肺癌是全世界肿瘤导致死亡的主要原因,非小细胞肺癌(NSCLC)大约占所有肺癌的80%,进一步可分为几个亚型,包括肺腺癌、肺鳞癌和肺大细胞癌。其中肺腺癌是所有肺癌中最常见的亚型,约占所有肺癌的40%[1],并且呈上升的趋势。肺癌的发生、发展过程极其复杂,过去的几十年一直致力于研究基因、蛋白质的作用;近年来,研究发现一种新的小分子microRNA(miRNA)也参与肺癌发生、发展的各个环节。
MicroRNA是内源的、非编码的小RNA分子,与AGO(Argonaute)蛋白结合形成RISC(RNA-induced silencing complex)复合体,指导AGO蛋白与互补的mRNA结合, 并进一步剪切互补的mRNA或者抑制mRNA的翻译[2]。许多miRNA被证明可以调控肿瘤增殖、凋亡、细胞周期、侵袭性等病理过程密切相关[3-7]。在肺癌、胃癌、宫颈癌、膀胱癌、前列腺癌中均发现了miR-126-5p(microRNA-126-5p)表达量的变化[8-10]。Jusufovic[11]发现miR-126-5p与肺癌患者预后相关。研究认为肺癌细胞中miR-126-5p可以通过吸附MDH1蛋白mRNA导致细胞死亡[12]。对miR-126-5p作用机制进行探索,发现其可以影响STAT3信号通路[13]。miRNA不仅在细胞、组织中稳定表达,在外周血中也能够表达,而且具有更强的稳定性,可能具有更广阔的应用前景[14]。然而,目前肺腺癌患者癌组织及外周血清miR-126-5p表达水平差异性及其作用机制仍然缺乏系统性研究。
BRCC3(breast cancer type 1 susceptibility protein/breast cancer type 2 susceptibility proteincontaining complex subunit 3)是一种E3泛素化连接酶[15]。在鼻咽癌中,BRCC3可以作为患者不良预后的指标,在乳腺癌中,BRCC3与G2/M细胞周期阻滞有关,可以减弱DNA损伤的修复[16-17]。研究BRCC3与卵巢癌的关系,结果提示BRCC3为独立的不良预后因素[18]。在神经胶质瘤细胞株中,发现敲减BRCC3可以提高肿瘤细胞对化疗药物的敏感度[19]。然而,关于BRCC3在肺癌中的作用,目前仍然缺乏相关的研究。
综上所述,miR-126-5p临床预后价值尚缺乏大样本数据的研究证明,本研究主要通过生物信息学方法阐述其临床预后作用,并且预测到其靶蛋白BRCC3,进一步论证了BRCC3在正常组织和肺癌中表达差异,为肺腺癌的临床治疗提供了新的靶点。
1 资料和方法 1.1 肺腺癌患者肿瘤组织与正常肺组织miR-126-5p表达水平差异STARBASE网站是一个在线非编码RNA研究数据库(http://starbase.sysu.edu.cn/panCancer.php),利用网站自带的PAN-CANCER软件可以分析32种肿瘤组织与正常组织间mircroRNA表达的差异性,纳入病人数据主要来源于TCGA(The Cancer Genome Atlas)RNA测序结果,数据库设定条件:Entrance of The Platform:miRNA differential expression; miRNA: mir-126-5p; Cancer: lung adenocarcinoma; Chart type: box plot; Data scale: log2-scale.结果使用箱状图来进行表示。
1.2 肺腺癌miR-126差异性表达对患者预后的影响Kaplan-Meier Plotters是一款在线分析肿瘤患者生存预后的软件(http://www.kmplot.com/analysis/index.php?P=service&cancer=pancancer_mirna),此软件miRNA Pan-cancer平台可分析差异miRNA肺癌患者的生存预后,患者主要来自于TCGA数据库和GEO(Gene Expression Omnibus)数据库。参数设定:Select all: Lung adenocarcinoma; Gene symbol: has-mir-126-5p; Split patients by: Auto select best cutoff; Survival: OS; Cancer type: lung adenocarcinoma; Stage: all; Race: all; Gender: all。
1.3 miR-126-5p在肺腺癌中靶基因预测使用STARBASE在线网站,利用miRNA-mRNA模块分析预测miR-126-5p在肺腺癌中靶基因,为了提高预测的准确性及缩小靶基因的范围,PREDICTED PROGRAM设定选择miRanda和miRmap。在线分析miR-126-5p和靶基因mRNA表达的相关性。
1.4 靶基因BRCC3在肺腺癌及正常组织中的表达量及其对预后的影响使用UALCAN在线网站(http://ualcan.path.uab.edu/index.html)分析TCGA数据库中BRCC3在肺腺癌及正常组织的表达量,在Kaplan-Meier Plotters在线网站中分析BRCC3对肺腺癌患者高表达及低表达患者中生存差异。
