克罗恩病(CD)在我国的发病率呈现逐年上升趋势[1-2],CD病程早期系统性的内科治疗,对抑制消化道的炎症反应,维持早期状态控制疾病进展至关重要[3]。原花青素B2(PCB2)因其具有极强的抗氧化活性和抑制炎症反应特性,已被广泛应用于药物领域[4-5]。研究显示,PCB2可抑制CD肠黏膜炎症反应中的重要促炎信号PI3k/AKT通路,提示其潜在的抗CD样肠炎的作用[6-7]。目前尚未发现有关PCB2对CD样肠炎治疗作用的研究。维持肠屏障功能和结构的完整性可能对缓解CD疾病进展具有重要作用,其与肠道炎症反应状态密切相关[8]。因此,本研究以三硝基苯磺酸(TNBS)诱导CD样肠炎小鼠模型,观察PCB2对CD样肠炎的治疗效果,并以肠屏障功能为主线分析药物可能的作用机制,发掘可用于CD治疗的天然植物提取物,以期为CD的药物治疗提供参考。
1 材料和方法 1.1 材料实验动物为6~8周龄雄性Balb/c小鼠(南京大学模式动物研究所),体质量为23.0±1.5 g,饲养于SPF环境,环境温度为23±2 ℃,避免强光和噪音刺激,进食和饮水自由。TNBS、PCB2、FITC-葡聚糖及Mac-Conkey's培养基(Sigma Chemicals,USA),ZO-1、claudin-1、PI3K、AKT及β-actin抗体(San Francisco, CA,USA),白介素- 1β(IL-1β)、肿瘤坏死因子-α(TNF-α)及白介素-10(IL- 10)、酶联免疫吸附法(ELISA)试剂盒(IBL-America, CA,USA)。
1.2 动物模型及干预方法建立TNBS结肠炎小鼠模型[9]:0.7%的戊巴比妥钠腹腔注射麻醉,麻醉满意后摆放小鼠头低脚高30°倾斜,将TNBS溶于50%乙醇溶液,配置成0.1 mL TNBS溶液用于单只小鼠给药(含TNBS 150 mg/kg/只),经肛门注入TNBS溶液0.1 mL,并保持倾斜体位30 min,TNBS模型小鼠在干预后7 d可形成稳定的CD样肠炎。将造模成功的小鼠随机分为PCB2治疗组及对照组,每组10只。治疗组给予PCB2治疗:以100 mg/kg的剂量将PCB2溶于0.2 mL生理盐水配置成单只小鼠的给药剂量,给药方式为灌胃[10];对照组小鼠给予0.2 mL/d生理盐水灌胃;干预周期为4周,干预结束后处死小鼠进行检测分析。
1.3 观察指标及检测方法 1.3.1 评估PCB2干预对TNBS模型小鼠疾病症状的影响采用结肠炎小鼠疾病活动度(DAI)评估小鼠结肠炎症状,包括:血便(1分)、直肠脱垂(1分)、稀便(1分)和竖立(1分),严重的腹泻及直肠脱垂另各加1分,累计相加总分为DAI得分[11]。记录并比较4周内两组小鼠体质量变化趋势。
1.3.2 评估PCB2干预对结肠炎模型小鼠肠道炎症的影响(1)采用小鼠结肠组织炎症评分:结肠组织石蜡切片经H&E染色后光学显微镜下观察,依据Spencer推荐的炎症评分评估两组小鼠结肠组织炎症程度,评分量表为0~4分,分数越高提示炎症越重;每张切片选择3处不重叠视野分别评估,取平均分数作为最终评分结果[12];(2)评估两组小鼠结肠组织炎症介质水平:将小鼠结肠粘膜组织匀浆,采用ELISA法检测IL-1β、TNF-α及IL-10表达水平。
1.3.3 评估PCB2干预对结肠炎模型小鼠肠屏障功能的影响(1)评估两组小鼠结肠组织通透性:小鼠禁食4 h后,给予小鼠灌胃异硫氰酸荧光素(FITC)-葡聚糖,剂量为600 mg/kg。4 h后通过颈椎脱臼法处死小鼠,并行心脏穿刺抽血1 mL,室温静置1 h后离心分离血清,荧光测定法检测血清FITC-葡聚糖水平[13];(2)评估两组小鼠淋巴结细菌易位:采用Mac-Conkey's琼脂培养基对无菌采集的小鼠肠系膜淋巴结和脾脏分别进行培养,以单位/g组织计数菌落数,当其超过102单位/g组织时认定结果为阳性[14]。
1.3.4 评估PCB2干预对结肠炎模型小鼠肠屏障结构的影响采用免疫荧光法[15]检测两组小鼠肠粘膜上皮细胞紧密连接蛋白ZO-1及claudin-1表达。同时采用Western blot定量检测ZO-1及claudin-1表达。
1.3.5 分析PCB2干预对TNBS模型小鼠肠粘膜PI3K/ AKT通路的影响采用Western blot法检测评估,结果采用Image J软件分析。
1.4 统计学方法采用SPSS23.0统计分析所得数据,计量资料以均数±标准差表示,组间比较采用t检验;率的比较采用卡方检验。P < 0.05为差异具有统计学意义。
