2. 西安交通大学第一附属医院精准外科与再生医学国家地方联合工程研究中心,陕西 西安 710061
2. National Local Joint Engineering Research Center for Precision Surgery and Regenerative Medicine, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, China
有关肿瘤细胞三维培养的研究越来越受到关注[1-2]。肿瘤细胞与间质细胞、细胞外基质、可溶性细胞因子等肿瘤微环境的相互作用均影响肝癌的恶性表型[3-5]。三维培养可刺激细胞的自分泌和旁分泌,并促进肿瘤进展相关的细胞行为。因此,体外构建合适的肝癌细胞三维培养模型具有重要意义。
所谓肿瘤组织工程即利用组织工程的手段构建复杂的细胞培养模型,以模拟体内的肿瘤微环境,进而研究肿瘤的发生、发展及治疗等[6]。与传统二维培养相比,基于壳聚糖-海藻酸钠[7]、丝素蛋白[8]和胶原凝胶[9]的肝癌模型其恶性程度及耐药性明显增加。但上述细胞外基质的成分及其超微结构仍与体内的有所差异。蛋白质组学分析发现,肝脏细胞外基质中含有58种基质蛋白[10],这比常用的细胞培养基质成分复杂得多。因此,在肝脏微环境中来自细胞外基质的信号是复杂的,这些可触发下游胞内信号通路的细胞-基质间作用无法通过单一组分的材料在体外进行模拟。
脱细胞支架保留了其天然的细胞外基质,已广泛应用于组织工程器官重建[11-13],同时为肿瘤研究提供了新方法[14-15]。考虑到种属差异,基于动物脱细胞支架的肿瘤模型可能无法真实模拟人的体内环境。因此,有关人体组织或器官脱细胞基质的研究越来越受到重视。到目前为止,对于人全肝脏脱细胞支架的研究仍不多,Mazza团队[16]和我们[17]先前的研究为人类正常肝脏脱细胞支架的制备提供了一些可行性方案,但是目前尚无关于人脂肪肝脱细胞的报道。本研究采用一种新的方法成功制备了人脂肪肝脱细胞支架(hFLM),并证实基于hFLM构建肝癌模型的可行性,同时体外三维培养的肝癌细胞与裸鼠成瘤组具有相似的生长模式及黏附分子表达。
1 材料和方法 1.1 人肝脏脱细胞本实验所用的三个人脂肪肝和一个人正常肝脏均为临床不适宜移植的DCD器官,脂肪肝病理诊断为重度大泡性脂肪变性。器官获取符合标准DCD器官获取流程及新英格兰器官库标准,实验经西安交通大学第一附属医院伦理委员会批准。脱细胞流程大致如下:肝脏经- 80 ℃保存后反复冻融2个循环,分别在肝动脉及门静脉插管并通过蠕动泵进行灌注,门静脉流量为100 mL/min,肝动脉流量为50 mL/min。依次灌注去离子水30 min、0.1%十二烷基硫酸钠(SDS)24 h、0.5%SDS 24 h、去离子水30 min、1%曲拉通(Triton X-100)/0.05% EGTA 24 h和PBS 6 h。经过上述流程,人正常肝脏脱细胞支架(NLM)制备完成,但是经0.1% SDS、1% SDS、1% Triton/0.05%EGTA灌注后人脂肪肝支架内(hFLM)仍残留大量的脂滴,因此将hFLM切成2 cm×2 cm×1 cm小块,按5~8块/瓶将其置入螺口瓶中行进一步脱细胞实验。将装有组织块的螺口瓶置于摇床中,室温条件下用去离子水震荡洗涤2 h(120 r/min),然后更换0.5 mol/L NaCl 3 h、1 mol/L NaCl 3 h、去离子水2 h、1%Triton/ 0.05%EGTA 72 h(每12 h更换液体)和PBS 6 h震荡洗涤,并成功制备hFLM。将上述制备的支架用0.1%过氧乙酸消毒,再分别用灭菌PBS和去离子水震荡洗涤1 h,将制备的支架组织置于含100 U/L青霉素、100 mg/L链霉素的PBS中4 ℃保存。