1.5 肺腺癌患者外周血清miR-126-5p表达差异性我们收集2017年皖南医学院附属弋矶山医院体检中心30位健康者(对照组)的外周全血,同时收集胸心外科30例肺腺癌患者(病例组)的外周全血。健康对照组年龄23~71岁(中位43岁),男性17例,女性13例,无肿瘤相关病史;肺腺癌患者29~77岁(中位47岁),男性17例,女性13例,其中TNMⅠ期患者6例,Ⅱ期11例,Ⅲ期8例,Ⅳ期5例;采血前患者无肿瘤相关治疗病史。采用乙二酸四乙酸(EDTA)-K2抗凝,将取得的对照组及病例组的全血分别进行离心获取血浆,分装至500 μL试管中,在-80 ℃冰箱保存。运用qRT-PCR技术分析对照组及病例组各个样本中miR-126-5p的表达情况。用2-ΔΔCt法计算miR-126-5p的相对表达量。
1.6 统计学处理研究采用了SPSS 18.0软件来进行统计学分析,数值变量数据使用均数±标准差表示,两组间数值变量的比较采用t检验,P < 0.05为差异有统计学意义,数据作图采用Graphpad Prism 5(Version 5.01)。
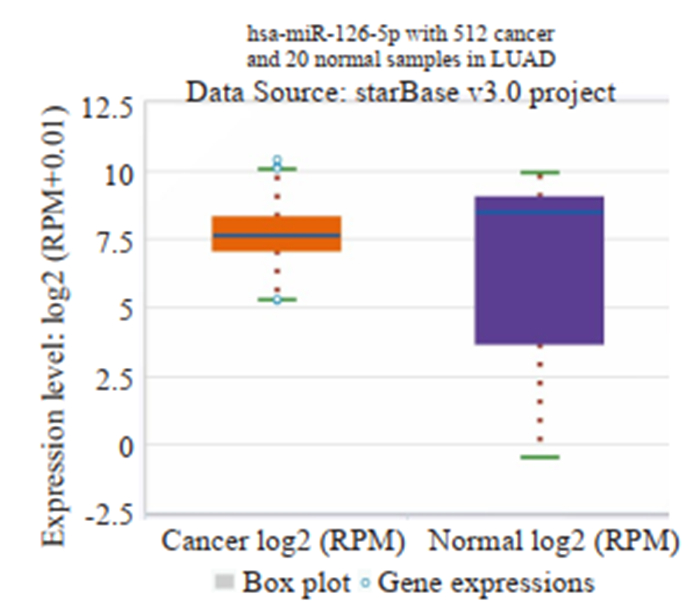
2 结果 2.1 肺腺癌患者肿瘤组织与正常肺组织miR-126-5p表达水平差异的大数据分析结果STARBASE数据库共分析512例肺腺癌患者组织样本,20例正常肺组织样本,肺腺癌组织中miR-126-5p表达水平低于正常肺组织(P < 0.05,图 1)。
|
图 1 miR-126-5p在肺腺癌组织和正常肺组织的表达水平对比 Fig.1 Comparison of the expression of miR-126-5p in lung carcinoma and normal lung tissue. |
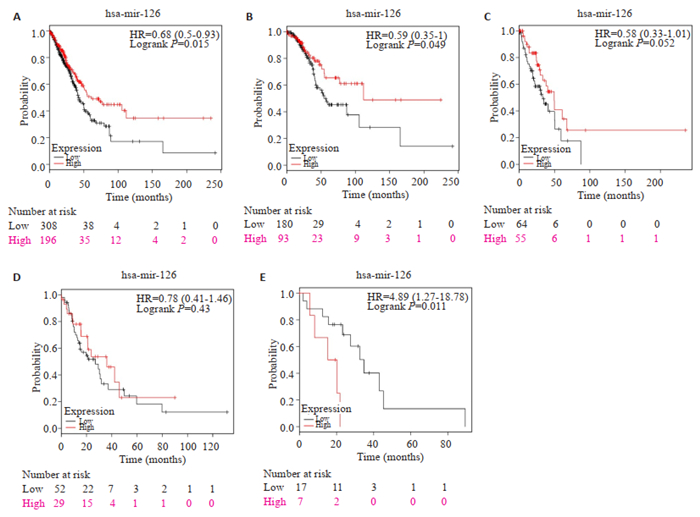
Kaplan-Meier Plotter数据库检索纳入了504例肺癌患者,其中miR-126-5p低表达组308例,高表达组196例;TNMⅠ期患者273例,Ⅱ期患者119例,Ⅲ期患者81例,Ⅳ期患者24例,未注明分期患者7例。男性患者236例,女性患者268例。高表达miR-126组患者生存预后明显优于低表达组,差异有明显统计学意义(HR=0.68,P=0.015),其生存曲线及各个TNM分期亚组生存曲线如图 2所示。
|
图 2 肺腺癌患者miR-126-5p高表达组和低表达组生存曲线 Fig.2 Survival curves of patients with lung adenocarcinoma in high mir-126-5p expression group and low expression group. A: Comparison of the overall survival curves of high-expression group and low-expression group; B: Survival curves for TNM stage Ⅰ patients; C: Survival curves for TNM stage Ⅱ patients; D: Survival curves for TNM stage Ⅲ patients; E: Survival curves for TNM stage Ⅳ patients. |
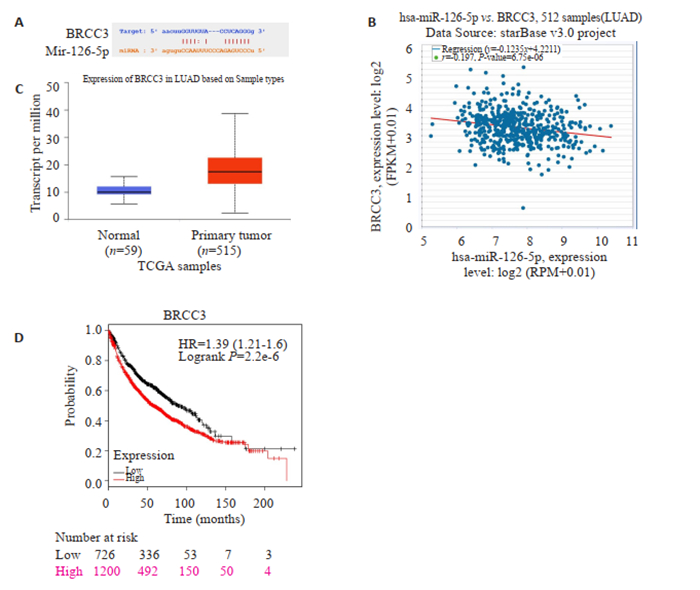
根据STARBASE软件的结果,预测到的靶基因为BRCC3,miR-126-5p可以与BRCC3 mRNA的3'UTR相结合(图 3A);miR-126-5p与BRCC3 mRNA的表达量呈负相关(r=-0.197,P < 0.05,图 3B)。
|
图 3 miR-126-5p靶基因预测及分析 Fig.3 Prediction and analysis of the target gene of miR-126-5p. A: Prediction of miR-126-5p binding sites in BRCC3 mRNA 3'UTR; B: Relationship between miR-126-5p expression level and BRCC3 mRNA level; C: BRCC3 mRNA level in normal lung tissue and lung adenocarcinoma; D: Relationship between BRCC3 mRNA level and the overall survival of patients with lung adenocarcinoma. |
基于TCGA数据在线网站分析提示肺腺癌患者BRCC3表达量明显高于正常组织,结果具有统计学意义(图 3C),为了进一步了解BRCC3对患者生存预后的影响,我们采用Kaplan-Meier Plotter数据库进行生存分析,结果提示高表达组1200人,低表达组726人,低表达组生存预后明显优于高表达组(HR=1.39,P < 0.05,图 3D)。