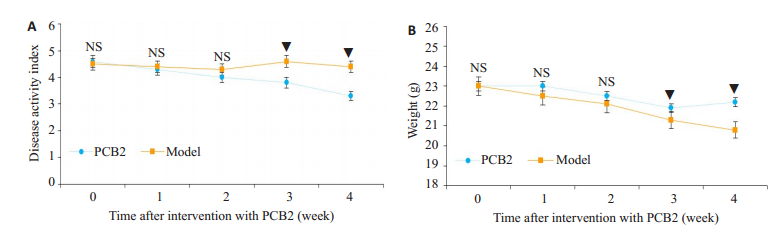
2 结果 2.1 PCB2干预改善了TNBS模型小鼠的疾病症状PCB2组小鼠在干预第3周起DAI评分低于对照组小鼠,组间比较差异具有统计学意义(P < 0.05)。PCB2组小鼠在干预第3周起平均体质量高于对照组小鼠(P < 0.05,图 1)。
|
图 1 PCB2干预对TNBS模型小鼠疾病症状的影响 Fig.1 Effect of PCB2 intervention on disease symptoms in mice with TNBS-induced colitis. A: Changes of DAI scores of the mice in the 2 groups; B: Changes of body weight in the 2 groups; NS: No significant difference between two groups; ▼P < 0.05 vs model group. |
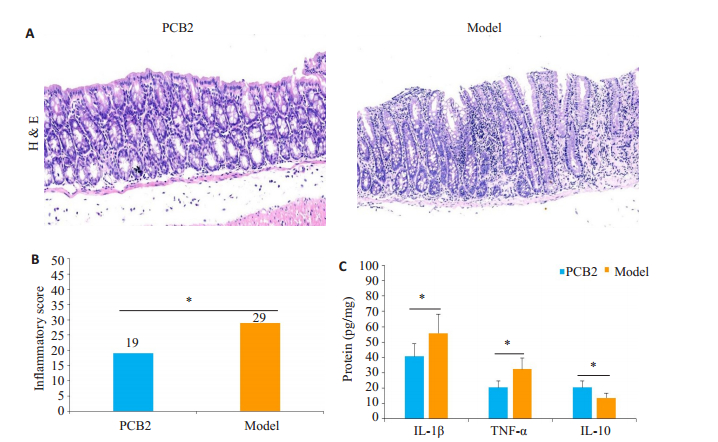
PCB2干预降低了TNBS模型小鼠结肠炎症,PCB2组小鼠结肠炎症评分低于对照组,差异具有统计学意义(P < 0.05)。同时,PCB2组小鼠结肠粘膜炎症介质IL-1β和TNF-α低于对照组,差异具有统计学意义(P < 0.05);而IL-10高于对照组,差异具有统计学意义(P < 0.05,图 2)。
|
图 2 PCB2干预对TNBS结肠炎模型小鼠肠道炎症的影响 Fig.2 Effect of PCB2 intervention on intestinal inflammation in mice with TNBS-induced colitis. A: HE staining of the colon tissue (Original magnification: ×200); B: Comparison of colonic inflammation scores between the 2 groups; C: Comparison of colonic mucosal inflammatory mediators. *P < 0.05. |
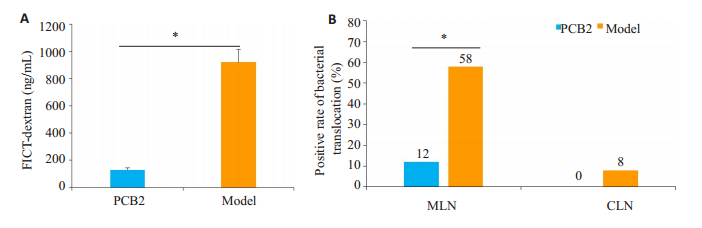
PCB2组小鼠对FITC-葡聚糖通透性低于对照组,差异具有统计学意义(P < 0.05);PCB2组小鼠肠系膜淋巴结细菌移位阳性率低于对照组,差异具有统计学意义(P < 0.05,图 3);而两组脾脏细菌移位阳性率差异无统计学意义(P>0.05)。
|
图 3 PCB2干预对TNBS模型小鼠肠粘膜屏障功能的影响 Fig.3 Effect of PCB2 intervention on intestinal mucosal barrier function in mice with TNBS-induced colitis. A: FITC-glucan permeability of the intestinal mucosa in the 2 groups; B: Positive rate of bacterial translocation in the lymph nodes in the 2 groups; *P < 0.05. |
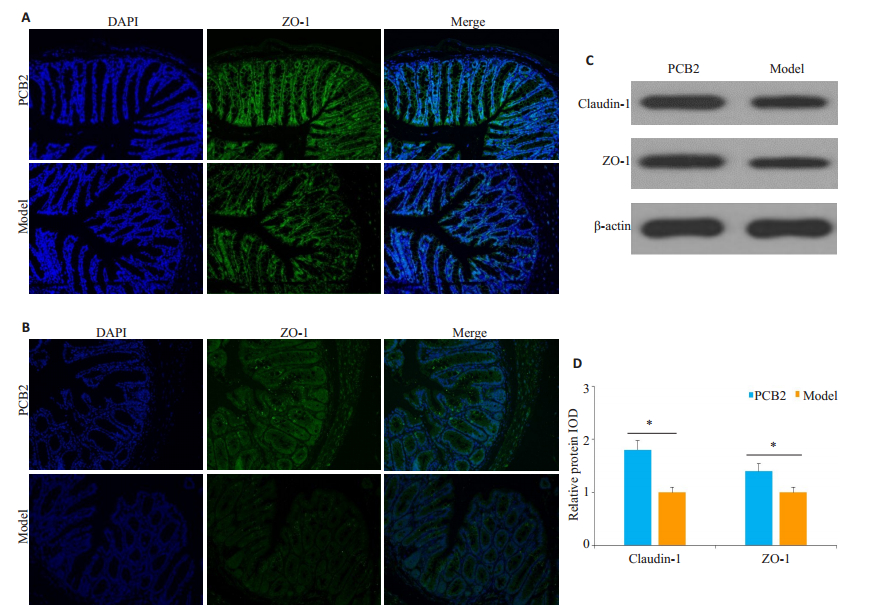
PCB2治疗改善了TNBS模型小鼠肠粘膜上皮细胞紧密连接蛋白claudin-1及ZO-1分布缺失及异位;Western blot检测及半定量分析显示,PCB2治疗提高了TNBS模型小鼠claudin-1及ZO-1的表达水平(P < 0.05,图 4)。
|
