2. 右江民族医学院科学实验中心,广西 百色 533000
2. Science Lab Center, Youjiang Medical University for Nationalities, Baise 533000, China
最新研究表明,作为肾素-血管紧张素系统(RAS)的主要效应分子之一,血管紧张素Ⅱ(AngⅡ)参与了心血管和其他疾病的病理过程,包括心血管疾病[1-2]和肾脏疾病[3]。富含半胱氨酸蛋白61(Cyr61)又被称为CCN1,是CCN家族成员之一,是由内皮细胞及成纤维细胞分泌产生的细胞基质蛋白[4]。Cyr61具有多种功能,近年来研究发现,在病理条件下,Cyr61通过调控细胞迁移、黏附、增殖、凋亡等参与了包括自身免疫性疾病、急慢性炎性疾病、心血管性疾病及肿瘤的发生与发展[5-7]。Cyr61还参与了包括胚胎发育、血管生成、伤口愈合等众多机体正常的生理活动[8-10]。目前有关AngⅡ和Cyr61之间的相互作用的研究甚少,有几项研究表明在血管平滑肌细胞中AngⅡ可以刺激Cyr61的表达并影响细胞的生长[11-12],但是其具体作用机制仍然不明确,所以深入研究Cyr61在AngⅡ相关性疾病中的变化及作用对疾病的诊断与治疗有着重要意义。
本研究采用CRISPR/Cas9敲除质粒敲低HEK293T细胞Cyr61基因,并用AngⅡ干预HEK293T细胞,利用流式细胞仪检测细胞凋亡,qRT-PCR及Western blot方法检测HEK293T细胞Cyr61及Bcl-2的表达变化,从而探索Cyr61在AngⅡ诱导HEK293细胞功能中的改变及作用。
1 材料和方法 1.1 材料和试剂HEK293T细胞株(南加利福尼亚大学Keck医学中心惠赠,冻存于右江民族医学院科学实验中心),Cyr61 CRISPR/Cas9 KO质粒、HDR转染质粒和Ultracruz转染试剂(Santa Cruz Biotechnolog),DMEM细胞培养基、胎牛血清(Gibco),AngⅡ(索莱宝),Annexin VFITC、PI凋亡试剂检测盒(BD),细胞周期检测试剂盒(凯基生物),兔抗Cyr61一抗(Santa Cruz)、兔抗Bcl-2一抗(Abcam)、鼠抗GAPDH一抗(Proteintech),羊抗鼠、羊抗兔IgG抗体(二抗)(碧云天)。蛋白酶抑制剂混合物(康为世纪),BCA蛋白定量试剂盒(碧云天)。RNAiso Plus RNA提取试剂盒(9108,TaKaRa),PrimeScriptTM RT reagent Kit with gDNA Eraser(RR047A,TaKaRa),Mir-X miRNA First Strand Synthesis Kit(638315,clontech),SYBR Premix Ex TaqTMII(RR820A,TaKaRa),PCR引物设计与合成由生工生物工程有限公司完成,GAPDH:上游引物:5'-GAGGAGGCATTG CTGATGAT-3',下游引物:5'-GAAGGCTGGGGCTC ATTT-3';Cyr61:上游引物:5'-GCGGTTCCGATGCA GAGATGG-3',下游引物:5'-GATGCTTGCGCTTCTC CTCTGTC-3';Bcl-2:上游引物:5'-TCCTTCCAGCCT GAGAGCAACC-3',下游引物:5'-TCACGACGGTAG CGACGAGAG-3'。
1.2 细胞培养正常HEK293T细胞和敲低Cyr61 HEK293T细胞在含10%胎牛血清的DMEM培养液,于37 ℃,5% CO2环境中传代培养。2~3 d换液1次。细胞融合至80%左右消化传代。
1.3 细胞转染及分组按每孔2×105/mL在六孔板接种HEK293T细胞,常规培养至70%融合时更换无血清培养液,将1.0 μg Cyr61 CRISPR/Cas9 KO质粒和1.0 μg HDR转染质粒加入到150 μL无血清无抗生素的培养基中并充分混匀,于室温下孵育5 min;将10 μL Ultracruz转染试剂加入140 μL无血清无抗生素的培养基中充分混匀,于室温下孵育5 min;将上述两种混合液一起混匀后于常温下混合孵育10 min后加入无抗生素含10%FBS的培养基使最终体积为2 mL加入含HEK293T细胞的六孔板内。转染48 h后于荧光显微镜下观察转染情况。转染成功后48~96 h观察细胞状态,生长状态良好时,加入8 μg/mL的嘌呤霉素(Puromycin)选择转染细胞,直至正常细胞完全死亡,得到稳定转染细胞系。并用Western blot法鉴定转染结果。将HEK293T细胞分为4组:对照组、Cyr61敲低组、AngⅡ组和Cyr61敲低+ AngⅡ组。并按照前面所述分组将两种类型细胞按照每孔2×105/mL接种于六孔板内,待细胞生长达到70%融合时,吸去原培养液并用冷的PBS洗涤2次,每孔更换新的含10%胎牛血清的DMEM培养基2 mL,并在对应孔内加入AngⅡ,使其终浓度为10-7 mol/L [13],在37 ℃、5%CO2饱和湿度条件下继续培养。培养48 h后收集各组细胞进行后续实验检测。
