2. 沈阳市第一人民医院,辽宁 沈阳 110041;
3. 辽宁中医药大学,辽宁 沈阳 110847
2. Department of Neurology, Shenyang First People's Hospital, Shenyang 110041, China;
3. Liaoning University of Traditional Chinese Medicine, Shenyang City, Liaoning Province, 110847
绝经后骨质疏松症(PMOP)影响数百万妇女,雌激素缺乏是导致PMOP发病的关键因素[1]。PMOP的治疗旨在保持骨骼健康,降低骨折风险。临床上,这些药物大多是抗破骨细胞吸收剂,即抑制破骨细胞骨吸收的药物[2]。在PMOP的发病过程中,破骨细胞的凋亡受到抑制是其重要的发病原因[3]。因此,选择能够调节破骨细胞凋亡的药物将是防治PMOP的重要方法[4-6]。
他汀类药物是常用降脂药物[7],其中辛伐他汀是最为经典的代表药物,广泛应用于临床治疗高血脂症。近年来研究发现,辛伐他汀除了降脂作用外,其具有抑制破骨细胞分化、防治PMOP的药效学作用,但对破骨细胞凋亡的作用目前还没有研究[8-9]。本研旨在阐明辛伐他汀对破骨细胞凋亡的影响,并探索其潜在的作用机制,为临床防治PMOP提供新思路及实验依据。
1 材料和方法 1.1 材料 1.1.1 细胞小鼠单核/巨噬细胞RAW264.7细胞(ATCC)。
1.1.2 试剂辛伐他汀(Simvastatin,SIM,Sigma);sRANKL(Peprotech);M- CSF(Peprotech);DMEM(Hychone);进口胎牛血清(Hychone);WST-1试剂盒(碧云天);TRAP染色试剂盒(Sigma);NFATc1一抗(Cell signaling Technology);VIVIT peptide (NFAT抑制剂,MCE);细胞凋亡检测试剂盒(碧云天)。
1.1.3 仪器细胞孵箱(Thermo);倒置相差显微镜(Olympus);免疫荧光显微镜(Olympus);流式细胞仪(BD);AMR-100酶标仪(杭州奥盛仪器),流式细胞仪(FACS CALBURC)。
1.2 实验方法 1.2.1 小鼠单核/巨噬细胞RAW 264.7培养及诱导分化小鼠单核/巨噬细胞RAW 264.7,在DMEM培养基(含10%胎牛血清,不含抗生素,37 ℃,5%CO2孵箱内培养)。细胞接种于10 cm2培养板内,加入诱导分化培养基(DMEM,含10%胎牛血清、15 ng/mL sRANKL+MCSF)继续培养7 d。
1.2.2 WST-1检测细胞增殖活性分组:对照组、10-5~10-9 mol/L的SIM组。细胞平铺(5×103/孔)96孔平板中,细胞经SIM处理3、12、24、48 h后,更换诱导分化培养基,诱导培养基培养7 d,每孔加20 μL WST-1检测剂。在37 ℃,5% CO2的细胞培养箱中孵育1 h。450 nm读取吸光度值。A450 nm=(处理后细胞A值-对照组细胞A值/N),n>3。
1.2.3 流式细胞仪检细胞周期及凋亡细胞周期检测:细胞分A组(Control组)、B组(sRANKL+M-CSF组)、C组(SIM+sRANKL+M-CSF组)、D组(VIVIT peptide(10-4 mol/L)+sRANKL+M-CSF组)、E组(SIM+VIVIT peptide+sRANKL+M-CSF组)。将264.7细胞培养在每孔1×105细胞的6孔培养皿中,每孔加入诱导分化培养基(15 ng/mL的sRANKL+M-SCF),或不含SIM(10-6 mol/L)诱导分化7 d。在4 ℃下用冰冻70%乙醇固定细胞,在37 ℃下与检测溶液孵育30 min后进行细胞周期检测。
细胞凋亡检测:根据试剂盒的实验步骤进行,在上述实验的基础上,收集细胞,加入FITC-Annexin V孵育30 min后DAPI核染色5 min,然后用FACS CALBURC流式细胞仪进行细胞周期分析,结果采用FCS软件进行处理。