图 4 PCB2干预对TNBS结肠炎模型小鼠肠屏障结构的影响 Fig.4 Effect of PCB2 intervention on intestinal barrier structure in the mice. A: Expression of tight junction protein claudin-1 in intestinal mucosal epithelial cells of the mice in the 2 groups (×200); B: Expression of tight junction protein ZO-1 in intestinal mucosal epithelial cells in the 2 groups (× 200); C: Western blotting for claudin-1 and ZO-1 in the intestinal mucosa in the 2 groups; D: Comparison of intestinal mucosal claudin-1 and ZO-1 expression levels; *P < 0.05. |
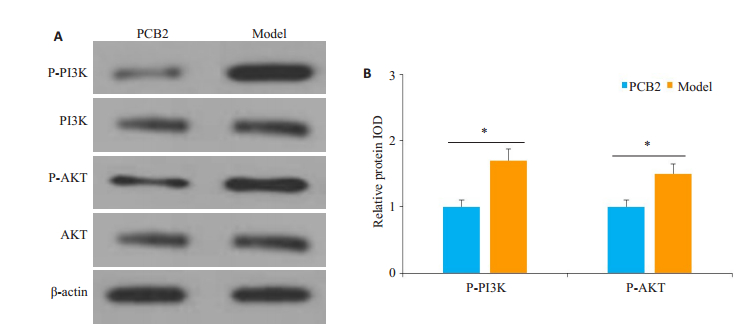
PCB2组小鼠肠粘膜p-PI3K及p-AKT表达水平低于对照组小鼠,差异具有统计学意义(P < 0.05,图 5)。
|
图 5 PCB2干预对TNBS结肠炎模型小鼠肠粘膜PI3K/AKT通路的影响 Fig.5 Effect of PCB2 intervention on intestinal mucosal PI3K/AKT pathway in the mouse models of colitis. A: Western blotting of PI3K and AKT expression levels in the intestinal mucosa of two groups of mice; B: Comparison of expression levels of p-PI3K and p-AKT between the two groups. *P < 0.05. |
本研究通过在体研究发现PCB2对TNBS诱导的CD样肠炎具有保护作用,其可能的机制为抑制PI3K/ AKT信号通路,而发挥抗炎及肠黏膜屏障保护作用。
本研究采用TNBS模型作为CD的动物模型,其具有诱导成功率高、肠炎模型稳定等优点[16-17],结果发现从第3周开始DAI评分量表PCB2组显著优于对照组,且体质量较对照组显著增加。同时,病理组织切片显示PCB2组小鼠肠壁组织炎细胞浸润显著减少,分子免疫学分析发现肠粘膜促炎介质IL-1β、TNF-α显著降低,而抗炎介质IL-10显著升高。这一组数据证实PCB2对TNBS小鼠显示出良好的在体抗肠炎作用。CD的肠黏膜免疫/炎症反应以Th1、Th17型免疫反应为主导,而IL-1β、TNF-α是Th1、Th17型T淋巴细胞下游的主要促炎介质,是多种药物治疗的主要抑制靶介质[18-20]。例如临床上使用的英夫利昔单抗的主要治疗原理即是通过抑制TNF-α所引发的炎症级联放大和慢性炎症维持作用[21-22]。
在获得PCB2对CD样肠炎的治疗效果后,我们进一步尝试分析其可能的作用途径。肠屏障受损是CD疾病发生发展的关键环节,维持肠粘膜屏障功能和结构的完整性是控制疾病进展的重要治疗措施[23]。通透性实验表明,PCB2组小鼠对FITC-葡聚糖通透性显著降低,且肠系膜淋巴结细菌移位比率亦显著降低,说明PCB2干预能够降低TNBS小鼠肠粘膜通透性及细菌易位。究其原因,从免疫荧光及免疫印迹分析法结果看,PCB2干预对减少TNBS小鼠肠粘膜紧密连接蛋白claudin-1及ZO-1的分布缺失和异位发挥了保护性作用,这一定程度上可以解释PCB2组通透性较差的原因[24]。保护肠黏膜屏障是治疗CD的重要途径之一,例如临床上使用的肠内营养[25-26]、谷氨酰胺[27-28]等制剂的作用途径均包含保护患者肠黏膜屏障功能和结构。既往研究证明,PI3K/AKT信号通路是是炎症调控的关键通路,在维持慢性炎症反应状态和诱发急性炎症发作的过程中发挥促进作用[6, 29]。本研究采用免疫印迹法分析了PCB2干预对小鼠肠粘膜PI3K/AKT通路的影响,结果显示PCB2干预能够显著抑制PI3K及AKT的磷酸化激活。这一结果导致包括IL-1β和TNF-α在内的促炎介质释放的减少,而IL-1β和TNF-α被证实可参与肠粘膜紧密连接蛋白的破坏,这可以部分解释PCB2对肠屏障功能和结构的保护机制。