1.2 三种肝癌模型构建对于体外三维培养,将hFLM进一步分割成5 mm× 5 mm×3 mm组织片,置于6孔板中,用培养基孵育过夜,然后将约5×105 HepG2细胞接种到hFLM内于培养箱培养30 min,再每孔加入3 mL培养基行进一步培养。HepG2细胞在hFLM中分别培养3、6、9、12和15 d(n=6)。培养基由DMEM培养基(Gibco)、10%胎牛血清(Gibco)、100 U/L青霉素及100 mg/L链霉素构成,隔天更换培养基。在二维培养模型中,将105 HepG2细胞接种于培养皿中(n=6),隔天更换培养基,直至细胞长满。根据各组样品的DNA含量绘制两种模型的生长曲线。在体内成瘤模型中,6周龄雄性裸鼠皮下注射5× 106 HepG2细胞悬液(n=6),注射后每隔4 d用游标卡尺测量肿瘤大小,体积估算约为(长×宽2)/2,并以此绘制生长曲线。在第16天处死小鼠,收集肿瘤组织并保存于-80 ℃行下一步分析。
1.3 组织学检测及免疫荧光组织样本用10%福尔马林固定,石蜡包埋,5 μm切片后行HE染色。免疫荧光染色,将样本低温切片丙酮固定,然后再水合,用1% BSA(Sigma)封闭,并在4 ℃孵一抗过夜。本研究所用抗体为anti-collagen Ⅰ,anticollagen Ⅲ, anti-collagen Ⅳ,anti-fibronectin(1:100,Abcam),anti-E-cadherin(1:50,CST)和anti-vimentin (1:100,CST)。然后,二抗37 ℃孵育1 h,DAPI复染,用Olympus BX53F成像观察。PCNA指数是通过计算每个标本的随机5个镜下图像中PCNA阳性细胞数的比值的均数(n=6)。
1.4 扫描电镜用2.5%戊二醛固定脂肪肝、脱细胞支架及含有HepG2细胞的支架标本,梯度浓度乙醇(50%、75%、80%、95%、100%)脱水,并干燥、喷金。使用S-450扫描电镜(Hitachi)观察支架超微结构及细胞形态。
1.5 DNA定量将新鲜肝组织、NLM、hFLM和含有HepG2细胞的支架组织(n=6)分别置于EP管中,冷冻干燥后称重计算组织干重,然后利用DNA提取试剂盒(TIANGEN Biotech)提取标本DNA,用多功能酶标仪Varioskan Flash(Thermo)行DNA定量,计算残余DNA/干重比。
1.6 Western blotting分别提取hFLM组、裸鼠成瘤组及二维培养组样本蛋白,用10%SDS-PAGE跑胶并转膜。在4 ℃孵一抗过夜,二抗37 ℃孵育1 h后通过成像系统(Universal Hood Ⅲ,Bio-Rad)观察各组蛋白表达情况。实验所用抗体为anti-E-cadherin, anti-vimentin(1:1000,CST)和anti-GAPDH(1:5000,Proteintech)。
1.7 统计分析所有数据均表示为均数±标准差,使用SPSS12.0软件进行统计学分析。应用Student's t test检验来判断实验组与对照组间的显著差异,P < 0.05为差异有统计学意义。
2 结果 2.1 人肝脏脱细胞正常人肝脏呈褐色,HE染色呈典型的肝板结构,在灌注SDS和Triton后,细胞成分逐渐沿血管周围区域至肝脏边缘脱除。通过门静脉和肝动脉双重灌注完成脱细胞支架制备耗时7 d。脱细胞后,正常肝脏变为半透明状,保留了肝脏形态,包膜完整,可见部分浅表血管走形。HE染色显示纤维状排列的肝板结构,无细胞成分残留(图 1B)。脂肪肝呈淡黄色且肿大,边缘圆钝,质地较正常肝组织软。HE染色提示肝细胞内含有大量脂肪空泡,汇管区有炎性细胞浸润。但是,经SDS和Triton灌注后,脂肪肝变为金黄色,质地更加柔软,HE染色提示支架内无细胞成分(图 1A)。HE染色过程中,二甲苯可使脂质溶解。我们推测,SDS灌注过程中,细胞膜及核膜完全溶解,细胞碎片被冲出,但是大量脂质粘附在细胞外基质上。鉴于SDS对于脂质的作用较弱,我们选用Triton行进一步脱细胞实验。此后,将hFLM切成小片,在Triton中震荡洗涤72 h,缓慢有效地去除残留的脂质。肝组织呈半透明状,HE染色同样证实支架内已无细胞成分。
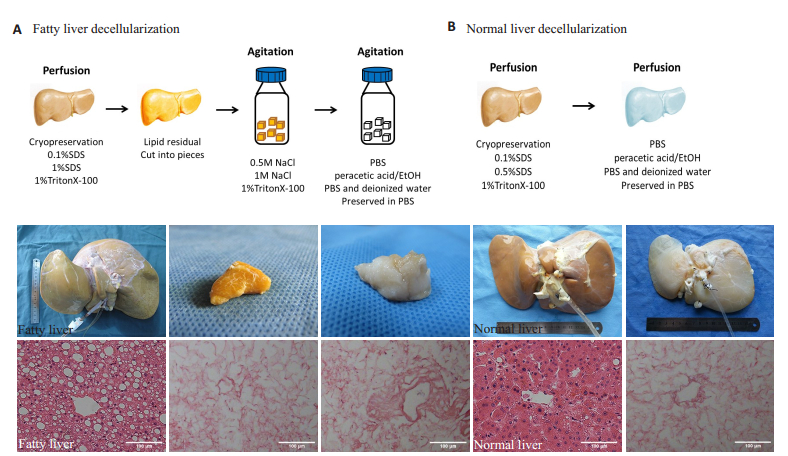
|