1.4 流式细胞术检测细胞凋亡依照分组用AngⅡ干预HEK293T 48 h后收集各孔上清液于离心管中备用,然后再将各组细胞用4 ℃ PBS清洗2次,胰酶消化后与前述上清液共同制成单细胞悬液,调整细胞密度为1×l06/mL,取l00 μL细胞悬液加入5 μLAnnexinV-FITC和5 μL PI溶液混匀,室温避光孵育l5 min,加入400 μL PBS,流式细胞仪进行检测。重复试验3次。
1.5 Quantitative Real-time PCR检测将转染Cyr61 CRISPR/Cas9 KO质粒的HEK293T细胞及正常HEK293T培养48 h后,按照RNAiso Plus RNA提取试剂盒操作说明提取总RNA。取1 μg总RNA反转录合成cDNA,具体操作按照试剂盒说明书进。使用LightCycler® 96实时荧光定量PCR仪进行检测。选取GAPDH作为内参,检测Cyr61、Bcl-2及GAPDH的mRNA表达。以2-△△CT法计算相对表达量[14],ΔΔCT=(CT实验组目的-CT实验组内参)-(CT对照组目的-CT对照组内参)。重复试验3次。
1.6 Western blot检测依照分组用AngⅡ干预HEK293T 48 h后收集各组细胞用预冷的PBS液洗2次,加入150 μL预冷PI裂解液,冰浴30 min,刮勺收集样本,4 ℃ 12 000 r/min离心10 min,取上清,以二喹琳甲酸(BCA)法测定提取蛋白质量浓度。每个样本取30~50 μg蛋白加入5×SDS上样缓冲液煮沸变性后,十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳,湿式电印迹法300 mA恒流转至PVDF膜,3%BSA室温封闭1 h,加TBST洗膜缓冲液洗3次,每次10 min,加入一抗孵育4 ℃过夜,加TBST洗膜缓冲液洗3次,每次10 min,加入HRP标记的羊抗兔或羊抗小鼠IgG(H+L),常温震荡孵育1 h,洗涤后采用ChemiDocXRS凝胶成像系统Qµnatity One软件采集图像,灰度值采用NIH Image软件(National Institute of Health, Bethesda, Md, USA)测定。以目的蛋白条带与GAPDH蛋白条带灰度值的比值表示其相对含量。重复试验3次。
1.7 统计学分析采用SPSS23.0统计软件进行数据分析,所有数据均进行正态分布和方差齐性检验。服从正态分布的数据以均数±标准差表示,两组间比较采用独立样本t检验,多组间采用单因素方差分析。非正态分布则以中位数和极值表示,采用秩和检验。每个实验组重复3次。P < 0.05为差异有统计学意义。
2 结果 2.1 转染细胞结果的鉴定按照Cyr61 CRISPR/Cas9 KO质粒操作书面转染细胞48 h后用倒置荧光显微镜观察,转染组细胞可见绿色荧光蛋白均匀分布于细胞内(图 1)。
|
图 1 转染后HEK293T细胞绿色荧光蛋白表达情况 Fig.1 Expression of green fluorescent protein in the transfected cells (Original magnification: ×100). A: Cell were observed in light filed; B: Transfected after 48 hours. |
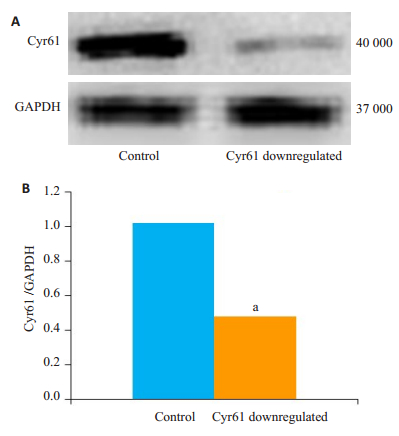
转染48 h后,收集细胞并提取细胞总蛋白,利用Western blot检测正常HEK293T细胞及敲低Cyr61的HEK293T细胞中Cyr61的表达量。结果显示两组HEK293T细胞中均有Cyr61的表达,Cyr61转染组细胞中Cyr61表达量显著低于对照组(aP < 0.05,图 2)。
|
图 2 敲低Cyr61后Cyr61蛋白表达变化 Fig.2 Expression of Cyr61 protein in HEK293T cells with Cyr61 knockdown (Mean ± SD, n=3). A: The diagram of primitive protein banding; B: Target protein relative gray value. aP < 0.05. |