本文研究结果具有一定潜在临床应用价值。CD是一类病因未明的透壁性肠道非特异性炎症反应性疾病,虽病变部位常见于末端回肠和附近结肠,却可呈节段性分布于全消化道,常反复发作难以彻底治愈[30]。CD患者在漫长的病程中常需外科手术切除病变严重肠管和清理腹腔脓肿[31-32]。因疾病反复发作,往往需要多次手术治疗[32]。因此,手术只是权宜之计,真正行之有效的治疗方法为药物对炎症的有效控制,维持疾病早期状态,避免严重并发症的发生[33]。现有针对CD肠道免疫/炎症系统的治疗药物多存在不足,例如水杨酸类、免疫抑制剂及生物制剂具有一定疗效,但仍然面临治疗效果不稳定、毒副作用大及药物抵抗等问题[34-35]。因此,急需研发治疗效果更佳及毒副反应较小的新型药物。PCB2是一种具有抗炎、抗氧化及免疫调节作用的植物提取单体,本研究发现其对CD动物模型具有治疗作用,提示其开发应用前景[36-37]。虽然我们并没有发现有采用PCB2治疗CD的先前报道,但重要提取物如雷公藤多苷等已然在国内应用于临床治疗,这提示重要提取物的潜在临床应用价值[38]。
本研究存在以下不足:我们虽然通过组织学和分子生物学等手段证实了PCB2对CD样肠炎具有治疗作用,且其作用机制可能是通过保护肠黏膜屏障,但PCB2还可能通过其他作用途径保护CD样肠炎;在信号通路分析中,我们发现PCB2抑制了经典炎症信号通路PI3K/AKT,但PCB2还可能通过影响其他信号通路发挥保护肠炎的效果。
综上,原花青素B2治疗能够抑制TNBS模型小鼠肠道炎症和保护肠粘膜细胞屏障功能和结构,这可能与抑制PI3K/AKT信号通路有关。
| [1] |
Torres J, Mehandru S, Colombel JF, et al. Crohn's disease[J]. Lancet, 2017, 389(10080): 1741-55. DOI:10.1016/S0140-6736(16)31711-1 |
| [2] |
钱家鸣, 杨红. 中国炎症性肠病研究的历史回顾、现状和展望[J]. 中国实用内科杂志, 2015, 36(9): 727-30. |
| [3] |
Heron V, Afif W. Update on therapeutic drug monitoring in crohn's disease[J]. Gastroenterol Clin North Am, 2017, 46(3): 645-59. DOI:10.1016/j.gtc.2017.05.014 |
| [4] |
Li W, Jiang Y, Sun T, et al. Supplementation of procyanidins B2 attenuates photooxidation-induced apoptosis in ARPE-19 cells[J]. Int J Food Sci Nutr, 2016, 67(6): 650-9. DOI:10.1080/09637486.2016.1189886 |
| [5] |
Stanger MC, Steffens CA, Soethe C, et al. Phenolic content and antioxidant activity during the development of 'brookfield' and 'mishima' apples[J]. Food Chem, 2017, 65(17): 3453-9. DOI:10.1021/acs.jafc.6b04695 |
| [6] |
Tokuhira N, Kitagishi Y, Suzuki M, et al. PI3K/AKT/PTEN pathway as a target for crohn's disease therapy (Review)[J]. Int J Mol Med, 2015, 35(1): 10-6. DOI:10.3892/ijmm.2014.1981 |
| [7] |
Byun EB, Ishikawa T, Suyama A, et al. A procyanidin trimer, C1, promotes NO production in rat aortic endothelial cells via both hyperpolarization and PI3K/Akt pathways[J]. Eur J Pharmacol, 2012, 692(1/3): 52-60. |
| [8] |
Holmberg FEO, Pedersen J, Jørgensen P, et al. Intestinal barrier integrity and inflammatory bowel disease: stem cell-based approaches to regenerate the barrier[J]. J Tissue Eng Regen Med, 2018, 12(4): 923-35. DOI:10.1002/term.v12.4 |