1.2.4 免疫荧光检测NFATc1核转位分组:A组(Control组)、B组(sRANKL+M-CSF组)、C组(SIM+sRANKL+M-CSF组)、D组(VIVIT peptide+sRANKL+M-CSF组)。细胞在盖玻片上并培养过夜,在含和不含SIM(10-6 mol/L)的条件下诱导分化培养基诱导分化7 d。细胞固定后,用NFATc1抗体孵育细胞(以1:400的比例稀释)过夜,用DAPI(5 min)染色细胞核,免疫荧光显微镜检测。
1.2.5 Western blot检测细胞分sRANKL + M- CSF、SIM+sRANKL+M-CSF组。细胞加入裂解液提取蛋白,BCA法定量蛋白。取20 μg蛋白样品,于10%聚丙烯酰胺凝胶上行SDS-PAGE电泳,电泳结束后100 V电转印至PVDF膜上。37 ℃封闭1 h、一抗4 ℃孵育过夜。辣根过氧化物酶(HRP)标记的稀释二抗孵育,ECL发光法曝光成像,扫描入电脑,n>3。Image J软件测定平均灰度值。
1.2.6 统计学方法采用SPSS13.0软件进行统计分析,组间比较采用单因素方差分析,数据以均数±标准差表示,P<0.05为差异有统计学意义。
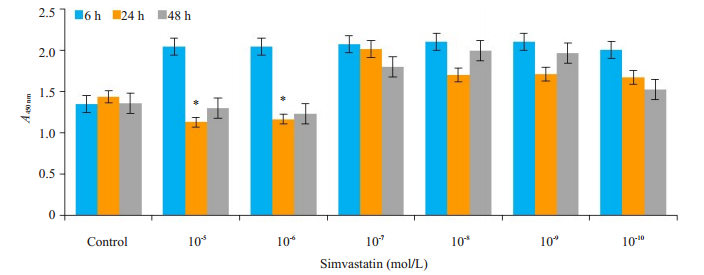
2 结果 2.1 SIM抑制RAW264.7细胞增殖活性WST-1方法对RAW264.7细胞增殖活性进行检测(图 1),与对照组相比较,SIM(10-5、10-6 mol/L)均能抑制破骨细胞的增殖活性(P=0.039、0.022,P<0.05),SIM(10-6 mol/L)作用24、48 h具有稳定抑制RAW264.7细胞的增殖活性。因此SIM(10-6 mol/L)作用24 h作为实验用药浓度及作用时间。
|
图 1 SIM抑制破骨细胞增殖 Fig.1 SIM inhibits proliferation of osteoclast. *P < 0.05 vs control group. |
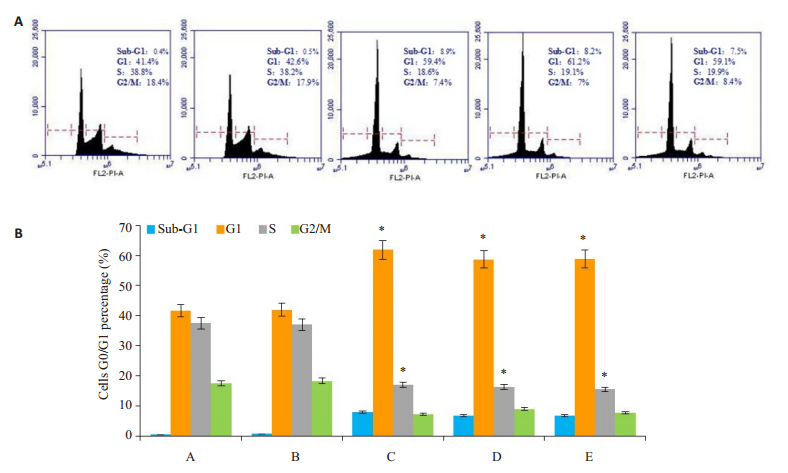
流式细胞术分析了细胞周期的变化,结果显示,C组G0/G1期细胞百分率显著增加至(59.4 ± 1.21)%(P= 0.041,P<0.05),A组为(41.4 ± 1.55)%,B组为(42.6 ± 2.14)%,D组为(34.6±1.14)%,E组为(34.2±1.12)%。同时,C组S期细胞百分率较对照组下降(18.6±1.11)%,A组为(38.8±2.21)%,B组为(38.82±0.81)%,D为(19.1± 1.04)%,E组为(19.9±1.16)%。同时,观察到SIM诱导的破骨细胞凋亡,C组的亚G1期比对照组增加了(8.9± 2.21)%(P=0.028,P<0.05),A组为(0.4±0.03)%,B组为(0.5±0.01)%,D为(8.2±0.14)%组,E组为(7.5±1.14)%(图 2A、B)。
|
图 2 SIM诱导破骨细胞G0/G1期抑制及凋亡 Fig.2 SIM induces inhibition of G0/G1- phase cells and promotes apoptosis in the osteoclasts. Group A: Control group; Group B: sRANKL+M-CSF; Group C: SIM+sRANKL+M-CSF; Group D: VIVIT+sRANKL+M-CSF; Group E: SIM+VIVIT+sRANKL+M-CSF. *P < 0.05 vs control group. |
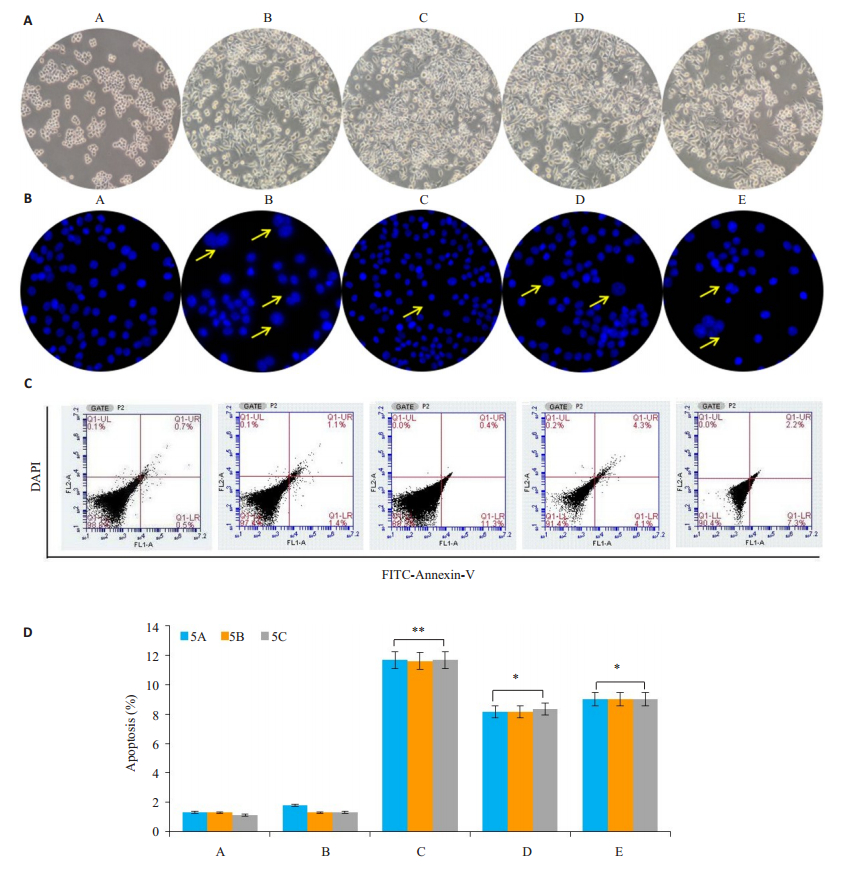
诱导分化培养基诱导细胞融合(图 3A),最后形成巨大的多核破骨细胞(核≥3个)。对照组的破骨细胞呈圆形,透光度好,边界清晰,贴于培养瓶底部。加入SIM后,细胞透光度下降,凋亡细胞皱缩,漂浮于培养基中。
|