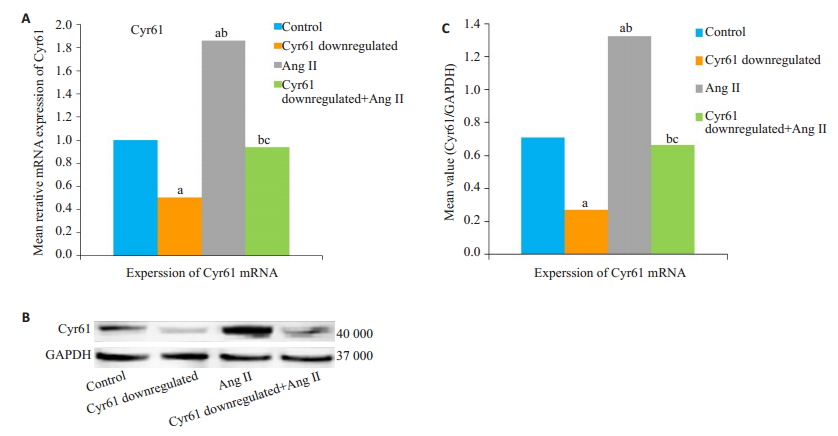
与对照组相比,AngⅡ干预组的Cyr61mRNA及蛋白表达量均明显升高(aP < 0.05)。与Cyr61敲低组相比,Cyr61敲低+ AngⅡ组的Cyr61mRNA及蛋白表达量均明显升高(bP < 0.05,图 3)。而与AngⅡ干预组相比明显降低(cP < 0.05)。
|
图 3 AngⅡ对HEK293T细胞Cyr61表达的影响 Fig.3 Effect of AngⅡ on Cyr61 expression in HEK293T cells (Mean±SD, n=3). A: Expression of Cyr61 mRNA; B: Western blotting of Cyr61 protein expression; C: Semi-quantitative analysis of Cyr61 protein expression relative to GAPDH. aP < 0.05 vs Control, bP < 0.05 vs Cyr61 doenregulated, cP < 0.05 vs AngⅡ. |
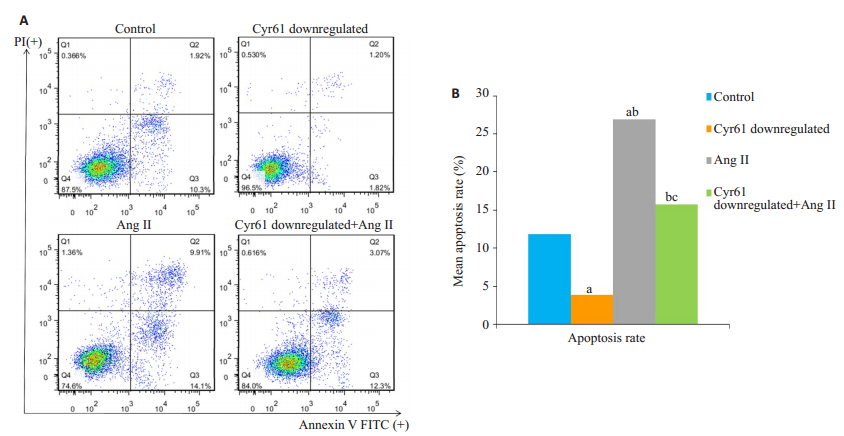
与对照组相比,Cyr61敲低组细胞凋亡率明显降低(aP < 0.05);Ang Ⅱ干预组的凋亡率明显升高(bP < 0.05)。Cyr61敲低+AngⅡ组的凋亡率较Cyr61敲低组增高(bP < 0.05),较AngⅡ组显著降低(cP < 0.05,图 4)。
|
图 4 敲低Cyr61对AngⅡ诱导的HEK293T细胞凋亡的影响 Fig.4 Effect of Cyr61 knockdown on AngⅡ-induced apoptosis in HEK293T cells (Mean±SD, n=3). aP < 0.05 vs Control, bP < 0.05 vs Cyr61 doenregulated, cP < 0.05 vs AngⅡ. |
与对照组相比,Cyr61敲低组Bcl-2蛋白表达量明增高(aP < 0.05);AngⅡ干预组的Bcl-2表达量明显降低(bP < 0.05)。Cyr61敲低+AngⅡ组的Bcl-2表达量较Cyr61敲低组降低(bP < 0.05),较AngⅡ组显著增高(cP < 0.05,图 5)
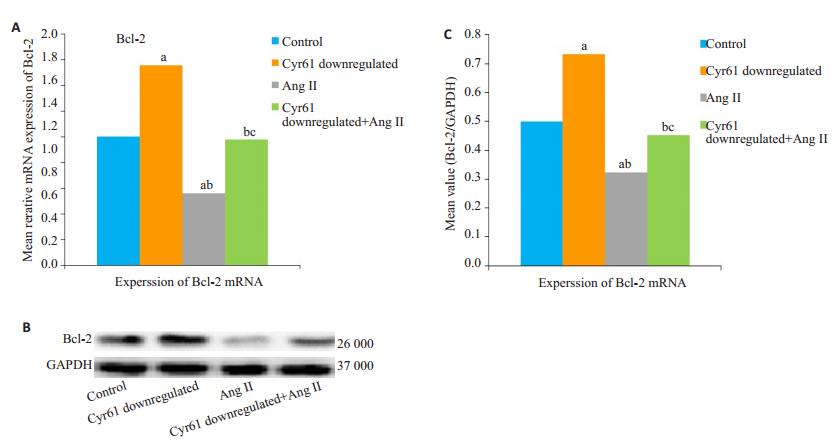
|
图 5 敲低Cyr61对AngⅡ干预下HEK293T细胞Bcl-2表达的影响 Fig.5 Effect of Cyr61 knockdown on Bcl-2 expression in AngⅡtreated HEK293T cells (Mean ± SD, n=3). A: Expression of Bcl-2 mRNA; B: Western blotting of Bcl-2 protein expression; C: Semiquantitative analysis of Bcl-2 protein expression relative to GAPDH. (aP < 0.05 vs Control, bP < 0.05 vs Cyr61 doenregulated, cP < 0.05 vs AngⅡ). |
本研究采用一种新的基因编辑技术---CRISPR/Cas9技术来敲低HEK293T细胞Cyr61基因,并用AngⅡ干预HEK293T细胞,利用流式细胞仪检测细胞凋亡,用qRT-PCR及Western blot方法检测细胞Cyr61及Bcl-2的表达变化,从而探索Cyr61在AngⅡ诱导HEK293细胞功能中的改变及作用。结果显示,CRISPR/Cas9KO质粒可以有效地敲低HEK293T细胞Cyr61基因。Cyr61表达量的上调与AngⅡ诱导的HEK293T细胞损伤有关。下调Cyr61的表达可以有效地保护AngⅡ诱导的细胞损伤。
