2. 西安交通大学第二附属医院 生物诊断治疗国家地方联合工程研究中心,陕西 西安 710004;
3. 西安交通大学第二附属医院 检验科,陕西 西安 710004
2. National & Local Joint Engineering Research Center of Biodiagnostics and Biotherapy, Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, China;
3. Clinical Laboratory, Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, China
乙肝e抗原(HBeAg)是乙型肝炎病毒(HBV)源性免疫抑制因子,在HBV免疫逃逸和不良病程转归中发挥重要作用[1-3]。HBeAg血清转换是判断病情发展趋势和药物疗效的重要指标,也是慢性乙肝治疗的中期目标[4-5]。HBeAg有可能成为慢性乙肝治疗的潜在新靶点。因此,研究HBeAg的作用机制具有重要的临床价值。但由于HBV具有严格的种属限制性和嗜肝细胞特性,缺乏理想的HBV动物模型一直是严重制约HBV基础研究和药物研发的共同瓶颈[6-8]。因此,构建能在肝脏特异性稳定表达HBeAg的转基因动物对于深入研究HBeAg的作用机制、评估以HBeAg为治疗靶点的相关药物/疫苗疗效具有重要意义。已有多种能表达HBV不同组份的HBV转基因(HBV-Tg)小鼠相继问世[9-14],采用HBeAg和HBc/HBeAg转基因小鼠已证实HBeAg比HBcg能诱导更强的T细胞免疫耐受。但现有模型仍旧存在制备技术复杂、繁育困难、遗传稳定性差、目标蛋白表达不稳定等缺点[15-17],并不能满足深入研究的需要[18-20]。
为此,本研究拟采用CRISPR/Cas9技术[21-22]构建一种新品系的HBeAg转基因小鼠,为深入研究HBeAg的生物学功HBeAg为靶点的乙肝治疗药物/疫苗等的临床疗效提供适宜的实验动物模型。
1 材料和方法 1.1 实验动物和材料本实验选用SPF级C57BL/6J小鼠(上海南方模式生物科技股份有限公司)。实验严格遵守国家实验动物福利伦理相关规定,实验中对动物处置符合科技部颁行的《关于善待实验动物的指导性意见》,满足动物保护、动物福利以及伦理原则的相关要求,严格遵守动物使用的3R原则。
sgRNA载体、pliver-HBeAg质粒(上海南方模式生物科技股份有限公司构建并完成)。DNA提取试剂盒(康为世纪生物有限公司),引物合成(上海博锐生物技术公司)。I2000SR化学发光免疫分析仪(美国雅培)。HBV相关抗原检测试剂盒(胶体金法)(厦门波生生物技术有限公司)。
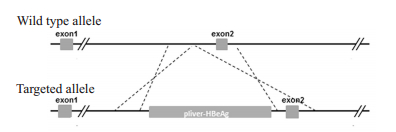
1.2 HBeAg基因小鼠的构建及鉴定 1.2.1 HBeAg基因的表达载体的构建及鉴定常规方法克隆乙型肝炎病毒HBeAg基因,采用CRISPR/Cas9技术[21-22],通过同源重组的方式将HBeAg基因在Rosa26基因位点定点插入pliver-HBeAg表达框(图 1),获得含有HBeAg基因的重组R26-e(Alb-HBeAg)1打靶载体。采用ScaⅠ酶切技术对该打靶载体中目的基因进行酶切电泳鉴定。
|
图 1 Rosa26基因位点定点插入相应表达框结构示意图 Fig.1 Map of the ROSA26 genomic region. The line indicates the genomic DNA drawn with the insertion position of pliver-HBeAg integration. |
将重组R26-e(Alb-HBeAg)1打靶载体进行酶切,得到含有HBeAg基因的线性DNA片段,并进行PCR和电泳鉴定,并获取想用目的片段。再采用显微注射技术将Cas9 mRNA、gRNA和重组R26-e(Alb-HBeAg)1打靶载体注射到C57BL/6J小鼠的受精卵中,然后将受精卵随后移植入C57BL/6J雌性代孕小鼠子宫腔内。采用长片段PCR技术,分别利用5'和3'同源臂PCR鉴定引物对出生的F0代幼鼠进行基因型鉴定。引物如下:
5'同源臂重组阳性F0代小鼠PCR鉴定引物:
Forward1: 5'-GCCGGGCCTCGTCGTCT-3'
Reverse2: 5'-TTTTTGGGGGTGATGGTGGTC-3'
3'同源臂重组阳性F0代小鼠PCR鉴定引物:
Forward3: 5'-TGCCCCTATCCTATCAACACTTCC-3'
Reverse4: 5'-IVGATCCATTGCCACCTTTCACTTAG-3'
1.3 HBeAg纯合子转基因小鼠的繁育和鉴定HBeAg转基因小鼠繁殖,将建系的F0代小鼠与野生型C57BL/6J小鼠交配,繁育获得F1代小鼠,并对F1代小鼠5'和3'同源臂进行PCR鉴定(方法同上),并采用sanger基因测序对鉴定阳性的F1代小鼠进行序列分析。