2.5 肺腺癌患者外周血清miR-126-5p表达差异性收集到的病例组肺腺癌患者30例,其中男性18例,女性12例,TNM分期Ⅰ期患者6例,Ⅱ期患者12例,Ⅲ期患者10例,Ⅳ期患者2例;对照组30例,其中男性14例,女性16例。肺腺癌患者外周血miR-126-5p表达水平明显低于对照组(0.63±0.12 vs 1.23±0.21),差异有统计学意义(P=0.02)。
3 讨论miRNA主要的功能是结合靶基因mRNA的3UTR区,对其进行降解,从而沉默靶基因[20]。既往研究认为miR-126-5p产生于内皮细胞,编码基因定位在人类9号染色体的表皮生长因子的基因区域内[21]。miR-126-5p可以通过AKT/ERK1/2信号通路来靶向抑制CXCR4基因,从而抑制肿瘤的增生[22]。有研究提示其表达水平可以被TUBEIMOSIDE-1抑制;研究A549肺癌细胞株表明miR-126-5p能够作用于STAT3信号通路,抑制癌细胞生长[23-24]。miR-126-5p可以被LncRNA PVT1-5调控,通过ceRNA机制来发挥生物学作用[25]。运用Meta分析回顾性研究了非小细胞肺癌病人临床预后与miR- 126-5p癌组织的差异性表达之间的关系,提示高表达miR-126-5p的患者具有明显的生存优势[26]。本研究纳入的病人数量较多,采用大数据分析方法提示肺腺癌组织miR-126-5p表达水平低于正常肺组织,肺癌组织中miR-126-5p表达量可以作为临床预后指标,高表达病人具有较好的临床预后,结果与Jusufovic的研究相符[11]。
在本研究中我们发现并首次提出miR-126-5p可以调控BRCC3基因,基于大数据样本分析,BRCC3与患者的生存预后明显相关,属于肺癌的不良预后因子,其对肺癌的增殖及转移的影响有待进行进一步的细胞和动物水平验证,是肺癌潜在的治疗靶点。
与组织学水平检测microRNA相比,外周血血清检测microRNA具有早期诊断、创伤小等特点,特别是对于肿瘤病人的筛查及术后病人的随访监测具有重要的临床价值[27]。既往研究认为miR-126-5p在组织学类型、TNM分析、吸烟状态方面存在差异性表达,并且可以作为肺癌死亡的独立的危险因素[28]。然而,目前血清中miR-126-5p应用于肺腺癌的临床诊断和预后评估的研究较少,因此,本研究还检测了外周血中miR-126-5p的表达,结果表明肺腺癌组表达量低于健康对照组,其可以作为临床检测的潜在生物标记物。
综上所述,肺腺癌组织及外周血清中miR-126-5p表达水平低于正常肺组织,miR-126-5p高表达肺腺癌患者预后较好,低表达患者预后较差,miR-126-5p可以作为肺腺癌的诊断和预后的标志物,其调控的BRCC3可以作为潜在的诊疗靶点用于临床实践。
| [1] |
Meng W, Ye Z, Cui R, et al. MicroRNA-31 predicts the presence of lymph node metastases and survival in patients with lung adenocarcinoma[J]. Clin Cancer Res, 2013, 19(19): 5423-33. DOI:10.1158/1078-0432.CCR-13-0320 |
| [2] |
Bica-Pop C, Cojocneanu-Petric R, Magdo LA, et al. Overview upon miR-21 in lung cancer: focus on NSCLC[J]. Cell Mol Life Sci, 2018, 75(19): 3539-51. DOI:10.1007/s00018-018-2877-x |
| [3] |
Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation[J]. Circ Res, 2007, 100(11): 1579-88. DOI:10.1161/CIRCRESAHA.106.141986 |
| [4] |
Bartel DP. MicroRNAs: target recognition and regulatory functions[J]. Cell, 2009, 136(2): 215-33. DOI:10.1016/j.cell.2009.01.002 |
| [5] |
Cui H, Song R, Wu J, et al. MicroRNA-337 regulates the PI3K/AKT and Wnt/β-catenin signaling pathways to inhibit hepatocellular carcinoma progression by targeting high-mobility group AT-hook 2[J]. Am J Cancer Res, 2018, 8(3): 405-21. |
