特立帕肽(TPTD)主要作用于骨骼、肾小管和肠道等系统,调节血清钙盐的稳态[1-3]。基础和临床研究证明间断小剂量的TPTD显著成骨[4],用于治疗严重骨质疏松。但大剂量持续应用时,犹如甲状旁腺素亢进状态,显著促进皮质骨和松质骨吸收[5]。研究如何降低TPTD破骨作用,保留或者促进成骨活性是有关PTH研究的一个重要方向[6-7],而TPTD的皮下持续泵入模型常常被用来研究其破骨作用。我们在研究hPTH(1-34)及其模拟肽的破骨作用时,意外观察到hPTH(1-34)增加去势小鼠松质骨量,与之前广泛报道的TPTD促进骨吸收的结果不符合。TPTD在去势小鼠的成骨作用或促进骨缺损愈合的研究常选用间断给药的方式,而不采用持续灌注的给药方式[8]。以往TPTD持续灌注促骨吸收的结果来自于正常小鼠的研究,但在去势后TPTD连续灌注是否还保持相同的促骨吸收作用并不明确。本文探讨TPTD的破骨作用在正常或去势小鼠并不完全相同,TPTD持续灌注的给药方式在去势小鼠身上可能没有显现促骨吸收的作用并分析可能的机制。
1 材料和方法 1.1 TPTD微量缓释泵的制备TPTD(重组人PTH(1-34))由广州特立生物科技有限公司合成,0.1%三氟乙酸(TFA)溶解成10-3 mol/L备用。用生理盐水将TPTD储存液按40μg(kg·d),共14 d量配制成0.2 mL,注入植入式胶囊渗透泵(ALZET)。空白对照渗透泵注入等量生理盐水。
1.2 微量泵持续泵入小鼠分组及模型的制备24只6周龄小鼠,适应性喂养1周后,随机分成2组,每组有12只小鼠,进行卵巢切除(OVX)或假手术处理(SHAM)。卵巢切除按常规方法进行[9],假手术进行切除卵巢以外的同样手术过程。1周后对OVX和SHAM小鼠再次随机分组,形成4个实验分组:(1)假手术对照(SHAM-VEH)组:SHAM后植入空白对照缓释泵;(2)假手术特立帕肽(SHAM+TPTD)组:SHAM后植入含TPTD溶液的胶囊缓释泵;(3)卵巢切除对照(OVXVEH)组:OVX后植入空白对照缓释泵;(4)卵巢切除特立帕肽(OVX+TPTD)组:OVX后植入含TPTD溶液的胶囊缓释泵。所有小鼠在植入胶囊缓释泵2周后处死。
饲养条件:实验动物分笼,每笼3只,饲养于室温18~29 ℃,相对湿度40%~70%的清洁环境中。换气次数8~10次/h,氨浓度≤l4 mg/m3,噪声≤60 db,昼夜明暗交替12/12 h。隔日更换垫料,自由摄食、摄水。
1.3 小鼠骨松质和骨皮质显微结构分析所有小鼠在植入胶囊缓释泵2周后,行牵引颈椎脱臼法处死,解剖获得双侧下肢,常温予以4%多聚甲醛固定24~48 h。分析前,生理盐水洗涤30 min,使其充分水化。显微CT扫描分析胫骨上段骨和股骨中段的骨量、骨小梁数目、骨小梁离散度,骨小梁连接系数和骨皮质面积等参数。显微CT(μCT80.Scanco Medical AG)参数设置:电压55 kV,电流1451 mA,层厚12μm。
1.4 胫骨骨松质组织学分析将多聚甲醛固定后的胫骨标本流水冲洗24 h,EDTA脱钙液脱钙30 d,经过50%、70%、80%、90%、95%及无水乙醇各处理60 min脱水,然后二甲苯透明、浸石蜡。常规包埋,保持胫骨切面与包埋盒底部平行。石蜡切片厚度5μm。
HE染色按常规进行。TRAP染色:采用SIGMAALDRICH 387A-1KT TRAP染色试剂盒,按照说明书流程染色。用甲基绿(建成生物工程研究所)复染2 min,甘油封片,显微镜下观察和计数破骨细胞数目,并以ImageJ软件分析每单位体积表面破骨细胞数目。测量胫骨生长板厚度。
1.5 血清钙离子浓度测定处死小鼠后即刻进行胸腔取血,置于1.5 mL的离心管中,室温静置30 min,直至血液凝结,4 ℃离心机离心15 min(2000 g),小心移取上层黄色液体到新的1.5 mL离心管,即刻置于冰槽里备用或放进-80 ℃冰箱里保存。试剂盒测量血清总钙离子浓度。
1.6 成骨细胞蛋白表达分析取新生C57BL小鼠颅骨以胶原酶消化法分离成骨细胞,取P2代细胞,培养于6 cm细胞培养皿。以成骨诱导培养基(DMEM+10%胎牛血清+50μg/mL维生素C+ 100 mmol/L磷酸甘油+100 nmol/L地塞米松)培养24 h后分为4组,分别接受10-8 mol/L TPTD,10-8 mol/L E2,10-8 mol/L(TPTD+E2)或等体积0.1%TFA,培养48 h后,注射器吸干培养基,PBS轻柔洗涤2次,每次3 min,随后吸干PBS,向每个培养皿内加入150 μL RIPA裂解液,静置15 min;然后将细胞裂解物移入1.5 mL离心管,超声粉碎仪充分裂解细胞(300 w,5 s,重复3次),最后离心(1.2 g×104 g)20 min,留取上清即细胞总蛋白。提取细胞蛋白全程冰上操作。