图 1 人肝脏脱细胞支架制备 Fig.1 Preparation of human liver decellularization. (A) Schematic representation of fatty liver decellularization protocol (top) and the representative macroscopic images and HE staining at different time points in the decellaularization process (bottom). A large number of lipid droplets were left in human fatty liver decellularized matrix (hFLM) after SDS and Triton X-100 perfusion, but HE staining revealed that all cell components had been removed after these steps. The fatty liver was cut into pieces for further decellularization. (B) Schematic representation of normal liver decellularization protocol (top). Representative macroscopic images and HE staining of normal liver decellularized matrix are shown at the bottom. Scale bars: 100 μm |
扫描电镜观察hFLM的超微结构,可见支架内原有的细胞成分被完全清除,仅留下蜂窝状结构,典型的门管三联结构被保留,血管横截面图像提示大血管基底膜及弹性纤维的完整性(图 2A)。新鲜脂肪肝和正常肝脏的DNA含量分别为3017.9±1043.5和3778.4± 1240.3 ng· mg-1干重,而hFLM和NLM内残余DNA含量为21.6±11.0和23.7±13.0 ng·mg-1干重。脂肪肝及正常肝脏的脱细胞支架内的DNA残留量均小于新鲜组织的1%(P < 0.001)。hFLM和NLM的DAPI染色也证实细胞核成分已被脱除(图 2B)。免疫荧光提示四种细胞外基质蛋白(Ⅰ型胶原、Ⅲ型胶原、Ⅳ型胶原及纤连蛋白)在脱细胞后均得以保留(图 2C)。
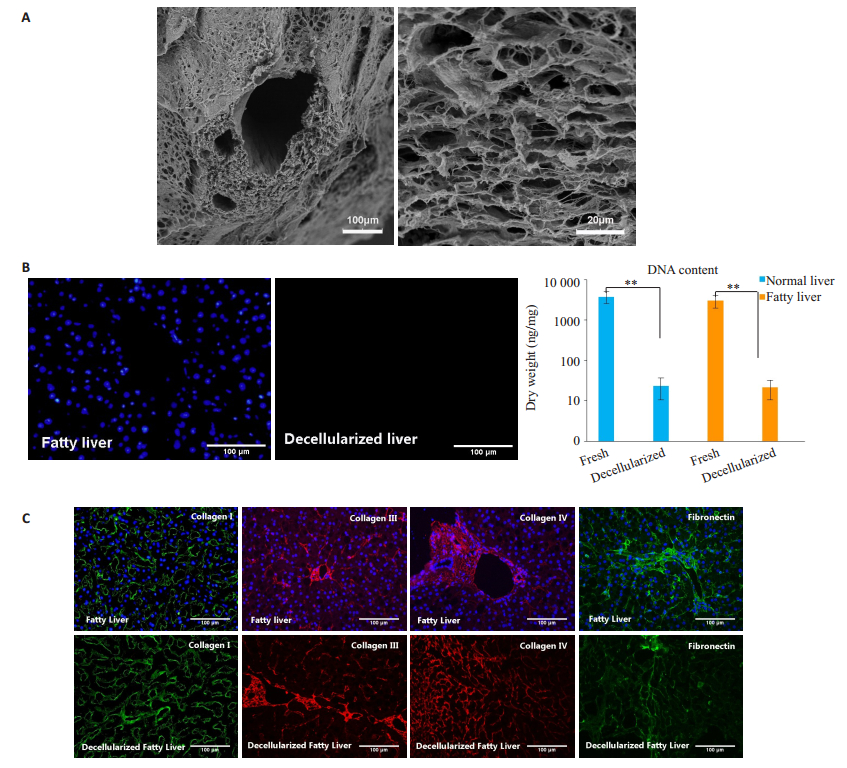
|
图 2 脱细胞支架鉴定 Fig.2 Characterization of hFLM scaffolds. (A) Scanning electron microscopy (SEM) of hFLM showing typical portal triad structure and porous features. (B) The presence of intact nuclear material was evaluated by staining the fatty liver and hFLM using DAPI. The residual DNA contents in hFLM was less than 1% of the fresh liver tissues (n=6). (C) Comparison of ECM before and after decellularization. Left to right: collagen Ⅰ, collagen Ⅲ, collagen Ⅳ and fibronectin. Scale bars: 100 μm (A left, B and C), 20μm (A right). **P < 0.001 |