| [9] |
Filipescu IE, Leonardi L, Menchetti L, et al. Preventive effects of bovine colostrum supplementation in TNBS-induced colitis in mice[J]. PLoS One, 2018, 13(8): 2029-41. |
| [10] |
Xiao Y, Dong J, Yin Z, et al. Procyanidin B2 protects against dgalactose-induced mimetic aging in mice: metabolites and microbiome analysis[J]. Food Chem Toxicol, 2018, 119(2): 141-9. |
| [11] |
Shin JS, Cho EJ, Choi H, et al. Anti-inflammatory effect of a standardized triterpenoid-rich fraction isolated from rubus coreanus on dextran sodium sulfate-induced acute colitis in mice and LPSinduced macrophages[J]. J Ethnopharmacol, 2014, 158(6): 291-300. |
| [12] |
Spencer DM, Veldman GM, Banerjee S, et al. Distinct inflammatory mechanisms mediate early versus late colitis in mice[J]. Gastroenterology, 2002, 122(1): 94-105. DOI:10.1053/gast.2002.30308 |
| [13] |
Zuo L, Li Y, Wang H, et al. Cigarette smoking is associated with intestinal barrier dysfunction in the small intestine but not in the large intestine of mice[J]. J Crohns Colitis, 2014, 8(12): 1710-22. DOI:10.1016/j.crohns.2014.08.008 |
| [14] |
Wang H, Zhang W, Zuo L, et al. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury[J]. Br J Nutr, 2013, 109(11): 1990-8. DOI:10.1017/S0007114512004308 |
| [15] |
Xi J, Ge S, Zuo L, et al. Protective role of green tea polyphenols in intestinal mucosal barrier function of mice with colitis induced by TNBS through inhibiting JAK2/STAT3 pathway[J]. Chin J Cell Mol Imm, 2018, 34(3): 237-41. |
| [16] |
Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: an overview[J]. Ann Med Surg, 2016, 11(3): 9-15. |
| [17] |
Chun CL, Xu Yu, et al. Recombinant expressed vasoactive intestinal peptide analogue ameliorates TNBS-induced colitis in rats[J]. World J Gastroenterol, 2018, 24(6): 706-15. DOI:10.3748/wjg.v24.i6.706 |