提取的蛋白经8% SDS-PAGE凝胶电泳分离,转聚偏二氟乙烯(PVDF)膜,兔抗鼠β-catenin和RANKL抗体(Abcam)和抗兔IgG(Abcam)依次孵育,化学发光仪对PVDF上相应的杂交条带曝光并记录。
1.7 统计学分析所得实验数据采用SPSS 23.0软件进行统计学分析,采用单因素方差分析检验(one-way ANOVA)进行组间比较检验。按P < 0.05为差异具有统计学意义。
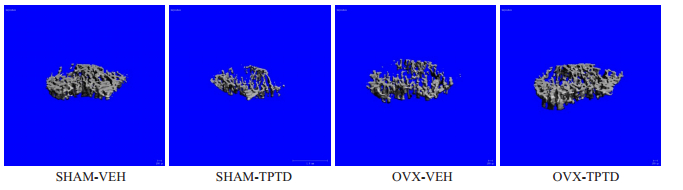
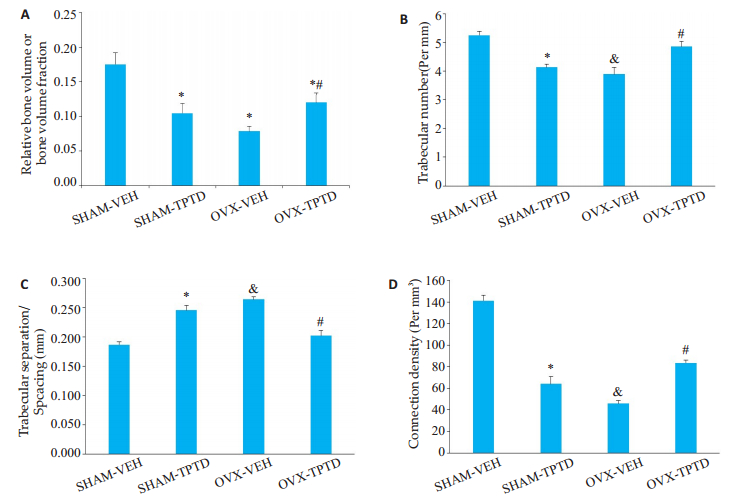
2 结果 2.1 TPTD持续泵入对正常或去势小鼠松质骨和皮质骨的影响与SHAM小鼠相比,OVX小鼠的胫骨上段骨小梁变得稀疏(图 1),骨密度(图 2A),骨微观结构参数股骨中段的骨量、骨小梁数目、骨小梁离散度和骨小梁连接系数明显下降(图 2)。在正常小鼠,TPTD持续作用使胫骨上段松质骨骨小梁稀疏(图 1),骨微观结构参数显著降低(图 2)。在OVX小鼠出现相反的现象,OVXTPTD组较OVX组小鼠骨量和骨微观参数显著升高(图 1,2)。
|
图 1 OVX和SHAM组小鼠受TPTD持续作用时胫骨骨小梁形态的改变 Fig.1 Morphological changes of tibia trabecular bone in OVX and SHAM groups following TPTD treatment. The trabecular bone was reduced in OVX group compared with SHAM group and in SHAM-TPTD group compared with SHAM-VEH group, and used increasd in OVX-TPTD group compared with OVX-VEH group. |
|
图 2 OVX和SHAM小鼠受TPTD持续作用时胫骨骨量的改变 Fig.2 Changes in tibial bone mass in OVX and SHAM mice following TPTD treatment. (*P < 0.05 vs SHAMVEH; #P < 0.05 vs OVX-VEH; & P < 0.05 vs SHAM-VEH). A: Relative bone volume or bone volume fraction in %; B: Trabecular number; C: Trabecular separation/Spcacing; D: Connection density |
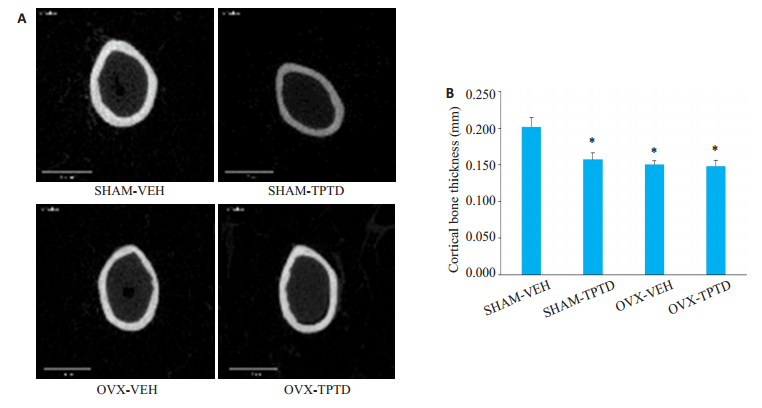
在股骨中段(图 3),OVX小鼠骨皮质显著低于SHAM小鼠,TPTD的作用使SHAM小鼠皮质骨变薄,但是TPTD并不引起OVX小鼠骨皮质厚度的进一步下降。
|
图 3 OVX和SHAM小鼠受TPTD持续作用时皮质骨厚度的改变 Fig.3 Changes in cortical bone thickness in OVX and SHAM mice after TPTD treatment. A: Micro-CT scan showing the midsection of the femur; B: Statistical results of measurements of femoral cortical bone thickness (*P < 0.05 vs SHAM-VEH) |
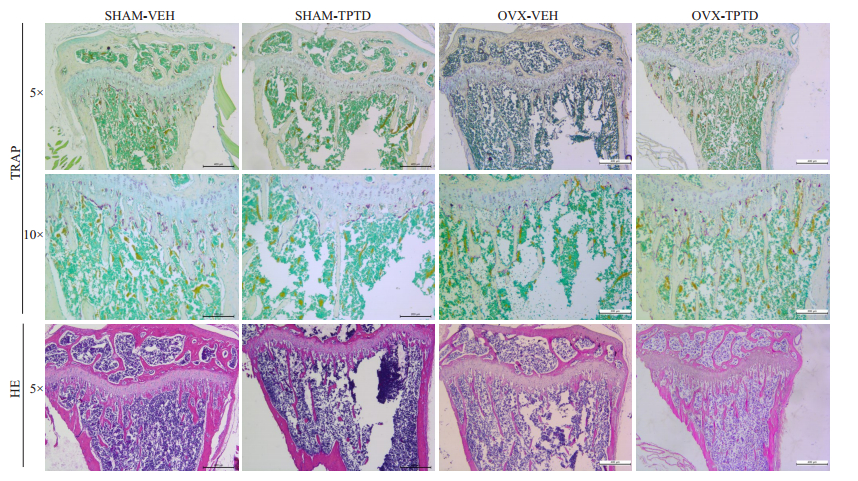
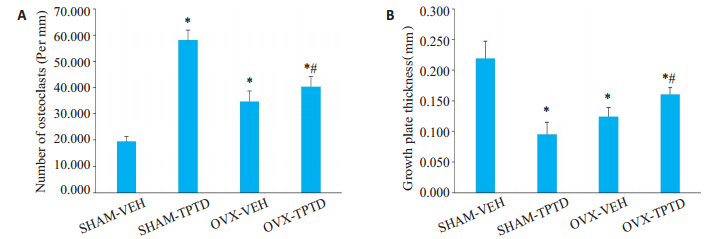
胫骨上段骨松质组织染色显示,在SHAM小鼠,TPTD连续泵入药两周后,骨量减少(图 4),骨小梁生长板的厚度变薄(图 5)。但在OVX小鼠,TPTD使松质骨骨小梁数量和生长板厚度增多。TRAP染色和计数显示,SHAM小鼠在TPTD连续刺激后,松质骨表面破骨细胞数显著增多(图 4和5,P < 0.05);但在OVX小鼠,TPTD连续刺激仅使松质骨表面破骨细胞数小幅度增加。提示TPTD泵入对破骨细胞的刺激效果明显受到去势的影响。
|