将HepG2细胞接种到hFLM内,以此评价支架是否适合构建肿瘤模型。HE染色提示HepG2细胞在脱细胞支架内存活良好,最初几天细胞在支架内稀疏分布,到培养后期增殖越来越密集。此外,外缘的肿瘤细胞呈现出沿细胞外基质向外扩散的趋势(图 3A)。PCNA染色提示大多数细胞在培养第6天时处于增殖状态。尽管细胞在第12天时排列更加紧密,但处于增殖状态的细胞数量显著减少(图 3B)。细胞培养第6天时的PCNA指数比第12天时高29.3%(P < 0.001,图 3D)。细胞增殖减缓可能与缺乏有效血管网提供营养有关。扫描电镜观察HepG2细胞在支架内的形态特征,可见细胞粘附于细胞外基质上,充满了脱细胞支架的网状空隙,培养第6天时细胞呈纺锤状形态,第12天时形成多细胞球体(图 3C)。通过检测支架内DNA含量变化及裸鼠成瘤中瘤体体积的变化来绘制3种肿瘤模型中细胞的生长曲线。在二维培养中,HepG2细胞贴壁后经过短暂的延迟期便迅速进入对数生长期,在第6天时增殖达高峰,细胞完全融合,之后部分贴壁细胞开始脱落。在裸鼠成瘤模型中,HepG2细胞经历了较长的延迟期,其细胞生长速度明显慢于二维模型中的细胞。有趣的是,在hFLM中培养的HepG2细胞呈现出一个较长的延迟期和较慢的生长速度,细胞增殖可维持15 d以上,这与体内模型接近(图 3E)。
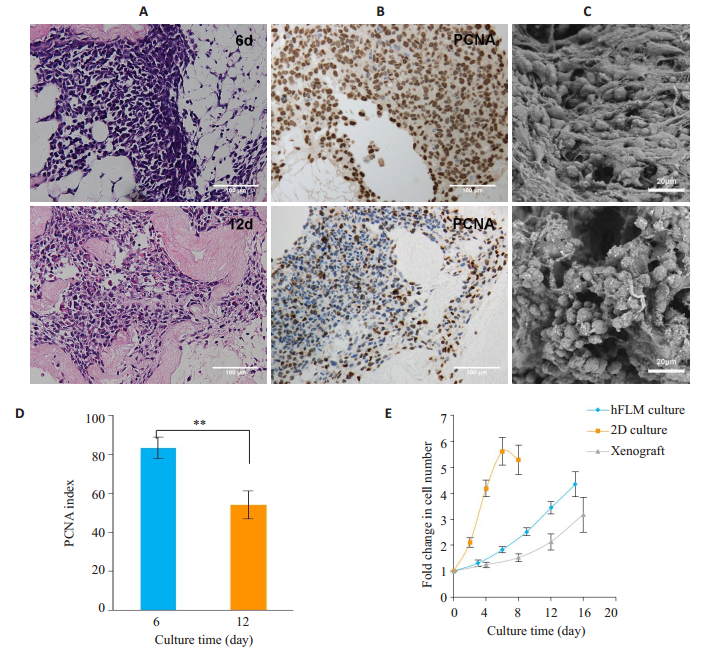
|
图 3 HepG2细胞在hFLM内的增殖 Fig.3 HepG2 cells cultured in hFLM. A: HE staining of HepG2 cells cultured in hFLM at 6 and 12 days. B: PCNA staining showed that cell proliferation slowed down at 12 days. C: SEM showed that the cells adhered to the extracellular matrix and formed multicellular spheroids. D: PCNA index of HepG2 cells cultured in hFLM at 6 and 12 days (n=6). E: Cell proliferation profiles of HepG2 cells in 2D culture, hFLM and xenografts. In the 2D culture model, the cells displayed a short lag phase followed by an exponential growth phase. However, HepG2 cells cultured in hFLM showed a longer lag phase and a slower growth rate, which more closely resembled those of the xenografts. Scale bars: 100 μm (A and B), 20 μm (C). **P < 0.001 |
在二维培养中,HepG2细胞高表达上皮细胞黏附分子E-cadherin却低表达间质细胞黏附分子vimentin。然而,在hFLM中培养的细胞高表达vimentin却低表达E-cadherin,这与裸鼠成瘤模型的表达更为接近(图 4)。
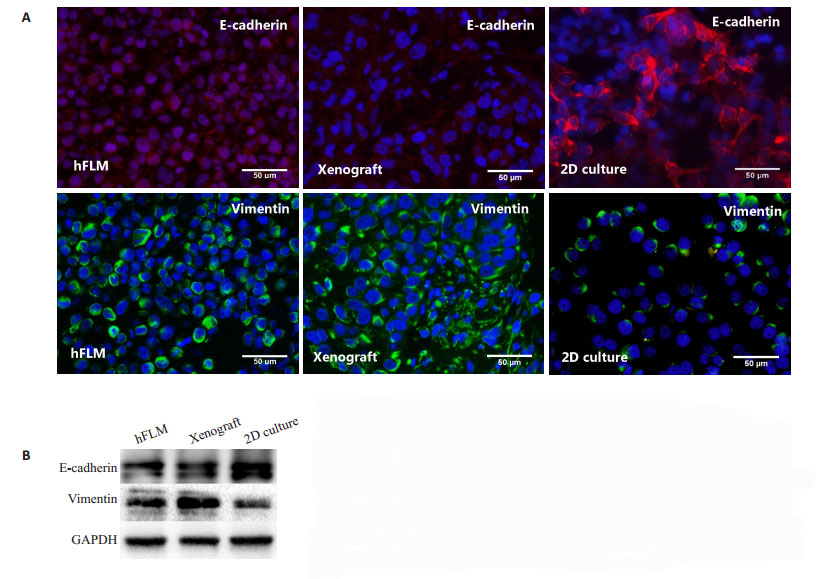
|
图 4 肿瘤模型内HepG2细胞差异性表达黏附分子 Fig.4 HepG2 cells cultured in hFLM showed altered expression of adhesion molecules compared with 2D culture. A: HepG2 cells in 2D culture model exhibit a high expression of epithelial cell adhesion marker E- cadherin and a low expression of mesenchymal adhesion molecule vimentin. Scale bars: 50 μm (A). However, the cells cultured in hFLM exhibit a low expression of E-cadherin and a high expression of vimentin, which is similar to that in tumors grown in vivo. B: Western blotting of E-cadherin and vimentin expression in HepG2 cells cultured in hFLM, 2D model and xenografts |