图 3 SIM诱导破骨细胞凋亡 Fig.3 SIM induces apoptosis of the osteoclasts. Group A: Control group; Group B: sRANKL+M-CSF; Group C: SIM+sRANKL+M-CSF; Group D: VIVIT+sRANKL+M-CSF; Group E: SIM+VIVIT+sRANKL+M-CSF. *P < 0.05, **P < 0.01 vs control group. |
在细胞周期检测细胞凋亡的基础上,我们应用DAPI染色及流式细胞仪进一步检测了SIM破骨细胞的凋亡的影响。DAPI染色显示SIM诱导破骨细胞的核凝聚和碎裂(图 3B),流式细胞仪检测结果显示SIM促进破骨细胞凋亡(图 3C、D),A组细胞的凋亡率为(1.2± 0.05)%,B组细胞的凋亡率为(2.4±0.05)%,加入SIM(C组)后破骨细胞的凋亡增加达(11.5±0.15)%(P=0.002,P<0.01),单独加入NFATc1抑制剂VIVIT peptide(D组)后对破骨细胞的(8.4±0.04)%(P=0.015,P<0.05),E组破骨细胞凋亡至(9.5±0.16)%(P=0.0018,P<0.05)。
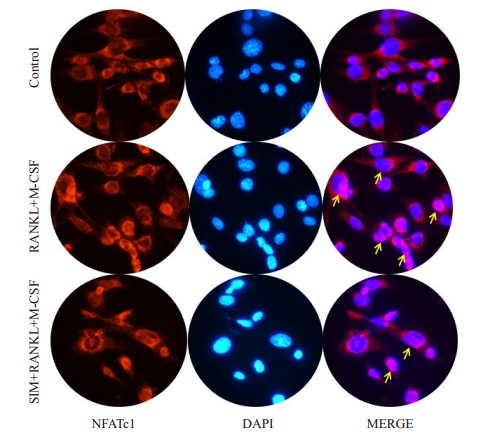
2.5 SIM抑制NFATc1细胞核转位细胞在含或不含SIM的sRANKL+M-CSF诱导分化培养基诱导7 d内,免疫细胞化学染色分析NFATc1从线粒体向细胞核的移位(图 4)。为了评估SIM在线粒体NFATc1释放和随后的核易位中的具体作用,我们分析了NFATc1易位到细胞核的状态,在存在或不存在SIM的情况下。破骨细胞分化后,细胞核周围线粒体聚集随后迅速释放NFATc1进入细胞核。加入SIM后NFATc1的易位受到抑制。
|
图 4 SIM抑制NFATc1细胞核转位 Fig.4 SIM inhibits NFATc1 nuclear transposition in the osteoclasts. |
进一步分析SIM抑制成骨细胞分化的潜在机制(图 5A、B),免疫印迹检测通路蛋白显示,sRANKL+M- CSF促进细胞NFATc1的磷酸化并向细胞核转位,加入SIM后NFATc1的细胞核内转位受到抑制(P=0.013,P<0.05)。
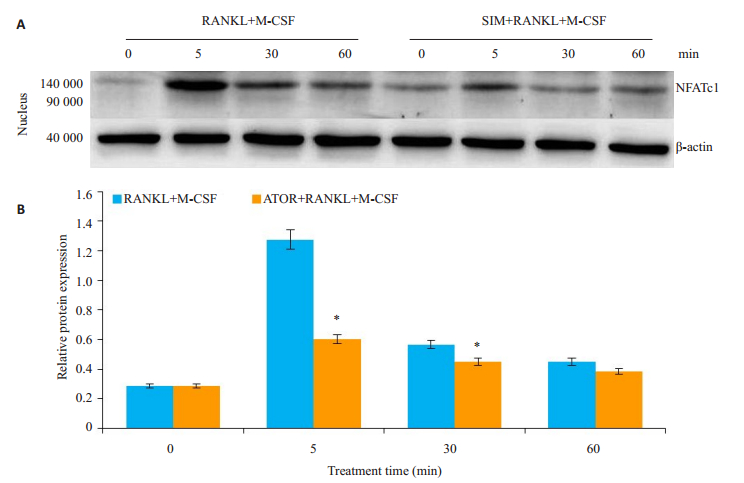
|
图 5 SIM抑制NFATc1磷酸化 Fig.5 SIM inhibits phosphorylation of NFATc1 in the osteoclasts. *P < 0.05 vs sRANKL+M-CSF group. |
PMOP是最常见的原发性骨质疏松症,雌激素缺乏是其发病的主要原因[10]。雌激素降低增加破骨细胞吸收活性而不相应增加成骨细胞的成骨活性,导致骨的吸收量大于骨的沉积,致骨量降低。此外,雌激素抑制破骨细胞的形成,促进破骨细胞的凋亡[1]。因此,在PMOP的发病过程中,破骨细胞的凋亡受到抑制是其重要的发病原因[3]。因此,促进破骨细胞的凋亡是防治PMOP的重要方法[4]。
研究表明,他汀类药物具有增加骨形成[11-12]、抑制破骨细胞形成[13]的药效学作用,但对破骨细胞凋亡的影响还不清楚[8]。因此,本研究的重点在于检测SIM对破骨细胞凋亡的影响。我们的研究结果表明,RAW264.7细胞在诱导分为破骨细胞的过程中,经SIM处理后,破骨细胞的增殖活性受到抑制,SIM(10-6 mol/L)作用24、48 h明显的抑制RAW264.7细胞的增殖活性。为了进一步检测RAW264.7细胞的增殖受到抑制的机制,我们应用流式细胞检测SIM对细胞周期的影响,结果显示,应用SIM后细胞停滞在G0/G1期、同时破骨细胞在Sub-G1期的细胞数量明显增加,加入NFATc1通路抑制VIVIT peptide后[14-15],VIVIT peptide所致的细胞周期抑制、Sub-G1期的细胞数量与SIM作用相似。因此,我们认为SIM能够诱导破骨细胞凋亡[16-17],并通过NFATc1通路进行调节的[18-19]。