图 4 胫骨上段松质骨切片HE染色与TRAP染色结果 Fig.4 HE staining and TRAP staining of the upper part of the tibial cancellous bone. In SHAM mice, continuous pumping of TPTD for 2 weeks significantly reduced the bone mass and growth plate thickness in the tibia (P < 0.05). In OVX mice, TPTD treatment significantly increased the number and thickness of the trabecular and bone growth plate thickness in the tibia (P < 0.05). The number of osteoclasts increased significantly in SHAM mice with TPTD treatment (P < 0.05); but the effect of TPTD was not obvious on the number of osteoclasts in OVX mice (P < 0.05) |
|
图 5 OVX和SHAM小鼠受TPTD持续作用时胫骨上段TRAP阳性细胞数目和胫骨生长板厚度的改变 Fig.5 Number of TRAP-positive cells in the upper tibia and growth plate thickness of the tibia in OVX and SHAM mice with or without continuous pumping of TPTD. A: TRAP positive cells counted on the cancellous bone surface of the upper tibia (*P < 0.05 vs SHAM-VEH group; #P < 0.05 vs OVX-VEH group); B: Comparison of the thickness of the growth plate among the 4 groups (*P < 0.05 vs SHAM-VEH group; #P < 0.05 vs OVX-VEH group) |
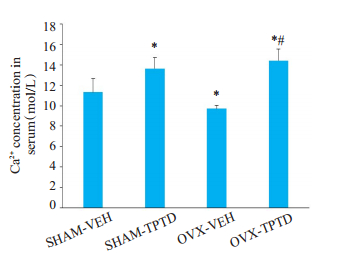
血清钙离子浓度测定显示(图 6),去势会导致小鼠血清钙离子浓度降低(P < 0.05),而连续注射TPTD后会导致小鼠的血清钙离子浓度出现显著的提升(P < 0.05)。在OVX组小鼠身上连续应用TPTD会导致血清钙离子浓度上升的绝对值大于在正常小鼠身上连续应用TPTD(P < 0.05)。
|
图 6 OVX和SHAM小鼠受TPTD持续作用时血清钙离子浓度的改变 Fig.6 Changes in serum Ca2+ concentration in OVX and SHAM mice with or without TPTD treatmnent for 2 weeks. *P < 0.05 vs SHAM-VEH group; #P < 0.05 vs OVXVEH group |
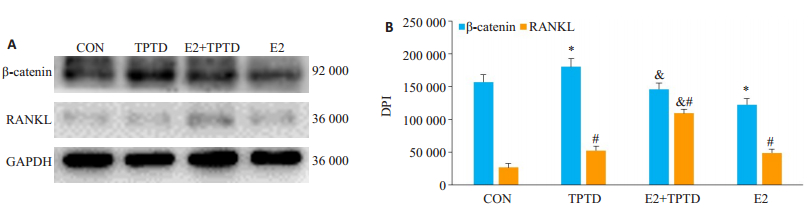
P2代颅骨成骨细胞加雌二醇和TPTD连续培养48 h后,TPTD使β-catenin和RANKL表达升高,E2的加入减弱了TPTD对β-catenin表达的效果,使RANKL表达更进一步升高(图 7)。
|
图 7 成骨细胞受到TPTD和雌二醇连续刺激48 h时蛋白表达的改变 Fig.7 Changes in protein expression of osteoblasts stimulated by TPTD and estradiol for 48 h.A: TPTD significantly up-regulated the expression of β-catenin in 48 h after continuous stimulation. After the addition of estrogen, the expression of β-catenin was inhibited, but the expression of RANKLwas up-regulated; B: Analysis of the gray value of the protein band using ImageJ *P < 0.05 vs CONgroup; #P < 0.05 vs CONgroup; & P < 0.05 vs TPTD group |