AngⅡ是RAS主要生物活性产物之一,在心血管调节中具有众所周知的作用,是肾脏炎症和纤维化以及血压和肾血流动力学的关键调节因子[15-17]。AngⅡ在不同的细胞中有着不同的作用,如在血管平滑肌细胞中可以促进其增殖而导致动脉粥样硬化[18],相反的,AngⅡ可以诱导足细胞、心肌细胞及脐静脉内皮细胞的凋亡[19-21]。可见AngⅡ的作用机制十分复杂,深入研究AngⅡ的作用机制对于AngⅡ参与的疾病的预防、诊断与治疗具有极其重要的意义。富含半胱氨酸的蛋白61(Cyr61)又称CCN1,是由内皮细胞、成纤维细胞和平滑肌细胞产生和分泌细胞外基质的组成部分[4]。Cyr61通过调节不同的信号通路,在不同的生物过程中发挥重要作用。Cyr61在胚胎发育过程中对心血管发育至关重要,而在成年期,它与炎症、伤口愈合、损伤修复以及纤维化和癌症等相关病理密切关[22]。近年来研究发现Cyr61参与了系统性红斑狼疮相关性肺动脉高压[23]、肾缺血-再灌注损伤[24]、心力衰竭[25]、冠心病[26]、心肌损伤[27]等疾病的发生与发展。另外,Shimura等[28]研究得出尿Cyr61可能成为结肠癌非侵袭性诊断标准。这些研究结果表明Cyr61与多种疾病的发生相关,可能成为临床相关疾病诊断和治疗的潜在靶点。有几项研究表明,AngⅡ可以通过直接调控Cyr61的表达参与多种疾病的病理发展。Hilfiker等[29]研究发现,用AngⅡ干预大鼠血管平滑肌细胞后Cyr61的表达明显上调并参与血管平滑肌细胞增殖的调节。Jin等[30]研究发现AngⅡ可以通过诱导Cyr61的表达诱导血管衰老,这种作用能够被AngⅡ受体-1阻滞剂抑制。Saikawa等[31]研究表明AngⅡ可以诱导Cyr61的表达参与了胆管癌病理发展。这些研究说明Cyr61可能是AngⅡ的下游信号分子。我们的研究发现,在AngⅡ作用下Cyr61的表达明显升高,因此,我们推测Cyr61也可能参与了AngⅡ诱导的HEK293T细胞损伤过程。为验证这个假说,我们应用AngⅡ干预体外培养的HEK293T细胞,并且观察了敲低Cyr61对AngⅡ诱导的HEK293T细胞凋亡的影响。我们通过流式细胞术检测各组细胞的凋亡率。结果发现,AngⅡ作用48 h后HEK293T细胞凋亡率明显增高,敲低Cyr61后HEK293T细胞凋亡率下降,与对照组及AngⅡ干预组相比差异有统计学意义。上述实验结果说明Cyr61参与了AngⅡ诱导的HEK293T细胞凋亡过程,敲低Cyr61能够有效降低AngⅡ诱导的HEK293T细胞凋亡程度。
细胞存活的调节由Bcl-2家族中抗凋亡因子及促凋亡因子的平衡决定[32]。所以为了进一步研究Cyr61在AngⅡ诱导HEK293T细胞凋亡的作用机制,本实验进一步观察了敲低Cyr61对HEK293T干预下HEK293T细胞Bcl-2表达的影响。我们研究结果发现AngⅡ作用48 h后HEK293T细胞Bcl-2表达水平明显下调,说明Bcl-2确实参与了AngⅡ诱导的HEK293T细胞凋亡过程,而敲低Cyr61则可以在基因及蛋白水平上调Bcl-2的表达。考虑到Bcl-2作为抗细胞凋亡因子起作用,我们的研究结果表明Cyr61通过Bcl-2途径调节AngⅡ诱导HEK293T细胞的凋亡。
综上所述,Cyr61高表达参与Ang Ⅱ诱导的HEK293T细胞损伤过程,敲低Cyr61能够明显减轻AngⅡ诱导的HEK293T细胞凋亡程度,Cyr61通过Bcl- 2途径调节AngⅡ诱导HEK293T细胞的凋亡。因此,阻断病理状况下Cyr61的高表达可能是治疗AngⅡ相关性疾病的新策略。
| [1] |
Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation[J]. Circ Res, 2010, 106(11): 1675-80. DOI:10.1161/CIRCRESAHA.110.217737 |
| [2] |
Cavalera M, Wang JH, Frangogiannis NO, et al. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities[J]. Transl Res, 2014, 164(4): 323-35. DOI:10.1016/j.trsl.2014.05.001 |
| [3] |
Saldanha D, Rodrigues PR, Lauar AO, et al. Renin angiotensin system and cytokines in chronic kidney disease: clinical and experimental evidence[J]. Protein Pept Lett, 2017, 24(9): 799-808. |
| [4] |
Emre Y, Imhof BA. Matricellular protein CCN1/CYR61: a new player in inflammation and leukocyte trafficking[J]. Semin Immunopathol, 2014, 36(2): 253-9. DOI:10.1007/s00281-014-0420-1 |
