesterol diet resulted in significantly increased plasma levels of TC, TG and LDL and decreased HDL-C level as compared with the rats with normal feeding (all P < 0.01). Atorvastatin treatment obviously reduced TC and LDL-levels and lowered TG levels to a lesser extent, and significantly increased HDL-C level in the rats with femoral ligation and high-cholesterol feeding.
| Tab.1 Blood lipid profile of the rats (mmol/L, Mean±SD, n=10) |
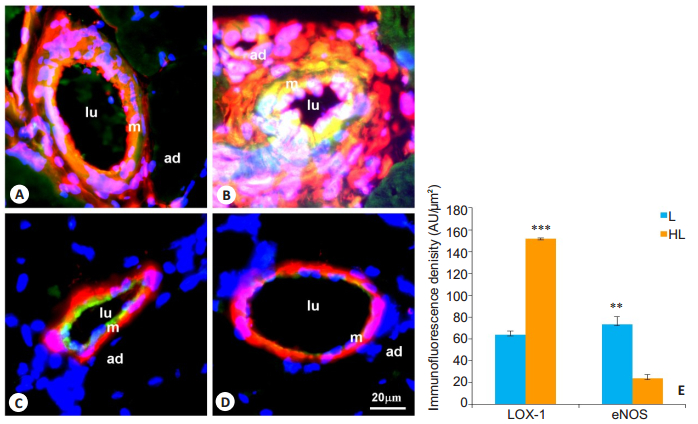
Two weeks after femoral artery ligation, only weak LOX-1 expression was detected in the neointima of the collateral vessels in rats with normal feeding (Fig. 1A). However, the collateral vessels of the hypercholesterolemic rats showed a significantly increased LOX-1 expression, distributed mainly in the neointimal and medial areas and co-localized with vimentin-positive cells (Fig. 1B). Quantitative analysis of LOX-1-stained sections revealed a substantially increased LOX-1 expression in the collateral vessels in HL group (P < 0.001, Fig. 1E). We also noted an induced expression of eNOS in the collateral vessels, likely localized in the endothelial cells as evidenced by a lack of α-SM-actin in the eNOSpositive areas. In the rats with normal feeding, the collateral vessels showed a high expression of eNOS, which was decreased by about 68% in hypercholesterolemic rats (P < 0.01, Fig. 1C, D, E).
|
Fig.1 Expression of LOX-1 and eNOS in collateral vessels in rats with femoral artery ligation (L group) and hypercholesterolemia+femoral ligation (HL group). A, B: Dual immunostaining of LOX-1 (green) and vimentin (red); C, D: Dual immunostaining of eNOS (green) and α-SM-actin (red). Specific fluorescence: Green for LOX-1 and eNOS, red for vimentin and α-SM-actin, and blue for the nuclei; A, C: L group; B, D: HL group; E: Quantitative analysis of immunofluorescence intensity of LOX-1 and eNOS in the collateral arteries (n=10). LOX-1 proteins are observed in the neointima and markedly increased in HL group as compared with L group. The expression of eNOS is observed in the endothelium and significantly reduced in HL group. Lu: Lumen; m: Media; ad: Adventitia; **P < 0.01, ***P < 0.001 vs L group. |
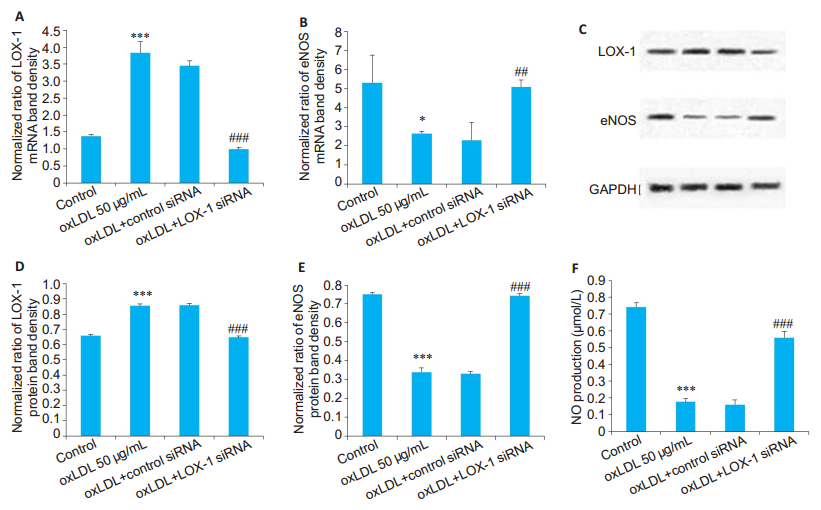
We further investigated the relationship between hypercholesterolemia and eNOS/NO in cultured HUVECs. As shown in Fig. 2, a 24-h incubation of HUVECs with oxLDL (50 μg/mL), a modified LDL often found in blood vessels of hypercholesterolemic patients with atherosclerosis, resulted in an increased expression of LOX-1 by ~4 folds at the mRNA level (P < 0.001) and by ~20% at the protein level (P < 0.001) compared with those in the control cells. Transfection with LOX-1-specific siRNA significantly reduced LOX-1 expression in HUVECs at both the mRNA and protein levels (both P < 0.001, Fig. 2). Interestingly, knocking down LOX-1 rescued oxLDL-induced reduction of eNOS expression at both the mRNA (P < 0.05) and protein (P < 0.001) levels. We also measured NO production in the cells spectrophotometrically, and as shown in Fig. 2F, oxLDL markedly reduced NO production in HUVECs (P < 0.001), which was rescued by knocking down LOX-1 (P < 0.001).