TPTD是目前公认最有效的抗骨质疏松作用的药物,但它兼具成骨和破骨功能。TPTD的促进成骨和破骨作用与其给药浓度及给药方式相关,间断小剂量使用的净作用表现为成骨[10],而连续大浓度使用的净作用表现为破骨[11]。TPTD抗骨质疏松的效用具有时间依赖性,时间窗大约为18~24月,超过这一时间窗,破骨作用增加,成骨作用减弱,导致其耐药性的产生[12]。因此探讨如何抑制其破骨功能是目前研究的热点之一。为最大限度的研究和了解TPTD及其相关蛋白的破骨功能,TPTD持续泵入小鼠动物模型模式常用于实验研究,连续泵入时间窗的选择,大多采用2周这一时间节点[13-15]。同时,小鼠去卵巢模型也是最常见的骨质疏松动物模型,研究中我们意外观察到持续应用TPTD对OVX小鼠骨骼无促破骨作用,本文对比研究了TPTD持续泵入对假手术和去势小鼠骨骼的影响,揭示TPTD持续刺激在正常与去势小鼠的骨代谢存在效果背离的现象,该现象与其破骨功能相关,并且与β-catenin、RANKL的表达水平相关。
持续TPTD刺激的效应最先从临床上甲状旁腺亢进的患者观察到,该类患者往往伴随出现严重的高血钙和骨质疏松,骨组织内破骨细胞显著增多,类骨质钙化缺陷。在正常的鼠动物模型中,TPTD或PTH(1-34)持续灌注引发同甲状旁腺功能亢进一样的骨丢失现象[16],并且与单核细胞趋化蛋白(MCP-1)相关。有趣的是,比较轻微的甲状旁腺亢进的患者,可以观察到明显骨形成现象[17-18]。在卵巢切除大鼠,PTH(1-34)灌注可以一定程度上防止松质骨骨量的减少,与雌激素联用时,效果更显著[19]。最近的报导显示,纳米羟基磷灰石搭载hPTH(1-34)靶向到去势大鼠骨组织发挥成骨作用[20],提示在去势动物,TPTD局部缓释可能促进骨形成。我们通过对比研究,明确了TPTD持续作用在去势小鼠(低雌激素水平)和正常小鼠的不同作用,证实去势小鼠不适于作为研究TPTD破骨作用的动物模型。
TPTD灌注在去势小鼠未体现出促骨吸收现象的机制目前不清楚,并且与TPTD间断皮下注射的实验结果存在很大的差异。去势雌性鼠是研究绝经后骨质疏松的经典模型,为许多抗骨质疏松药物的研究提供了有力的依据[21-24]。TPTD间断皮下注射临床上能有效治疗绝经后骨质疏松,在去势大、小鼠模型,间断TPTD具有最强的防骨丢失作用。细胞学和体内研究都证明TPTD和雌激素联合应用能够更有效地促进骨生长与治疗骨质疏松[25-27]。雌激素通过抑制环腺苷酸(cAMP)信号途径而抑制TPTD间断注射引起的破骨细胞的形成和骨面贴附[26, 28]。我们通过组织学观察发现,持续TPTD泵入使小鼠骨质内破骨细胞数目增多,去势之后再泵入TPTD,其破骨细胞增加的量显著低于在SHAM小鼠的实验结果。而OVX引起的生长潜能(生长板厚度)的降低也会被TPTD的作用逆转。
进一步的实验提示,出现上述现象的原因既存在骨组织局部的因素,也存在全身的因素。Wnt/β-catenin是介导TPTD影响骨代谢的主要和直接信号通路[29],承担促骨形成和抑制破骨吸收(抑制RANKL的表达)的作用。本实验中,我们对原代成骨细胞进行48 h TPTD连续刺激,比较存在或缺乏雌激素情况下成骨细胞合成β-catenin和RANKL的情况,希望探究TPTD连续刺激时,雌激素对骨代谢相关细胞的影响。结果表明,TPTD连续刺激48 h显著上调细胞β-catenin的表达,加入雌激素后,β-catenin的表达受到抑制,但RANKL表达上调。这一结果提示,在低雌激素水平(去势)的情况下,TPTD持续刺激引起的β-catenin上调,虽然RANKL表达也有一定程度上调,但最终净作用体现为骨组织内破骨细胞的量减少,骨吸收减弱,骨量丢失不明显,出现小鼠松质骨骨量不降反升,与正常小鼠的表现背离。通过动物实验,我们发现在正常小鼠,TPTD持续应用使胫骨上段松质骨骨小梁稀疏,骨微观结构参数显著降低,证明TPTD在长时间连续应用时确实有发挥破骨作用的可能,这与以往文献报道的结果是相符的。小鼠血清钙离子水平的表现则体现全身因素的作用。去势之后,血清钙离子浓度出现轻度降低,TPTD灌注在正常小鼠引起血钙增加,在去势小鼠引起更明显的血钙增高(与OVX比)。血清钙源来自于肠道吸收、肾脏回吸收和骨骼释放,有利于骨量的保持。在骨质无明显吸收的情况下,OVX-TPTD小鼠出现明显的血钙浓度增加,提示肠源性和肾源性因素与雌激素水平相关并影响持续TPTD对骨骼的作用。