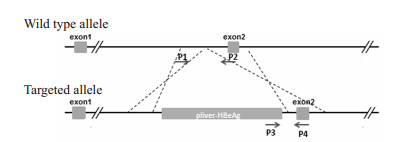
1.4 BeAg纯合子转基因小鼠的繁育和鉴定再次将携带HBeAg基因的F1代转基因小鼠(基因敲入杂合子)进行交配,获得子代(杂合子和纯合子)HBeAg转基因小鼠。并采用短片段PCR对其基因型(纯合子、杂合子和野生型)进行鉴定,将携带HBeAg基因的F1代转基因小鼠再次回交,逐步繁育纯化,直至获得纯合子子代转基因小鼠。用于小鼠基因型鉴定的PCR引物序列和P1、P2、P3、P4引物位置如下(图 2)。
|
图 2 小鼠基因型鉴定PCR引物位置示意图 Fig.2 Position of PCR primers for identification of the genotype of the mice. |
P1-F:5'-TCAGATTCTTTTATAGGGGACACA-3'
P2-R:5'-TAAAGGCCACTCAATGCTCACTAA-3'
P3-F:5'-CTTCTAGATACCGCCTCAGC-3'
P4-R:5'-AGCCATGTTTTATATTCCTTACC-3'
1.5 HBeAg转基因小鼠中HBeAg和HBeAb的检测小鼠尾静脉和球后采血,以野生型C57BL/6J小鼠为对照,采用雅培i2000SR全自动化学发光免疫分析仪及配套的HBeAg、HBeAb试剂检测1、2、6、10、11、14号F1代小鼠中HBeAg、HBeAb的表达;采用胶体金法HBV检测试剂盒(厦门市波生生物技术有限公司),检测HBeAg转基因小鼠中HBsAg、HBsAb、HBeAg(双抗体夹心法)和HBeAb、HBcAb(竞争法)的表达;采用Ventana Bench Mark XT全自动免疫组化染色仪检测HBeAg转基因小鼠各脏器组织中HBeAg的表达。具体操作和结果判定参照仪器和试剂说明书。
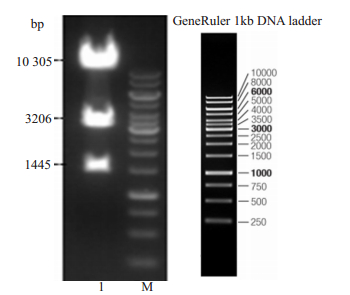
2 结果 2.1 HBeAg基因的表达载体的构建和酶切鉴定对重组R26-e(Alb-HBeAg)1打靶载体进行酶切和PCR电泳鉴定,结果显示目的片段与理论条带大小(1445、3206、10 305 bp)一致(图 3~4),其中:gRNA:5'ggggacacactaagggagct-3'。
|
图 3 重组R26-e(Alb-HBeAg)1打靶载体酶切鉴定电泳图 Fig.3 Agarose gel electrophoresis of enzyme digested recombinant r26-e (alb-hbeag)1 plasmid. Lane1: Recombinant r26-e (alb-hbeag)1 plasmid; M: 1kD marker. |

|
图 4 体外转录gRNA、Cas9电泳结果 Fig.4 Agarose gel electrophoresis of the PCR products for gRNA and Cas9 plasmid in vitro. Left: gRNA (Lanes 1-4: Guide RNA1; Lane 5: 1 kB DNA marker); Right: t7-Cas 9 plasmid (lane 1), lined-t7-Cas 9 plasmid (lane 2), Cas 9 (lane 3), and 1 kB marker (lane 4). |
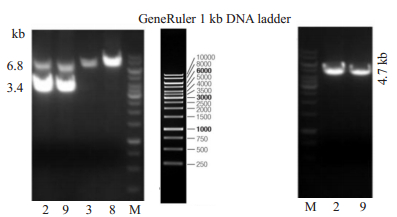
采用长片段PCR结果显示,2,9号为双臂同源重组阳性的F0代小鼠,已经获得HBeAg基因正确同源重组的阳性F0代建系小鼠(图 5)。
|
图 5 同源重组F0代小鼠PCR鉴定电泳图 Fig.5 Agarose gel electrophoresis of the PCR products from F0 offspring homologous recombinant HBeAg transgenic mice. Left: 3.4 kb and 6.8 kb fragments amplified from the 5'-arm homologous recombinant positive genome (Lanes 1-4 are No. 2, 9, 3 and 8 HBeAg transgenic mice, respectively); Right: 4.7 kb fragment amplified from 3 'homologous arm of HBeAg transgenic mice (Lanes 1 and 2 are No. 2 and 9 HBeAg transgenic mice, respectively. M: 1 kb DNAmarker). |
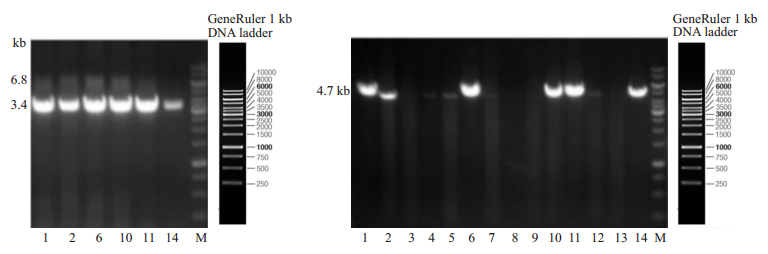