|
Fig.2 Expressions of LOX-1 and eNOS and NO production in HUVECs cultured in the presence of oxLDL with or without LOX-1 siRNA transfection. A, B: Relative mRNA levels of LOX-1 and eNOS, respectively (n=1 μg); C: Expression of LOX-1 and eNOS determined by Western blotting; D, E: Relative protein levels of LOX-1 and eNOS, respectively (n=1.5 μg). Treatment with oxLDL (50 μg/mL) increases the expression of LOX-1 mRNA and protein and decreases the expression of eNOS mRNA and protein. Transfection with LOX-1 siRNA (50 nmol/L) significantly decreases oxLDL- induced up-regulation of LOX-1 and restores the expression of eNOS; F: Quantitative analysis of NO production in the HUVECs following oxLDL incubation and LOX-1 siRNA transfection (n=50 μL). *P < 0.05, ***P < 0.001 vs control; ##P < 0.01, ###P < 0.001 vs control siRNA. |
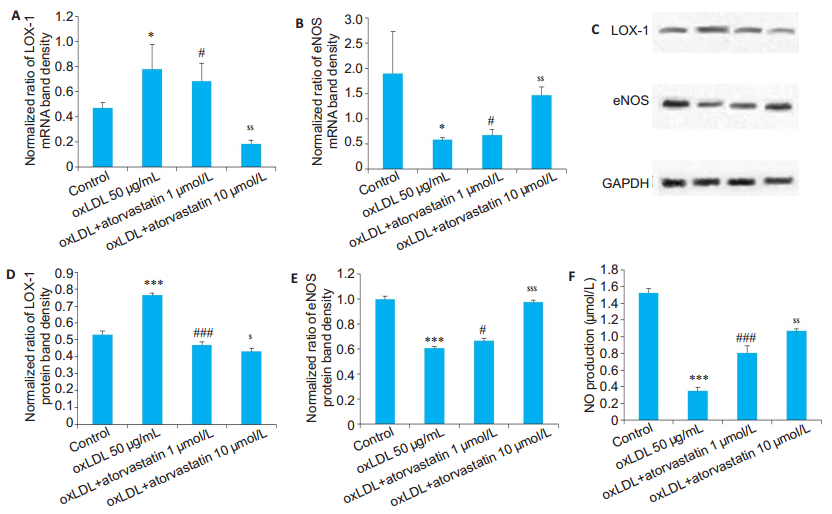
We determined the effects of statins on the HUVECs treated with atorvastatin at a low (1 μmol/L) and a high (10 μmol/L) dose for 30 min prior to oxLDL treatment. As shown in Fig. 3, atorvastatin pretreatment, especially at the high dose, obviously decreased LOX-1 expression in oxLDL-treated HUVECs. Similarly, atorvastatin dosedependently restored the expression of eNOS mRNA (P < 0.05) and protein (P < 0.05) in oxLDL-treated HUVECs (Fig. 3). Atorvastatin also significantly inhibited oxLDLinduced reduction of NO production (P < 0.001), showing a stronger effect at the high dose (P < 0.01, Fig. 3F).