倒置相差显微镜、细胞核DAPI染色、流式细胞仪进一步检测SIM对细胞凋亡的影响。光学显微镜下,SIM诱导成熟的破骨细胞死亡、漂浮于培养基中。DAPI染色显示SIM诱导破骨细胞的核凝聚和碎裂,流氏细胞仪显示SIM促进成熟破骨细胞凋亡,单独或与SIM共同加入NFATc1抑制剂VIVIT peptide后亦可促进破骨细胞的凋亡,结果与单独加入SIM相似,说明SIM发挥这类似NFATc1通路抑制VIVIT peptide的药效学作用[20-21]。
免疫荧光染色及Western blot进一步显示了,SIM对NFATc1细胞核内转位的抑制。RAW264.7细胞未向破骨细胞分化时,NFATc1分布在细胞核周围,加入破骨细胞分化诱导剂后,NFATc1向细胞核内转位[22-24]。
活化的T细胞C1(NFATc1)的核因子是NFAT转录因子家族的成员[25-26]。NFATc1是由钙信号调节,是NFAT家族的经典成员[27]。NFATc1被称为破骨细胞形成和功能的主调节器[29-30]。当NFATc1被激活时,其转位到细胞核并调节转录。我们的研究结果显示,在RAW264.7细胞内NFATc1仅有微量的表达,当细胞向破骨细胞分化时,NFATc1的表达明显增加,SIM下调破NFATc1表达,结合流式细胞仪、免疫荧光染色检测结果,我们有理由相信SIM抑制NFATc1的磷酸化及核内转移,进而促进破骨细胞的凋亡。
目前研究骨质疏松症的发病机制虽然较多,但是基于NFATc1信号通路,从破骨细胞凋亡作为研究的切入点,探索SIM对破骨细胞凋亡的影响及机制的相关研究较少。因此,本研究对于临床应用SIM防治PMOP具有一定的意义。
| [1] |
Tella Sh GJ. Treatment of postmenopausal osteoporosis[J]. J steroid biochem Mol Biol, 2014, 142: 155-70. DOI:10.1016/j.jsbmb.2013.09.008 |
| [2] |
Gambacciani M, Levancini M. Management of postmenopausal osteoporosis and the prevention of fractures[J]. Panminerva Med, 2014, 56(2): 115-31. |
| [3] |
Chen FP, Fu TS, Yc L, et al. Risk factors and quality of Life for the occurrence of hip fracture in postmenopausal women[J]. Biomed J, 2018, 41(3): 202-8. |
| [4] |
Kamiński A, Bogacz A, Uzar I, et al. Association betweenGREM2 gene polymorphism with osteoporosis and osteopenia in postmenopausalwomen[J]. Eur J Obstet Gynecol Reprod Biol, 2018, 228: 238-42. DOI:10.1016/j.ejogrb.2018.07.009 |
| [5] |
Ma W, Dou Q, Ha X. Let-7a-5p inhibits BMSCs osteogenesis in postmenopausal osteoporosis mice[J]. Biochem Biophys Res Commun, 2019, 510(1): 53-8. DOI:10.1016/j.bbrc.2019.01.003 |
| [6] |