在本研究中,我们介绍了一种新的人脂肪肝脱细胞方案,包括反复低温冻融、通过门静脉及肝动脉灌注梯度浓度的SDS、1%Triton和Triton反复震荡洗涤组织。脱细胞过程保留了肝脏细胞外基质的成分及三维超微结构。在hFLM中培养的HepG2细胞能维持存活15 d,并表达与体内生长肿瘤相似的细胞黏附分子。
在大鼠[18-19]及大型动物模型[20]中,肝脏脱细胞支架制备已经是一项成熟的技术,但较少有关于人肝脏脱细胞的研究报道,尚无人脂肪肝的报道。从大鼠器官到人体器官,体积增加了几十倍,脱细胞的难度和时间成本也成倍增加。Jacques[21]比较了大鼠和人的肺脏、心脏和肾脏脱细胞的时间消耗,大鼠器官脱细胞平均耗时4~5 d,而人体器官平均耗时12~14 d。Mazza[16]花了14 d完成肝段(1~3段)的脱细胞,通过上下腔静脉灌注花了6周完成人类整肝脱细胞支架制备。本研究通过门静脉和肝动脉双重灌注梯度浓度的SDS制备人整肝脱细胞支架,共耗时约7 d,大大缩短了脱细胞时间。因此,双重灌注可有效提高脱细胞效率。
然而,同样的方法在hFLM的制备中失败了,因为在灌注SDS后支架中残留了大量的脂质,延长灌注时间仍无法将其脱除。事实上,Triton和SDS在脱细胞过程中各有特点。Triton是一种非离子型去污剂,可破坏DNA与蛋白质间的连接以及脂质与脂质蛋白间的连接[22],脱细胞效果较温和。而SDS是一种阴离子去污剂,可溶解细胞膜及核膜,更适合于致密组织的脱细胞,但容易使蛋白质变性,破坏关键生长因子成分[23]。尽管SDS已广泛应用于大型器官脱细胞研究,但是Triton对脂肪组织的去除效果显著优于SDS。考虑到用Triton行全肝灌注脱细胞效率低,于是将脂肪肝切成小块后行后续处理,最终成功制备了hFLM。保留于支架内部完整的血管网有助于细胞养分的输送,但是由于支架被分割成片,血管网的完整性遭破坏,所以采用将组织片浸入培养基中培养以替代灌注法。在培养后期,细胞养分获取受限,当细胞量到达一定水平后肿瘤组织中心的HepG2细胞增殖减缓。
在二维培养中,细胞可获得足够的营养和氧气,代谢产物易扩散到培养基中被稀释和替换,但在体内环境中,这些代谢产物可在局部高浓度蓄积,影响细胞的增殖和迁移[24]。在我们的研究中,HepG2细胞在贴壁后迅速生长,直至完全融合。然而,在hFLM中培养的细胞生长速度较慢,与体内肿瘤生长速度接近,且三维培养后期处于增殖状态的细胞数减少,它们多位于细胞团外缘,呈向外侵袭的趋势。细胞-基质间相互作用也可以影响胞内细胞骨架蛋白机械力进而影响细胞形态[25-26]。在人脱细胞脂肪组织支架中培养的乳腺癌细胞比在二维环境培养下的细胞形态更圆[27],脱细胞支架中的脂肪干细胞也发现了类似的形态学变化[28]。在hFLM模型中,HepG2细胞也形成了多细胞球体并粘附于细胞外基质上,其形态与二维培养明显不同。上皮间质转化是促进肿瘤转移的一个重要过程[29-31]。黏附分子是维持细胞形态及功能的重要成分,影响细胞生长、接触抑制和侵袭等。本研究检测分析了上皮间质转化中两个关键黏附分子,同二维培养相比,hFLM培养的HepG2细胞高表达vimentin、低表达E-cadherin。有趣的是,这两种黏附分子在体内成瘤组织中的表达水平与hFLM组相似。由此可见,hFLM为肝癌细胞提供了合适的微环境来模拟体内肿瘤的增殖和侵袭等生物学行为。
综上所述,我们介绍了一种新的人脂肪肝脱细胞方案,并证实利用这种支架体外培养肝癌细胞的可行性。hFLM培养的HepG2细胞与体内培养的肿瘤细胞具有相似的生长模式和黏附分子表达。在疾病发展过程中细胞外基质保留了真实的病理改变,这是人工合成材料难以模拟的。因此,人体器官或组织脱细胞基质可作为体外三维培养的一种潜在支架,这将有助于研究细胞外基质与肿瘤发生发展的关系。
| [1] |