|
Fig.3 Expression of LOX-1 and eNOS and NO productions in HUVECs treated with atorvastatin. A, B: Relative mRNA levels of LOX-1 and eNOS (n=1 μg); C: Expression of LOX-1 and eNOS determined by Western blotting; D, E: Relative protein levels of LOX-1 and eNOS, respectively (n=1.5 μg). Atorvastatin (1 μmol/L) decreases oxLDL-induced up-regulation of LOX-1, and the high concentration (10 μmol/L) resulted in a more pronounced effect. Pretreatment of HUVECs with atorvastatin attenuates oxLDL-induced downregulation of eNOS, and the effect is more pronounced with the high concentration of atorvastatin; F: Quantitative analysis of NO production in the HUVECs in the presence of 1 and 10 μmol/L atorvastatin (n=50 μL). *P < 0.05, ***P < 0.001 vs control; #P < 0.05, ###P < 0.001 vs oxLDL; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs the low concentration. |
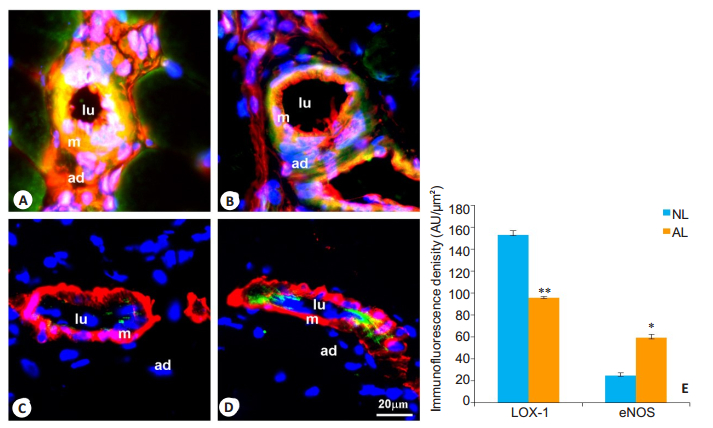
We also examined the effect of atorvastatin on collateral vessel formation in rats with femoral artery ligation and high-cholesterol feeding. Intragastric administration of atorvastatin for 2 weeks effectively prevented hypercholesterolemia-induced up-regulation of LOX-1 (P < 0.01) and down-regulation of eNOS (P < 0.05) in the collateral vessels of the hypercholesterolemic rats (Fig. 4).
|
Fig.4 Expression of LOX-1 and eNOS in the collateral vessels of the rats in hypercholesterolemia + atorvastatin + femoral ligation (AL) group and hypercholesterolemia+normal saline+femoral ligation (NL) group. A, B: Dual immunostaining of LOX-1 (green) and vimentin (red), respectively; C, D: Dual immunostaining of eNOS (green) and α-SM-actin (red), respectively. Specific fluorescence: green for LOX-1 and eNOS, red for vimentin and α-SM-actin, and blue for the nuclei. A, C: NL group; B, D: AL group; E: Quantitative analysis of immunofluorescence intensity of LOX-1 and eNOS in NL and AL groups (n=10). LOX-1 expression is significantly decreased in AL group as compared with that in NL group. The expression of eNOS is markedly increased in AL group. Lu: Lumen; m: Media; ad: Adventitia. *P < 0.05, **P < 0.01 vs NL group. |
Atherosclerosis is the major factor contributing to the increased incidence of coronary heart disease and peripheral arterial disease in the industrialized world [14]. Hypercholesterolemia is considered an important risk factor in the development of atherosclerosis. In addition, oxLDL is well recognized as an atherogenic factor that causes the formation and progression of atherosclerosis[15]. A recent study indicated that oxLDL causes damages of macrophage autophagy to aggravate atherosclerosis [16], and oxLDL cholesterol-associated oxidative stress impairs the function of eNOS[17].
LOX-1 is a single transmembrane receptor that mediates the uptake of oxLDL, and is expressed mainly on endothelial cells and detected also in other cell types such as smooth muscle cells and macrophages [18, 19]. Accumulating evidence indicates the role of LOX-1 in promoting vascular dysfunction and atherosclerosis[20]. In this study, we found that in hypercholesterolemic rats, the expression of LOX-1 was increased in the neointima of the collateral vessels, localized primarily in the endothelial cells and vascular smooth muscle cells; the expression of eNOS, however, was reduced in the endothelial cells of the collateral vessels. In the in vitro experiment, pretreatment of HUVECs with oxLDL obviously down-regulated the expression of eNOS and suppressed NO production. These results suggested that oxLDL uptake in the neointima of the collateral vessels caused endothelial activation and injury. We further used a LOX-1 siRNA to confirm that oxLDL reduced eNOS/NO pathway via regulating LOX-1. oxLDL was originally identified to decrease the activation of Akt[21] and phosphorylation of MAPK[22]in endothelial cells, and LOX-1 was found to mediate monocyte adhesion-triggered NAPDH oxidase-dependent Akt/eNOS signaling pathway[23]. Interestingly, Zhou et al[24] demonstrated that oxLDL mediated the dephosphorylation of Akt and eNOS by LOX-1-induced endoplasmic reticulum stress, suggesting that LOX-1/Akt/eNOS signaling is an essential pathway to mediate oxLDL-induced endothelial function damages and also plays a key role in hypercholesterolemia-induced impairment of the collateral vessels.