Eriksen EF, Halse J, Moen MH. New developments in the treatment of osteoporosis[J]. Acta Obstet Gynecol Scand, 2013, 92(6): 620-36. DOI:10.1111/aogs.2013.92.issue-6 |
| [7] |
Moshiri A, Shahrezaee M, Shekarchi B, et al. Three-Dimensional porous Gelapin-Simvastatin scaffolds promoted bone defect healing in rabbits[J]. Calcif Tissue Int, 2015, 96(6): 552-64. DOI:10.1007/s00223-015-9981-9 |
| [8] |
Zhang P, Han F, Li YX, et al. Local delivery of controlled-release simvastatin to improve the biocompatibility of polyethylene terephthalate artificial ligaments for Reconstruction of the anterior cruciate ligament[J]. Int J Nanomedicine, 2016, 11(1): 465-78. |
| [9] |
Zhu S, He H, Zhang C, et al. Effects of pulsed electromagnetic fields on postmenopausal osteoporosis[J]. Bioelectromagnetics, 2017, 38(6): 406-24. |
| [10] |
Wilches-Buitrago L, Viacava PR, Cunha FQ, et al. Fructose 1, 6-bisphosphate inhibits osteoclastogenesis by attenuating RANKLinduced NF-κB/NFATc-1[J]. Inflamm Res, 2019, 68(5): 415-21. |
| [11] |
Shifang Z, Jue S, Fuming H, et al. Design and in vitro evaluation of simvastatin-hydroxyapatite coatings by an electrochemical process on Titanium surfaces[J]. J Biomed Nanotechnol, 2014, 10(7): 1313-9. DOI:10.1166/jbn.2014.1859 |
| [12] |
Lorenzo J. The many ways of osteoclast activation[J]. J Clin Invest, 2017, 127(7): 2530-2. DOI:10.1172/JCI94606 |
| [13] |
Yan Q, Xiao LQ, Tan L, et al. Controlled release of simvastatinloaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: in vitro and in vivo characteristics[J]. J Biomed Mater Res A, 2015, 103(11): 3580-9. DOI:10.1002/jbm.v103.11 |
| [14] |
Miao W, Gao H, Hou X. Magnesium lithospermate B inhibits Titanium particles-induced osteoclast formation by c-fos and inhibiting NFATc1 expression[J]. Connect Tissue Res, 2019, 25: 1-8. |