Rijal G, Li WM. Native-mimicking in vitro microenvironment: an elusive and seductive future for tumor modeling and tissue engineering[J]. J Biol Eng, 2018, 12: 20. DOI:10.1186/s13036-018-0114-7 |
| [2] |
Herrmann D, Conway JR, Vennin C, et al. Three-dimensional cancer models mimic cell-matrix interactions in the tumour microenvironment[J]. Carcinogenesis, 2014, 35(8): 1671-9. DOI:10.1093/carcin/bgu108 |
| [3] |
Yan L, Xu F, Dai CL. Relationship between epithelial-tomesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2018, 37(1): 203. DOI:10.1186/s13046-018-0887-z |
| [4] |
Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression[J]. Nat Med, 2011, 17(3): 320-9. DOI:10.1038/nm.2328 |
| [5] |
Birgani MT, Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy[J]. Int J Mol Sci, 2017, 18(2): Pii: E405. DOI:10.3390/ijms18020405 |
| [6] |
Ghajar CM, Bissell MJ. Tumor engineering: the other face of tissue engineering[J]. Tissue Eng Part A, 2010, 16(7): 2153-6. DOI:10.1089/ten.tea.2010.0135 |
| [7] |
Leung M, Kievit FM, Florczyk SJ, et al. Chitosan-Alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance[J]. Pharm Res, 2010, 27(9): 1939-48. DOI:10.1007/s11095-010-0198-3 |
| [8] |
Kundu B, Saha P, Datta K, et al. A silk fibroin based hepatocarcinoma model and the assessment of the drug response in hyaluronan-binding protein 1 overexpressed HepG2 cells[J]. Biomaterials, 2013, 34(37): 9462-74. DOI:10.1016/j.biomaterials.2013.08.047 |
| [9] |
Hou J, Hong ZX, Feng F, et al. A novel chemotherapeutic sensitivitytesting system based on collagen gel droplet embedded 3D-culture methods for hepatocellular carcinoma[J]. BMC Cancer, 2017, 17(1): 729. DOI:10.1186/s12885-017-3706-6 |
| [10] |
Li QY, Uygun BE, Geerts S, et al. Proteomic analysis of naturallysourced biological scaffolds[J]. Biomaterials, 2016, 75: 37-46. DOI:10.1016/j.biomaterials.2015.10.011 |