| [5] |
Borkham-Kamphorst E, Steffen BT, Van De Leur E, et al. CCN1/CYR61 overexpression in hepatic stellate cells induces ER stressrelated apoptosis[J]. Cell Signal, 2016, 28(1): 34-42. DOI:10.1016/j.cellsig.2015.10.013 |
| [6] |
Liu Y, Zhang F, Zhang Z, et al. High expression levels of Cyr61 and VEGF are associated with poor prognosis in osteosarcoma[J]. Pathol Res Pract, 2017, 213(8): 895-9. DOI:10.1016/j.prp.2017.06.004 |
| [7] |
Zhu XJ, Song YF, Wu CL, et al. Cyr61 participates in the pathogenesis of acute lymphoblastic leukemia by enhancing cellular survival via the AKT/NF-kappa B signaling pathway[J]. Sci Rep, 2016, 6: 34018-27. DOI:10.1038/srep34018 |
| [8] |
Mo FE, Muntean AG, Stolz DB, et al. CYR61 (CCN1) is essential for placental development and vascular integrity[J]. Mol Biol Cell, 22(24): 8709-20. DOI:10.1128/MCB.22.24.8709-8720.2002 |
| [9] |
Estrada R, Li N, Sarojini H, et al. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61[J]. J Cell Physiol, 2009, 219(3): 563-71. DOI:10.1002/jcp.v219:3 |
| [10] |
Lee HY, Chung JW, Youn SW, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia[J]. Circ Res, 2007, 100(3): 372-80. DOI:10.1161/01.RES.0000257945.97958.77 |
| [11] |
Gao BB, Stuart L, Feener EP. Label-free quantitative analysis of onedimensional PAGE LC/MS/MS proteome: application on angiotensin Ⅱ-stimulated smooth muscle cells secretome[J]. Mol Cell Proteomics, 2008, 7(12): 2399-409. DOI:10.1074/mcp.M800104-MCP200 |
| [12] |
付鹏, 牛铁生, 孙英贤. 血管紧张素Ⅱ上调血管平滑肌细胞富含半胱氨酸蛋白61的表达[J]. 中国动脉硬化杂志, 2007, 15(6): 431-4. DOI:10.3969/j.issn.1007-3949.2007.06.008 |
| [13] |
Yang Q, Ma YQ, LiuYP, et al. Angiotensin Ⅱ down-regulates nephrinAkt signaling and induces podocyte injury: role of c-Abl[J]. Mol Biol Cell, 2016, 27(1): 197-208. DOI:10.1091/mbc.E15-04-0223 |
| [14] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method[J]. Methods, 2001, 25(4): 402-8. DOI:10.1006/meth.2001.1262 |