采用长片段PCR技术对携带HBeAg基因的F1代转基因小鼠(图 6),结果显示编号为1、2、6、10、11、14号的F1代小鼠阳性,且基因测序结果显示HBeAg基因序列正确。
|
图 6 F1代HBeAg转基因小鼠PCR鉴定电泳图 Fig.6 Agarose gel electrophoresis of the PCR products for F1 offspring homologous recombinant HBeAg transgenic mice. Left: 3.4 kb fragments amplified from the 5'-arm homologous recombinant positive genome (Lanes 1-6 are No. 1, 2, 6, 10, 11 and 14 HBeAg transgenic mice, respectively); Right: 4.7 kb fragment amplified from 3' homologous arm of HBeAg transgenic mice (Lanes 1-14 are No, 1-14 HBeAg transgenic mice, respectively. M: 1 kb DNAmarker). |
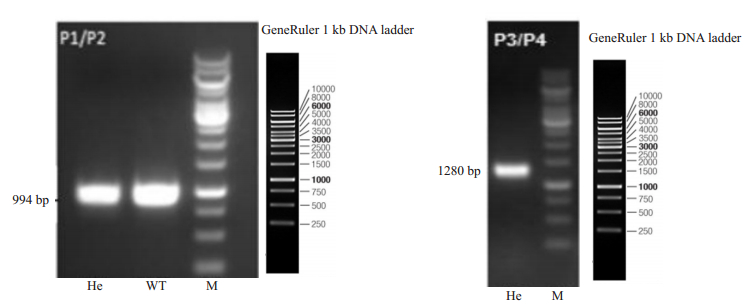
采用短片段PCR对F1代转基因小鼠交配所获得子代(杂合HBeAg转基因小鼠的基因型进行鉴定,结果显示其中P1、P2、P3、P4为所使用的引物。截至目前,共获得22只F2代纯合子和29只杂合子HBeAg转基因小鼠(图 7)。
|
图 7 PCR鉴定HBeAg转基因小鼠基因型 Fig.7 PCR for identification of the genotype of HBeAg transgenic mice. Left: 994 bp fragments amplified from heterozygous (He) HBeAg transgenic mice and wild-type (WT) mice by PCR usingP1/P2 primer; Right: 1280 bp fragments amplified from heterozygous HBeAg transgenic mice by PCR usingP3/P4 primer. M: 1 kb DNAmarker. |
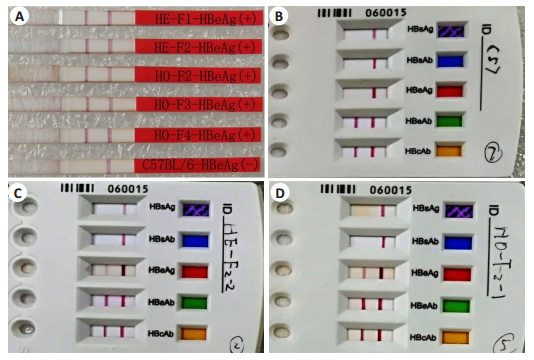
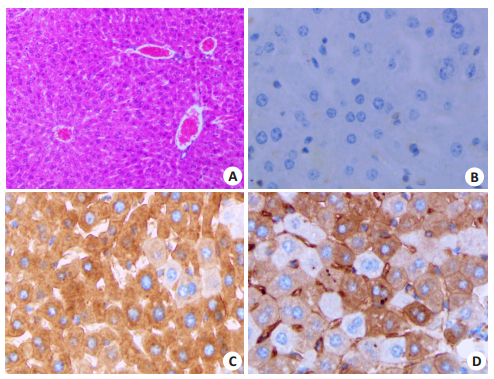
化学发光免疫分析和胶体金法检测,雅培i2000SR全自动化学发光定量分析结果显示杂合子和纯合子HBeAg转基因小鼠静脉血浆中HBeAg效价均大于1300 PEIU/mL;胶体金法检测结果显示HBeAg阳性,HBsAg、HBsAb、和HBeAb、HBcAb均呈阴性(图 8)。免疫组化方法检测结果证实杂合子和纯合子HBeAg转基因小鼠肝脏肝细胞可高表达HBeAg蛋白(图 9)。
|
图 8 胶体金法检测HBeAg转基因小鼠中HBeAg和HBeAb的表达 Fig.8 Expression of HBeAg and HBeAb in HBeAg transgenic mice detected by colloidal gold immunoassay method. A: HBeAg was positively expressed in peripheral blood of HE-F1, HE-F2, HO-F2, HO-F3, HO-F4 HBeAg transgenic mice, while HBeAg was negative in C56BL/6J mice; B: HBsAg, HBsAb, HBeAg, HBsAb and HBcAb were all negative in the plasma of C57BL/6J mice; C, D: HBeAg was positive in the plasma of heterozygous (C) and homozygous (D) HBeAg transgenic mice, while HBsAg, HBsAb, HBeAb and HBcAb were all negative. |
|