| [15] |
Sakamoto M, Fukunaga T, Sasaki K, et al. Ibration enhances osteoclastogenesis by inducing RANKL expression via NF-κB signaling in osteocytes[J]. Bone, 2019, 20(123): 56-66. |
| [16] |
Liang X, Hou Z, Xie Y, et al. Icariin promotes osteogenic differentiation of bone marrow stromal cells and prevents bone loss in OVX mice via activating autophagy[J]. J Cell Biochem, 2019. DOI:10.1002/jcb.28585 |
| [17] |
Han SY, Kim YK. Berberine suppresses RANKL-Induced osteoclast differentiation by inhibiting c-Fos and NFATc1 expression[J]. Am J Chin Med, 2019, 47(2): 439-55. |
| [18] |
Zhuang JL, Li X, Zhang Y, et al. Sema6A-plexin-A2 axis stimulates RANKL-induced osteoclastogenesis through PLC gamma-mediated NFATc1 activation[J]. Life Sci, 2019, 222(222): 29-35. |
| [19] |
Ma JD, Jing J, Wang JW, et al. Activation of the PGC-1β/NFATc-1 pathway in circulating osteoclast precursors associated with bone destruction in rheumatoid arthritis[J]. Arthritis Rheumatol, 2019. DOI:10.1002/art.40868 |
| [20] |
Moon HJ, Kim SE, Yun YP, et al. Simvastatin inhibits osteoclast differentiation by scavenging reactive Oxygen species[J]. Exp Mol Med, 2011, 43(11): 605-12. DOI:10.3858/emm.2011.43.11.067 |
| [21] |
Yamashita M, Otsuka F, Mukai T, et al. Simvastatin inhibits osteoclast differentiation induced by bone morphogenetic protein-2 and RANKL through regulating MAPK, AKT and Src signaling[J]. Regul Pept, 2010, 162(1/3): 99-108. |
| [22] |
Wang L, Liu YY, Zhou YE, et al. Zoledronic acid inhibits the growth of Cancer stem cell derived from cervical Cancer cell by attenuating their stemness phenotype and inducing apoptosis and cell cycle arrest through the Erk1/2 and Akt pathways[J]. J Exp Clin Cancer Res, 2019, 38(1): 93. DOI:10.1186/s13046-019-1109-z |