| [11] |
Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds[J]. Annu Rev Biomed Eng, 2011, 13: 27-53. DOI:10.1146/annurev-bioeng-071910-124743 |
| [12] |
Mei J, Yu YL, Li MZ, et al. The angiogenesis in decellularized scaffold-mediated the renal regeneration[J]. Oncotarget, 2016, 7(19): 27085-93. |
| [13] |
Zheng XL, Xiang JX, Wu WQ, et al. Using a decellularized splenic matrix as a 3D scaffold for hepatocyte cultivation in vitro: a preliminary trial[J]. Biomed Mater, 2015, 10(4): 045023. DOI:10.1088/1748-6041/10/4/045023 |
| [14] |
Li WF, Hu XE, Wang SP, et al. Detection and evaluation of AntiCancer efficiency of astragalus polysaccharide via a tissue engineered tumor model[J]. Macromol Biosci, 2018, 18(11): e1800223. DOI:10.1002/mabi.201800223 |
| [15] |
Liu G, Wang B, Li SB, et al. Human breast cancer decellularized scaffolds promote epithelial-to-mesenchymal transitions and stemness of breast cancer cells in vitro[J]. J Cell Physiol, 2019, 234(6): 9447-56. DOI:10.1002/jcp.27630 |
| [16] |
Mazza G, Rombouts K, Hall AR, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation[J]. Sci Rep, 2015, 5: 13079. DOI:10.1038/srep13079 |
| [17] |
Zheng X, Xiang J, Wu W, et al. J[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2015, 35(7): 1028-33. |
| [18] |
Soto-Gutierrez A, Zhang L, Medberry C, et al. A whole-organ regenerative medicine approach for liver replacement[J]. Tissue Eng Part C Methods, 2011, 17(6): 677-86. DOI:10.1089/ten.tec.2010.0698 |
| [19] |
Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix[J]. Nat Med, 2010, 16(7): 814-20. DOI:10.1038/nm.2170 |
| [20] |
Yagi H, Fukumitsu K, Fukuda K, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach[J]. Cell Transplant, 2013, 22(2): 231-42. |
| [21] |