图 9 免疫组织化学法检测HBeAg转基因小鼠中HBeAg的表达 Fig.9 Expression of HBeAg in HBeAg transgenic mice detected by immunohistochemistry. A: HE staining of the liver tissue of HBeAg transgenic mice (Original magnification: ×10); B: Negative HBeAg expression in the liver of wild- type C57BL/6J mice (× 20); C: High expression of HBeAg in the liver cells of homozygous HBeAg transgenic mice (× 20); D: High expression of HBeAg in the liver cells of heterozygous HBeAg transgenic mice (×20). |
由于HBV的严格种属限制性和嗜肝细胞特性以及HBeAg在HBV感染和慢性乙肝进展中的重要作用,构建能在肝脏特异性稳定高表达HBeAg的转基因动物具有重要的临床价值,而近年来,CRISPR/Cas9技术凭借其高效的基因编辑能力已在细胞治疗中发挥出了重要作用,也为转基因动物的制备提供了新技术[23-24]。本研究采用CRISPR/Cas9技术将HBeAg基因定点插入带有白蛋白启动子的pliver-HBeAg表达框,以期实现HBeAg在肝细胞中特异性地高表达。长片段PCR鉴定结果证实共获得2只同源重组的F0代小鼠;再将F0代小鼠交配繁殖,采用短片段PCR对F1代转基因小鼠的基因型进行鉴定共获得6只阳性的F1代小鼠,利用F1代杂合子小鼠进行回交共获得22只F2代纯合子和29只杂合子HBeAg转基因小鼠。可见,与传统技术相比较采用CRISPR/Cas9技术构建转基因动物具有很好的优势。
同时,本研究还采用显微注射技术在C57BL/6J小鼠的受精卵环节进行基因编辑操作,从而降低HBeAg的免疫原性。而且采用免疫化学发光法和胶体金检测试剂盒检测杂合子和纯合子HBeAg转基因小鼠外周血中HBV相关抗原和抗体(包括HBsAg、HBsAb、HBeAg、HBeAb和HBcAb的表达,结果证实所有F1~F4代杂合子和纯合子HBeAg转基因小鼠外周中均能检出高滴度HBeAg蛋白,而HBsAg、HBsAb、HBeAb和HBcAb均未检出。同时,免疫组化染色结果证实杂合子和纯合子HBeAg转基因小鼠能在肝脏肝细胞中特异性高表达HBeAg蛋白,而在肝脏其他细胞和其他器官、组织中呈阴性表达。同时,HBeAg并非弥漫性表达于所有肝细胞中,而是特异性表达于肝小叶内部分肝细胞中,这种HBeAg表达的组织细胞学分布与人自然感染HBV后HBeAg蛋白在肝细胞中的表达模式完全一致。截至目前,该HBeAg转基因小鼠已经繁育到F4代,其遗传学特征稳定,仍能在肝细胞稳定高表达HBeAg蛋白且对HBeAg免疫耐受,并能在最大程度上接近并模拟人感染HBV后初期HBeAg的在体免疫耐受状态和生物功能。显然,该HBeAg转基因小鼠显然优于既往建立的HBeAg转基因小鼠[25-26],且尚未见文献报道,属于一种新品系的HBeAg转基因小鼠。
如前所述,由于HBeAg是HBV分泌性非颗粒状病毒衣壳蛋白,是HBV特有的免疫耐受因子,是引起HBV免疫耐受、慢性乙肝迁延不愈、并出现不良结局的重要原因之一[1-3]。而HBeAg血清转换(HBeAg转阴)已被认为是乙肝患者良好转归的里程碑事件,是判断病情发展趋势和药物疗效的一个重要指标,是最终彻底清除HBV、根治乙肝的基础,也是慢性乙肝治疗的中期目标[4-5]。因此,该新品系HBeAg转基因小鼠的构建无疑对于深入研究HBeAg的作用机制、评估以HBeAg为治疗靶点相关药物/疫苗的疗效具有重要的临床价值。
综上所述,该模型小鼠的建立为深入研究HBeAg感染机制、评价慢性乙型肝炎治疗性药物/疫苗疗效提供了新的适宜的实验动物模型,尤其适用于从原位细胞学和组织器官水平深入研究HBeAg的生物功能、HBeAg对肝脏局部器官免疫微环境作用及免疫耐受的分子机制研究。
| [1] |
Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-63. |
| [2] |
Tseng TC, Kao JH. Treating immune-tolerant hepatitis B[J]. J Viral Hepat, 2015, 22(2): 77-84. DOI:10.1111/jvh.12370 |
| [3] |
Kang EH, Kown TY, Oh GT, et al. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen[J]. Antiviral Res, 2006, 72(2): 100-6. DOI:10.1016/j.antiviral.2006.04.006 |
| [4] |
Fong TL, Tien A, Jo KJ, et al. Durability of hepatitis B e antigen seroconversion in chronic hepatitis B patients treated with entecavir or tenofovir[J]. Dig Dis Sci, 2015, 60(11): 3465-72. DOI:10.1007/s10620-015-3775-9 |