Statin therapy has been shown to prevent the progression of atherosclerotic decreases, reduce cardiovascular mortality by lowering LDL-C level[25], and enhance ischemia-induced angiogenesis[26]. In a clinical study of 354 subjects with a mean age of 68 years, the patients with intermittent claudication and hypercholesterolemia who receivd atorvastatin therapy at the daily dose of 80 mg showed significant improvement in pain-free treadmill walking distance and communitybased physical activities by as much as 40% compared with the placebo group at 1-year follow-up[27]. Sata et al[12] found that cerivastatin significantly augmented the recovery of blood flow in ApoE-/- mice by increasing the capillary density. Statins also increase the number of circulating endothelial progenitor cells [28] to promote ischemia-induced neovascularization [29]. Our results demonstrate that atorvastatin markedly reduces oxLDL uptake by effectively decreasing LOX-1 and restore the eNOS/NO pathway to promote collateral artery growth. Therefore, our findings also support the close correlation between LOX-1 and eNOS in the course of atorvastatin therapy. Moreover, Fukuda et al[30] reported that statins have an anti-proliferative effect in vascular smooth muscle cells. We assume that the protective effect of atorvastatin against hypercholesterolemia is possibly attributed to its activity to promote collateral blood flow to the ischemic tissues in a cholesterol-independent manner.
Given its important role in regulating oxLDL-eNOS/ NO-mediated vascular reactivity, LOX-1 may provide a potential therapeutic target for endothelial dysfunction and cardiovascular diseases. Atorvastatin can provide a potentially new treatment strategy for promoting the repair of collateral vessels injury in ischemic diseases.
| [1] |
Leyon JJ, Jaiveer S, Connolly DL, et al. Statin prescription is essential in peripheral vascular disease[J]. J Vasc Interv Radiol, 2010, 21(2): 175-7. DOI:10.1016/j.jvir.2009.12.381 |
| [2] |
Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries[J]. Microcirculation, 2003, 10(1): 83-97. |
| [3] |
Abacı A, Oğuzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels[J]. Circulation, 1999, 99(17): 2239-42. DOI:10.1161/01.CIR.99.17.2239 |
| [4] |
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis[J]. Nat Med, 2000, 6(4): 389-95. DOI:10.1038/74651 |
| [5] |
Lusis AJ. Atherosclerosis[J]. Nature, 2000, 407(6801): 233-41. DOI:10.1038/35025203 |
| [6] |
Stocker R, Keaney JJ. Role of oxidative modifications in atherosclerosis[J]. Physiol Rev, 2004, 84(8): 1381-478. |
| [7] |
Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the american heart association/ american stroke association[J]. Stroke, 2011, 42: 227-76. DOI:10.1161/STR.0b013e3181f7d043 |
| [8] |
Duggan ST. Pitavastatin: a review of its use in the management of hypercholesterolaemia or mixed dyslipidaemia[J]. Drugs, 2012, 72(4): 565-84. DOI:10.2165/11207180-000000000-00000 |
| [9] |
Tsai NW, Lee LH, Huang CR, et al. Statin therapy reduces oxidized low density lipoprotein level, a risk factor for stroke outcome[J]. Crit Care, 2014, 18(1): R16. |
| [10] |
Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells[J]. J Clin Invest, 1998, 101(12): 2711-9. DOI:10.1172/JCI1500 |
| [11] |
Dncer I, Ongun A, Turhan S, et al. Association between the dosage and duration of statin treatment with coronary collateral development[J]. Coron Artery Dis, 2006, 17(6): 561-5. DOI:10.1097/00019501-200609000-00010 |
| [12] |