| [23] |
Bae SJ, Shin MW, Son T, et al. Ninjurin1 positively regulates osteoclast development by enhancing the survival of prefusion osteoclasts[J]. Exp Mol Med, 2019, 51(1): 7. |
| [24] |
Xue Y, Liang ZE, Fu XM, et al. IL-17A modulates osteoclast precursors' apoptosis through autophagy-TRAF3 signaling during osteoclastogenesis[J]. Biochem Biophys Res Commun, 2019, 508(4): 1088-92. DOI:10.1016/j.bbrc.2018.12.029 |
| [25] |
Wang S, Liu Z, Wang J, et al. The triptolide-induced apoptosis of osteoclast precursor by degradation of cIAP2 and treatment of rheumatoid arthritis of TNF-transgenic mice[J]. Phytother Res, 2019, 33(2): 342-9. |
| [26] |
Pereverzev A, Komarova SV, Korcok JA, et al. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway[J]. Bone, 2008, 42(1): 150-61. DOI:10.1016/j.bone.2007.08.044 |
| [27] |
Weider M, Starost LJ, Groll KA, et al. Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning[J]. Nat Commun, 2018, 9(1): 899. |
| [28] |
Matsuike R, Tanaka H, Nakai K, et al. Continuous application of compressive force induces fusion of osteoclast-like RAW264.7 cells via upregulation of RANK and downregulation of LGR4[J]. Life Sci, 2018, 15: 30-6. |
| [29] |
Peng Q, Luo A, Zhou Z, et al. Interleukin 29 inhibits RANKLinduced osteoclastogenesis via activation of JNK and STAT, and inhibition of NF-κB and NFATc1[J]. Cytokine, 2018(18): 30282-5. |
| [30] |
Kim HJ, Park C, Kim GY, et al. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-κB and activation of the Nrf2/HO-1 signaling pathway[J]. Biosci Trends, 2018, 12(3): 257-65. DOI:10.5582/bst.2018.01107 |
 2019, Vol. 39
2019, Vol. 39