| [5] |
Fan R, Sun J, Yuan Q, et al. Original article: Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues[J]. Gut, 2016, 65(2): 313-20. DOI:10.1136/gutjnl-2014-308546 |
| [6] |
Dupinay T, Gheit T, Roques P, et al. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among macaca fascicularis from mauritius island[J]. Hepatology, 2013, 58(5): 1610-20. DOI:10.1002/hep.26428 |
| [7] |
杨悦, 许智慧, 刘妍, 等. HBV动物模型研究进展[J]. 传染病信息, 2016, 29(4): 236-41. |
| [8] |
Dandri M, Petersen J. Animal models of HBV infection[J]. Best Pract Res Clin Gastroenterol, 2017, 31(3): 273-9. DOI:10.1016/j.bpg.2017.04.014 |
| [9] |
Hwang JR, Park SG. Mouse models for hepatitis B virus research[J]. LabAnim Res, 2018, 34(3): 85-91. |
| [10] |
Guo WN, Zhu B, Ai L, et al. Animal models for the study of hepatitis B virus infection[J]. Zool Res, 2018, 39(1): 25-31. DOI:10.24272/j.issn.2095-8137.2018.013 |
| [11] |
Zhang R, Real CI, Liu C, et al. Hepatic expression of oncogenes Bmi1 and Dkk1 is up-regulated in hepatitis B virus surface antigentransgenic mice and can be induced by treatment with HBV particles or lipopolysaccharides in vitro[J]. Int J Cancer, 2017, 141(2): 354-63. DOI:10.1002/ijc.30742 |
| [12] |
Liu YM, Xu QY, Chen JX, et al. Investigation on effects of the nourishing kidney and eliminating toxicity decoction on immunological imbalance of Th1, Th17 and Treg in HBV transgenic mice[J]. Int J Clin Exp Med, 2015, 8(5): 6735-42. |
| [13] |
Mancini-Bourgine M, Guillen G, Michel ML, et al. Impact of the immunogen nature on the immune response against the major HBV antigens in an HBsAg and HLA-humanized transgenic mouse model[J]. Euroasian J Hepatogastroenterol, 2014, 4(1): 36-44. |
| [14] |
Buchmann P, Dembek C, Kuklick L, et al. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice[J]. Vaccine, 2013, 31(8): 1197-203. DOI:10.1016/j.vaccine.2012.12.074 |
| [15] |
Levander S, Sallberg M, Ahlen G, et al. A non-human hepadnaviral adjuvant for hepatitis C virus-based genetic vaccines[J]. Vaccine, 2016, 34(25): 2821-33. DOI:10.1016/j.vaccine.2016.04.030 |