Sata M, Nishimatsu H, Osuga J, et al. Statins augment collateral growth in response to ischemia but they do not promote cancer and atherosclerosis[J]. Hypertension, 2004, 43(6): 1214-20. DOI:10.1161/01.hyp.0000126186.29571.41 |
| [13] |
Wu Y, Li J, Wang J, et al. Anti-atherogenic effects of centipede acidic protein in rats fed an atherogenic diet[J]. J Ethnopharmacol, 2009, 122(3): 509-16. DOI:10.1016/j.jep.2009.01.017 |
| [14] |
Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals[J]. Circ Res, 2007, 101(1): 27-39. DOI:10.1161/CIRCRESAHA.107.151621 |
| [15] |
Ishigaki Y, Katagiri H, Gao J, et al. Impact of plasma oxidized lowdensity lipoprotein removal on atherosclerosis[J]. Circulation, 2008, 118(1): 75-83. DOI:10.1161/CIRCULATIONAHA.107.745174 |
| [16] |
Fan X, Wang J, Hou J, et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway[J]. J Transl Med, 2015, 13: 92. DOI:10.1186/s12967-015-0450-z |
| [17] |
Yan FX, Li HM, Li SX, et al. The oxidized phospholipid POVPC impairs endothelial function and vasodilation via uncoupling endothelial nitric oxide synthase[J]. J Mol Cell Cardiol, 2017, 112: 40-8. DOI:10.1016/j.yjmcc.2017.08.016 |
| [18] |
Moriwaki H, Kume N, Sawamura T, et al. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein[J]. Arterioscler Thromb Vasc Biol, 1998, 18(10): 1541-7. DOI:10.1161/01.ATV.18.10.1541 |
| [19] |
Yoshida H, Kondratenko N, Green S, et al. Identification of the lectinlike receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor[J]. Biochem J, 1998, 334(Pt 1): 9-13. |
| [20] |
Xu S, Ogura S, Chen J, et al. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers[J]. Cell Mol Life Sci, 2013, 70(16): 2859-72. DOI:10.1007/s00018-012-1194-z |
| [21] |
Li DY, Chen HJ, Mehta JL. Statins inhibit oxidized-LDL-mediated LOX-1 expression, uptake of oxidized-LDL and reduction in PKB phosphorylation[J]. Cardiovasc Res, 2001, 52(1): 130-5. |
| [22] |
Mehta JL, Li DY, Chen HJ, et al. Inhibition of LOX-1 by statins may relate to upregulation of eNOS[J]. Biochem Biophys Res Commun, 2001, 289(4): 857-61. DOI:10.1006/bbrc.2001.6070 |
| [23] |
Sakamoto N, Ishibashi T, Sugimoto K, et al. Role of LOX-1 in monocyte adhesion-triggered redox, Akt/eNOS and Ca2 + signaling pathways in endothelial cells[J]. J Cell Physiol, 2009, 220(3): 706-15. DOI:10.1002/jcp.21818 |
| [24] |
Zhou J, Abid MDN, Xiong Y, et al. ox-LDL downregulateseNOS activity via LOX-1-mediated endoplasmic reticulum stress[J]. Int J Mol Med, 2013, 32(6): 1442-50. |
| [25] |
Wadhera RK, Steen DL, Khan I, Giugliano RP, Foody JM. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality[J]. J Clin Lipidol, 2016, 10(3): 472-89. DOI:10.1016/j.jacl.2015.11.010 |
| [26] |
Sasaki K, Murohara T, Ikeda H, et al. Evidence for the importance of angiotensin Ⅱ type 1 receptor in ischemia-induced angiogenesis[J]. J Clin Invest, 2002, 109(5): 603-11. DOI:10.1172/JCI0213055 |
| [27] |
Mohler ER, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease[J]. Circulation, 2003, 108(12): 1481-6. DOI:10.1161/01.CIR.0000090686.57897.F5 |
| [28] |
Llevadot J, Murasawa S, Kureishi Y, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells[J]. J Clin Invest, 2001, 108(3): 399. DOI:10.1172/JCI200113131 |
| [29] |
Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization[J]. J Clin Invest, 2000, 105(11): 1527-36. DOI:10.1172/JCI8296 |
| [30] |
Fukuda K, Matsumura T, Senokuchi T, et al. Statins meditate antiatherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-gamma activation[J]. Biochem Biophys Res Commun, 2015, 457(1): 23-30. DOI:10.1016/j.bbrc.2014.12.063 |
 2019, Vol. 39
2019, Vol. 39