| [18] |
Bassolas-Molina H, Raymond E, Labadia M, et al. An RORγt oral inhibitor modulates IL-17 responses in peripheral blood and intestinal mucosa of crohn's disease patients[J]. Front Immunol, 2018, 9(11): 2307-19. |
| [19] |
Bourgonje AR, von Martels JZH, de Vos P, et al. Increased fecal calprotectin levels in crohn's disease correlate with elevated serum Th1- and Th17-associated cytokines[J]. PLoS One, 2018, 13(2): e0193202-15. DOI:10.1371/journal.pone.0193202 |
| [20] |
Savić Mlakar A, Hojsak I, Jergović M, et al. Pediatric crohn disease is characterized by Th1 in the terminal ileum and Th1/Th17 immune response in the colon[J]. Eur J Pediatr, 2018, 177(4): 611-6. DOI:10.1007/s00431-017-3076-8 |
| [21] |
Park JJ, Boutillier L, Cruz JE, et al. Effect of standardized infliximab dose rounding on an outpatient infusion center[J]. J Manag Care Spec Pharm, 2018, 24(10): 1028-33. |
| [22] |
Langford T, Arkir Z, Chalkidou A, et al. The clinical and costeffectiveness of 4 enzyme-linked immunosorbent assay kits for monitoring infliximab in crohn disease patients: protocol for a validation study[J]. JMIR Res Protoc, 2018, 7(10): e11218-29. DOI:10.2196/11218 |
| [23] |
Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases[J]. Inflamm Bowel Dis, 2011, 17(1): 362-81. DOI:10.1002/ibd.21403 |
| [24] |
Landy J, Ronde E, English N, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer[J]. World J Gastroenterol, 2016, 22(11): 3117-26. DOI:10.3748/wjg.v22.i11.3117 |
| [25] |
Levine A. Exclusive enteral nutrition: clues to the pathogenesis of crohn's disease[J]. Nestle Nutr Inst Workshop Ser, 2014, 79(2): 131-40. |
| [26] |
Wall CL, Gearry RB, Day AS. Treatment of active crohn's disease with exclusive and partial enteral nutrition: a pilot study in adults[J]. Inflamm Intest Dis, 2018, 2(4): 219-27. |
| [27] |