| [16] |
Yang D, Liu L, Zhu D, et al. A mouse model for HBV immunotolerance and immunotherapy[J]. Cell Mol Immunol, 2014, 11(1): 71-8. |
| [17] |
Chen M, Jagya N, Bansal R, et al. Prospects and progress of DNA vaccines for treating hepatitis B[J]. Expert Rev Vaccines, 2016, 15(5): 629-40. DOI:10.1586/14760584.2016.1131615 |
| [18] |
李凤磊, 郝晓磊, 田志刚. HBV小鼠模型与肝脏免疫学研究进展[J]. 中国免疫学杂志, 2016, 34(2): 145-53. DOI:10.3969/j.issn.1000-484X.2016.02.001 |
| [19] |
Larkin J, Clayton MM, Liu J, et al. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice[J]. Hepatology, 2001, 34(4, 1): 792-7. |
| [20] |
Yu DB, Liu H, Shi S, et al. A novel dendritic-cell-targeting DNA vaccine for hepatitis B induces T cell and humoral immune responses and potentiates the antivirus activity in HBV transgenic mice[J]. Immunol Lett, 2015, 168(2, SI): 293-9. DOI:10.1016/j.imlet.2015.10.007 |
| [21] |
Shen B, Zhang J, Wu H, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting[J]. Cell Res, 2013, 23(5): 720-3. DOI:10.1038/cr.2013.46 |
| [22] |
Mali P, Yang L, Esvelt KM, et al. RNA-Guided human genome engineering via Cas9[J]. Science, 2013, 339(6121): 823-6. DOI:10.1126/science.1232033 |
| [23] |
Watakabe I, Hashimoto H, Kimura Y, et al. Highly efficient generation of knock-in transgenic medaka by CRISPR/Cas9-mediated genome engineering[J]. Zoolog Letters, 2018, 4(1): 3-15. |
| [24] |
Kimura Y, Hisano Y, Kawahara A, et al. Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering[J]. Sci Rep, 2014, 52(4): 6545-57. |
| [25] |
Milich DR, Wolf SF, Hughes JL, et al. Interleukin 12 suppresses autoantibody production by reversing helper T-cell phenotype in hepatitis B e antigen transgenic mice[J]. Proceed National Acad Sci, 1995, 92(15): 6847-51. DOI:10.1073/pnas.92.15.6847 |
| [26] |
Merkle H, Deutschle T, Gastrock-Balitsch I, et al. H-2d mice born to and reared by HBeAg-transgenic mothers do not develop T cell tolerance toward the hepatitis B virus core gene products[J]. Virology, 2000, 273(1): 149-59. DOI:10.1006/viro.2000.0391 |
 2019, Vol. 39
2019, Vol. 39