| [6] |
Fu X, Wen HQ, Jing L, et al. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway[J]. Cancer Sci, 2017, 108(4): 620-31. DOI:10.1111/cas.13177 |
| [7] |
Van Sinderen M, Griffiths M, Menkhorst E, et al. Restoration of microRNA-29c in type Ⅰ endometrioid Cancer reduced endometrial Cancer cell growth[J]. Oncol Lett, 2019, 18(3): 2684-93. |
| [8] |
Feng R, Chen X, Yu Y, et al. miR-126 functions as a tumour suppressor in human gastric cancer[J]. Cancer Lett, 2010, 298(1): 50-63. |
| [9] |
Feng RH, Sah BK, Li JF, et al. miR-126: an indicator of poor prognosis and recurrence in histologically lymph node-negative gastric cancer[J]. Cancer Biomark, 2018, 23(3): 437-45. DOI:10.3233/CBM-181526 |
| [10] |
Wang CL, Zhou B, Liu M, et al. miR-126-5p restoration promotes cell apoptosis in cervical cancer by targeting Bcl212[J]. Oncol Res, 2017, 25(4): 463-70. DOI:10.3727/096504016X14685034103879 |
| [11] |
Jusufovic E, Rijavec M, Keser D, et al. let-7b and miR-126 are Down-Regulated in tumor tissue and correlate with microvessel density and survival outcomes in Non-Small-Cell lung cancer[J]. PLoS One, 2012, 7(9): e45577. DOI:10.1371/journal.pone.0045577 |
| [12] |
Lima Queiroz A, Zhang B, Comstock DE, et al. miR-126-5p targets Malate Dehydrogenase 1 in non-small cell lung carcinomas[J]. Biochem Biophys Res Commun, 2018, 499(2): 314-20. DOI:10.1016/j.bbrc.2018.03.154 |
| [13] |
Queiroz AL, Zhang BX, Comstock DE, et al. miR-126-5p targets malate dehydrogenase 1 in non-small cell lung carcinomas[J]. Biochem Biophys Res Commun, 2018, 499(2): 314-20. DOI:10.1016/j.bbrc.2018.03.154 |
| [14] |
Zhang Z, Wang J, Cheng J, et al. Effects of miR-126 on the STAT3 signaling pathway and the regulation of malignant behavior in lung cancer cells[J]. Oncol Lett, 2018, 15(6): 8412-6. |
| [15] |
Xu S, Yang F, Liu RW, et al. Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis[J]. Oncol Rep, 2018, 39(5): 2376-84. |
| [16] |
Dong Y, Hakimi MA, Chen X, et al. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair[J]. Mol Cell, 2003, 12(5): 1087-99. DOI:10.1016/S1097-2765(03)00424-6 |
| [17] |