本文通过对比研究,明确了TPTD持续作用在去势小鼠和正常小鼠的不同作用,为TPTD及其相关肽破骨作用动物模型的选择提供了实证依据,证实去势小鼠不适于作为研究TPTD破骨作用的动物模型。我们分析了产生效果背离现象的骨组织和全身性因素,其机制有待进一步开展。
| [1] |
Renkema KY, Alexander RT, Bindels RJ. Calcium and phosphate homeostasis: concerted interplay of new regulators[J]. Ann Med, 2008, 40(2): 82-91. DOI:10.1080/07853890701689645 |
| [2] |
Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations[J]. Best Pract Res Clin Rheumatol, 2008, 22(1): 129-48. DOI:10.1016/j.berh.2007.11.006 |
| [3] |
Pettway GJ, Meganck JA, Koh AJ, et al. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation[J]. Bone, 2008, 42(4): 806-18. DOI:10.1016/j.bone.2007.11.017 |
| [4] |
Morley P, Whitfield JF, Willick GE. Anabolic effects of parathyroid hormone on bone[J]. Trends Endocrinol Metab, 1997, 8(6): 225-31. DOI:10.1016/S1043-2760(97)00060-X |
| [5] |
Palmer M, Adami HO, Krusemo UB, et al. Increased risk of malignant diseases after surgery for primary hyperparathyroidism.A nationwide cohort study[J]. AmJ Epidemiol, 1988, 127(5): 1031-40. DOI:10.1093/oxfordjournals.aje.a114879 |
| [6] |
Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis a randomized clinical trial[J]. JAMA, 2016, 316(7): 722-33. DOI:10.1001/jama.2016.11136 |
| [7] |
Makino A, Takagi H, Takahashi Y, et al. Abaloparatide exerts bone anabolic effects with less stimulation of bone resorption-related factors: a comparison with teriparatide[J]. Calcif Tissue Int, 2018, 103(3): 289-97. DOI:10.1007/s00223-018-0422-4 |
| [8] |
贺行文, 陶同善, 柳维, 等. 特立帕肽对去势大鼠骨缺损修复影响的实验研究[J]. 中国骨质疏松杂志, 2015, 21(6): 669-73, 690. DOI:10.3969/j.issn.1006-7108.2015.06.007 |
| [9] |
李微, 张博, 徐红丹, 等. 左归丸对卵巢切除骨质疏松症模型小鼠骨代谢的影响[J]. 中华中医药杂志, 2018, 33(7): 2807-10. |
| [10] |
Langdahl BL, Andersen JD. Treatment of osteoporosis: unmet needs and emerging solutions[J]. J Bone Metab, 2018, 25(3): 133-40. DOI:10.11005/jbm.2018.25.3.133 |
| [11] |
Cheloha RW, Gellman SH, Vilardaga J. PTH receptor-1 signallingmechanistic insights and therapeutic prospects[J]. Nat Rev Endocrinol, 2015, 11(12): 712-24. DOI:10.1038/nrendo.2015.139 |