Akobeng AK, Elawad M, Gordon M. Glutamine for induction of remission in crohn's disease[J]. Cochrane Database Syst Rev, 2016, 2(9): CD007348-60. |
| [28] |
Yamamoto T, Shimoyama T, Kuriyama M. Dietary and enteral interventions for crohn's disease[J]. Curr Opin Biotechnol, 2017, 44(1): 69-73. |
| [29] |
Huang XL, Xu J, Zhang XH, et al. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis[J]. Inflamm Res, 2011, 60(8): 727-34. DOI:10.1007/s00011-011-0325-6 |
| [30] |
朱振浩, 邱琛, 张明, 等. 英夫利西单抗治疗的小肠克罗恩病患者不同肠段黏膜愈合情况分析[J]. 南方医科大学学报, 2017, 37(1): 44-8. |
| [31] |
郭振, 朱维铭. 克罗恩病并发症内外科治疗的整合[J]. 中国实用内科杂志, 2018, 36(3): 180-3. |
| [32] |
朱维铭. 克罗恩病并发症外科治疗的基本原则及目标[J]. 中华消化外科杂志, 2016, 15(12): 1146-9. DOI:10.3760/cma.j.issn.1673-9752.2016.12.003 |
| [33] |
朱秀丽, 王巧民. 克罗恩病治疗的新目标:深度缓解[J]. 胃肠病学和肝病学杂志, 2016, 25(10): 1094-7. |
| [34] |
Wright EK, Ding NS, Niewiadomski O. Management of inflammatory bowel disease[J]. Med J Aust, 2018, 209(7): 318-23. DOI:10.5694/mja2.2018.209.issue-7 |
| [35] |
Stein J, Aksan A, Farrag K, et al. Management of inflammatory bowel disease-related anemia and iron deficiency with specific reference to the role of intravenous iron in current practice[J]. Expert Opin Pharmacother, 2017, 18(16): 1721-37. DOI:10.1080/14656566.2017.1391790 |
| [36] |
Martinez-Micaelo N, González-Abuín N, Pinent M, et al. Procyanidin B2 inhibits inflammasome-mediated IL-1β production in lipopolysaccharide-stimulated macrophages[J]. Mol Nutr Food Res, 2015, 59(2): 262-9. DOI:10.1002/mnfr.201400370 |
| [37] |
Mendoza-Wilson AM, Castro-Arredondo SI, Espinosa-Plascencia A, et al. Chemical composition and antioxidant-prooxidant potential of a polyphenolic extract and a proanthocyanidin-rich fraction of apple skin[J]. Heliyon, 2016, 2(2): e00073-82. DOI:10.1016/j.heliyon.2016.e00073 |
| [38] |
陶庆松, 任建安, 嵇振岭, 等. 雷公藤多甙在维持术后克罗恩病临床缓解中的作用[J]. 中华胃肠外科杂志, 2009, 12(5): 491-3. DOI:10.3760/cma.j.issn.1671-0274.2009.05.022 |
 2019, Vol. 39
2019, Vol. 39