Guyette JP, Gilpin SE, Charest JM, et al. Perfusion decellularization of whole organs[J]. Nat Protoc, 2014, 9(6): 1451-68. DOI:10.1038/nprot.2014.097 |
| [22] |
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs[J]. Biomaterials, 2006, 27(19): 3675-83. |
| [23] |
Reing JE, Brown BN, Daly KA, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds[J]. Biomaterials, 2010, 31(33): 8626-33. DOI:10.1016/j.biomaterials.2010.07.083 |
| [24] |
Saglam-Metiner P, Gulce-Iz S, Biray-Avci C. Bioengineeringinspired three-dimensional culture systems: Organoids to create tumor microenvironment[J]. Gene, 2019, 686: 203-12. DOI:10.1016/j.gene.2018.11.058 |
| [25] |
Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression[J]. Cold Spring Harb Perspect Biol, 2011, 3(1): a003228. |
| [26] |
Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate[J]. Science, 2005, 310(5751): 1139-43. DOI:10.1126/science.1116995 |
| [27] |
Dunne LW, Huang Z, Meng W, et al. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments[J]. Biomaterials, 2014, 35(18): 4940-9. DOI:10.1016/j.biomaterials.2014.03.003 |
| [28] |
Wang LA, Johnson JA, Zhang QX, et al. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering[J]. Acta Biomater, 2013, 9(11): 8921-31. DOI:10.1016/j.actbio.2013.06.035 |
| [29] |
Li L, Li W. Epithelial-mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation[J]. Pharmacol Ther, 2015, 150: 33-46. DOI:10.1016/j.pharmthera.2015.01.004 |
| [30] |
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol, 2019, 29(3): 212-26. DOI:10.1016/j.tcb.2018.12.001 |
| [31] |
Oliveras-Ferraros C, Corominas-Faja B, Cufi S, et al. Epithelial-tomesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin)[J]. Cell Cycle, 2012, 11(21): 4020-32. DOI:10.4161/cc.22225 |
 2019, Vol. 39
2019, Vol. 39