| [12] |
Pazianas M. Anabolic effects of PTH and the anabolic window'[J]. Trends in Endocrinol Metabol, 2015, 26(3): 111-3. DOI:10.1016/j.tem.2015.01.004 |
| [13] |
Zhang LX, Balani YM, Trinh S, et al. Differential effects on bone and mesenchymal stem cells caused by intermittent and continuous PTH administration[J]. ZhonghuaYi Xue Za Zhi, 2018, 98(10): 781-7. |
| [14] |
Yukata K, Kanchiku T, Egawa H, et al. Continuous infusion of PTH1- 34 delayed fracture healing in mice[J]. Sci Rep, 2018, 8(1): 13175. DOI:10.1038/s41598-018-31345-1 |
| [15] |
Choudhary S, Santone E, Yee SP, et al. Continuous PTH in male mice causes bone loss because it induces serum amyloid a[J]. Endocrinology, 2018, 159(7): 2759-76. DOI:10.1210/en.2018-00265 |
| [16] |
Siddiqui JA, Johnson J, Le Henaff C, et al. Catabolic effects of human PTH (1-34) on bone: requirement of monocyte chemoattractant protein-1 in murine model of hyperparathyroidism[J]. Sci Rep, 2017, 7(1): 15300. DOI:10.1038/s41598-017-15563-7 |
| [17] |
Dempster DW, Parisien M, Silverberg SJ, et al. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism[J]. J Clin Endocrinol Metab, 1999, 84(5): 1562-6. |
| [18] |
Christiansen P, Steiniche T, Vesterby A, et al. Primary hyperparathyroidism: iliac crest trabecular bone volume, structure, remodeling, and balance evaluated by histomorphometric methods[J]. Bone, 1992, 13(1): 41-9. DOI:10.1016/8756-3282(92)90360-9 |
| [19] |
Zhou H, Shen V, Dempster DW, et al. Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat[J]. J Bone Miner Res, 2001, 16(7): 1300-7. DOI:10.1359/jbmr.2001.16.7.1300 |
| [20] |
Dave JR, Dewle AM, Mhaske ST, et al. Hydroxyapatite nanorods loaded with parathyroid hormone (PTH) synergistically enhance the net formative effect of PTH anabolic therapy[J]. Nanomedicine, 2019, 15(1): 218-30. |
| [21] |