Tu ZW, Xu BQ, Qu C, et al. BRCC3 acts as a prognostic marker in nasopharyngeal carcinoma patients treated with radiotherapy and mediates radiation resistance in vitro[J]. Radiation Oncology, 2015, 10(2): 331-8. DOI:10.1186/s13014-015-0427-3 |
| [18] |
Rebbeck TR, Mitra N, Wan F, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer[J]. JAMA, 2015, 313(13): 1347-61. DOI:10.1001/jama.2014.5985 |
| [19] |
Chai KM, Wang CY, Liaw HJ, et al. Downregulation of BRCA1-BRCA2-containing complex subunit 3 sensitizes glioma cells to temozolomide[J]. Oncotarget, 2014, 5(21): 10901-15. DOI:10.18632/oncotarget.2543 |
| [20] |
Hosseinahli N, Aghapour M, Duijf PH. Treating cancer with microRNA replacement therapy: A literature review[J]. J Cell Physiol, 2018, 233(8): 5574-88. DOI:10.1002/jcp.26514 |
| [21] |
Ebrahimi F, Gopalan V, Smith RA, et al. miR-126 in human cancers: clinical roles and current perspectives[J]. Exp Mol Pathol, 2014, 96(1): 98-107. DOI:10.1016/j.yexmp.2013.12.004 |
| [22] |
Liu YL, Zhou Y, Feng X, et al. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways[J]. Int J Oncol, 2014, 44(1): 203-10. DOI:10.3892/ijo.2013.2168 |
| [23] |
Shi HB, Bi HX, Sun XY, et al. Tubeimoside-1 inhibits the proliferation and metastasis by promoting miR-126-5p expression in non-small cell lung cancer cells[J]. Oncol Lett, 2018, 16(3): 3126-34. DOI:10.3892/ol.2018.9051 |
| [24] |
Zhang ZY, Wang JH, Cheng J, et al. Effects of miR-126 on the STAT3 signaling pathway and the regulation of malignant behavior in lung cancer cells[J]. Oncol Lett, 2018, 15(6): 8412-6. |
| [25] |
Li H, Chen S, Liu J, et al. Long non-coding RNA PVT1-5 promotes cell proliferation by regulating miR-126/SLC7A5 axis in lung cancer[J]. Biochem Biophys Res Commun, 2018, 495(3): 2350-5. DOI:10.1016/j.bbrc.2017.12.114 |
| [26] |
Zheng W, Zhou Y, Lu J, et al. The prognostic value of miR-126 expression in non-small-cell lung cancer: a meta-analysis[J]. Cancer Cell Int, 2017, 17(1): 71. DOI:10.1186/s12935-017-0440-8 |
| [27] |
曲莹, 何海燕, 侯健. 循环microRNA在恶性血液病中的研究进展[J]. 中国实验血液学杂志, 2014, 22(1): 219-22. |
| [28] |
张玉洁, 王微微, 商安全, 等. 非小细胞肺癌患者血清miR-22和miR-126的检测及其临床意义[J]. 中国肿瘤生物治疗杂志, 2017, 24(12): 1403-8. DOI:10.3872/j.issn.1007-385X.2017.12.011 |
 2019, Vol. 39
2019, Vol. 39