| [15] |
Santos RA, Ferreira AJ, Verano-Braga T, et al. Angiotensinconverting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system[J]. J Endocrinol, 2013, 216(2). |
| [16] |
Xu Z, Li WX, Han JB, et al. Angiotensin Ⅱ induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2)[J]. Sci Rep, 2017, 7: 44911. DOI:10.1038/srep44911 |
| [17] |
Miyajima A, Kosaka T, Kikuchi E, et al. Renin-angiotensin system blockade: Its contribution and controversy[J]. International Journal of Urology, 2015, 22(8): 721-30. DOI:10.1111/iju.12827 |
| [18] |
Hussain M, Awan FR. Hypertension regulating angiotensin peptides in the pathobiology of cardiovascular disease[J]. Clin Exp Hypertens, 2018, 40(4): 344-52. DOI:10.1080/10641963.2017.1377218 |
| [19] |
Cardoso VG, Goncalves GL, Thieme K, et al. Angiotensin Ⅱ-induced podocyte apoptosis is mediated by endoplasmic reticulum stress/PKC-delta/p38 MAPK pathway activation and trough increased Na+/H+ exchanger isoform 1 activity[J]. BMC Nephrol, 2018, 19(1): 179-91. DOI:10.1186/s12882-018-0968-4 |
| [20] |
Qi J, Wang F, Yang P, et al. Mitochondrial fission is required for angiotensin Ⅱ-Induced cardiomyocyte apoptosis mediated by a Sirt1-p53 signaling pathway[J]. Front Pharmacol, 2018, 9: 00176. DOI:10.3389/fphar.2018.00176 |
| [21] |
Zhou XL, Liang LW, Zhao Y, et al. Epigallocatechin-3-Gallate ameliorates angiotensin Ⅱ-Induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/caspase-3 signaling[J]. J Vasc Res, 2017, 54(5): 299-308. DOI:10.1159/000479873 |
| [22] |
Kim KH, Won JH, Cheng NY, et al. The matricellular protein CCN1 in tissue injury repair[J]. J Cell Commun Signal, 2018, 12(1): 273-9. DOI:10.1007/s12079-018-0450-x |
| [23] |
FAN Y, ZHAO J, QIAN J, et al. Cysteine-rich protein 61 as a novel biomarker in systemic lupus erythematosus-associated pulmonary arterial hypertension[J]. Clin Exp Rheumatol, 2018 Dec 20. [Epub ahead of print]. https://www.ncbi.nlm.nih.gov/pubmed/30620287