Martins Chavarry NG, Perrone D, Fleiuss Farias ML, et al. Alendronate improves bone density and type I collagen accumulation but increases the amount of pentosidine in the healing dental alveolus of ovariectomized rabbits[J]. Bone, 2019, 120: 9-19. DOI:10.1016/j.bone.2018.09.022 |
| [22] |
Chen SY, Yu HT, Kao JP, et al. An NMR metabolomic study on the effect of alendronate in ovariectomized mice[J]. PLoS One, 2014, 9(9): e106559. DOI:10.1371/journal.pone.0106559 |
| [23] |
Sasaki H, Miyakoshi N, Kasukawa Y, et al. Effects of combination treatment with alendronate and vitamin K-2 on bone mineral density and strength in ovariectomized mice[J]. J Bone Miner Metab, 2010, 28(4): 403-9. DOI:10.1007/s00774-009-0148-5 |
| [24] |
MostW, Schot L, Ederveen A, et al. In vitro and ex vivo evidence that estrogens suppress increased bone resorption induced by ovariectomy or PTH stimulation through an effect on osteoclastogenesis[J]. J Bone Miner Res, 1995, 10(10): 1523-30. |
| [25] |
Lin Y, Liu LJ, Murray T, et al. Effect of raloxifene and its interaction with human PTH on bone formation[J]. J Endocrinol Invest, 2004, 27(5): 416-23. DOI:10.1007/BF03345284 |
| [26] |
Liu BY, Wu PW, Bringhurst FR, et al. Estrogen inhibition of PTHstimulated osteoclast formation and attachment in vitro: Involvement of both PKA and PKC[J]. Endocrinology, 2002, 143(2): 627-35. DOI:10.1210/endo.143.2.8614 |
| [27] |
Rattanakul C, Lenbury Y, Krishnamara N, et al. Modeling of bone formation and resorption mediated by parathyroid hormone: response to estrogen/PTH therapy[J]. Biosystems, 2003, 70(1): 55-72. DOI:10.1016/S0303-2647(03)00040-6 |
| [28] |
Kaji H, Sugimoto T, Kanatani M, et al. Estrogen blocks parathyroid hormone (PTH)-stimulated osteoclast-like cell formation by selectively affecting PTH-responsive cyclic adenosine monophosphate pathway[J]. Endocrinology, 1996, 137(6): 2217-24. DOI:10.1210/endo.137.6.8641168 |
| [29] |
Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives[J]. J Clin Endocrinol Metab, 2012, 97(2): 311-25. DOI:10.1210/jc.2011-2332 |
 2019, Vol. 39
2019, Vol. 39