|
| [24] |
Li C, Zhao L, Wang Y, et al. Cysteine-rich protein 61, a specific ultraearly biomarker in kidney ischemia/reperfusion injury[J]. Nephrology (Carlton), 2018, 513(10): 47-58. |
| [25] |
Zhao JJ, Zhang CY, Liu J, et al. Prognostic significance of serum cysteine-rich protein 61 in patients with acute heart failure[J]. Cell Physiol Biochem, 2018, 48(3): 1177-87. DOI:10.1159/000491984 |
| [26] |
Deng JA, Qian XX, Li JP, et al. Evaluation of serum cysteine-rich protein 61 levels in patients with coronary artery disease[J]. Biomark Med, 2018, 12(4): 329-39. DOI:10.2217/bmm-2017-0390 |
| [27] |
Klingenberg R, Aghlmandi S, Liebetrau CA, et al. Cysteine-rich angiogenic inducer 61 (Cyr61): a novel soluble biomarker of acutemyocardial injury improves risk stratification after acute coronary syndromes[J]. Eur Heart J, 2017, 38(47): 3493-3501. DOI:10.1093/eurheartj/ehx640 |
| [28] |
Shimura T, Iwasaki H, Kitagawa M, et al. Urinary Cysteine-Rich protein 61 and trefoil factor 3 as diagnostic biomarkers for colorectal cancer[J]. Transl Oncol, 2019, 12(3): 539-44. DOI:10.1016/j.tranon.2018.12.006 |
| [29] |
HilfikerA, Hilfiker-Kleiner D, Fuchs M, et al. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin Ⅱ[J]. Circulation, 2002, 106(2): 254-60. DOI:10.1161/01.CIR.0000021426.87274.62 |
| [30] |
Kim I, Park CS, Lee HY. Angiotensin Ⅱ type 1 receptor blocker, fimasartan, reduces vascular smooth muscle cell senescence by inhibiting the CYR61 signaling pathway[J]. Korean Circ J, 2019, 49(7): 615-26. DOI:10.4070/kcj.2018.0379 |
| [31] |
Saikawa S, Kaji K, Nishimura N, et al. Angiotensin receptor blockade attenuates cholangiocarcinoma cell growth by inhibiting the oncogenic activity of Yes-associated protein[J]. Cancer Lett, 2018, 434: 120-9. DOI:10.1016/j.canlet.2018.07.021 |
| [32] |
Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology[J]. Fertil Steril, 2012, 98(3): 520-8. DOI:10.1016/j.fertnstert.2012.06.021 |
 2019, Vol. 39
2019, Vol. 39

