2. Laboratory of Molecular Medicine, Hubei University of Arts and Science, Xiangyang 441053, China
2. 湖北文理学院分子医学实验室,湖北 襄阳 441053
Rheumatoid arthritis (RA) is an autoimmune disease characterized by activation of T lymphocytes, synovium inflammation and bone erosion [1]. Although the pathogenesis of RA is unclear, the abnormal proliferation and apoptosis of auto-reactive effector T lymphocytes is thought to participate in the pathological process [2, 3], where the activated T cells appear to be dysfunctional with continuous proliferation and suppressed cell death[4]. Pro-inflammatory cytokines secreted by activated T lymphocytes also contribute to the pathological process of RA [5]. Activation-induced cell death (AICD), a form of apoptosis or programmed cell death, may help to selectively eliminate activated auto-reactive effector T cells and inhibit inflammatory responses that trigger the clinical symptoms of RA [6-8].
Programmed cell death 5 (PDCD5) is initially identified as an apoptosis-related gene cloned from TF-1 cells [9] and promotes cell apoptosis in response to various stimuli [10-12]. Growing numbers of researchers have focused their attention on the immune-modulating effect of PDCD5[13-15]. As an apoptosis-related protein, recombinant human PDCD5 (rhPDCD5) induces apoptosis of MOG-specific CD4 + T lymphocytes in experimental autoimmune encephalomyelitis mouse model by inducing activation of caspase-3 [16]. In a previous study we have shown that rhPDCD5 exerts anti-inflammatory effects in rats with collagen-induced arthritis (CIA) [17]. In this study, we aimed to further investigate the effects of rhPDCD5 on collagen-activated or anti-CD3- and anti-CD28-activated lymphocytes derived from the spleen of a rat model of CIA.
MATERIALS AND METHODS Reagents and antibodiesBovine type Ⅱ collagen (CII) and complete Freund's adjuvant (CFA) were purchased from Chondrex (Redmond, WA, USA). The monoclonal antibodies anti-CD3 and anti-CD28 were purchased from BD (San Diego, CA, USA). The enzyme-linked immunosorbent assay (ELISA) kits for cytokine detection were purchased from eBioscience (San Diego, CA, USA). Recombinant human tumor necrosis factor-α receptor IgG Fc fusion protein (rhTNFR:Fc) was obtained from Shanghai CP Guojian Pharmaceutical Co., Ltd (Shanghai, China). Recombinant human PDCD5 protein was supplied by Beijing Biosea Biotechnology Co., Ltd (Beijing, China). The endotoxin activity of the PDCD5 protein was < 10 EU/mg as detected using the limulus amebocyte lysate assay, and the purity of the rhPDCD5 protein was >95%.
Establishment and treatment of CIA modelsFemale Wistar rats (150 to 200 g) were obtained from the Department of Laboratory Animal Science of Peking University Health Science Center (Beijing, China). They were kept under pathogen-free conditions and had free access to food and water during the experiment. The experiments were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and in line with the Institutional Animal Care and Use Committee guidelines. The rats were randomly divided into 4 groups, namely the normal control group, CIA+ ovalbumin (OVA) group, CIA+rhTNFR: Fc group, and CIA+rhPDCD5 group. On day 0, the rats in all but the normal control groups were intradermally injected with 0.5 mL CFA emulsion containing 200 μg CII at the back and the base of the tail, followed by a booster injection 7 days later in the same manner. The normal control rats were injected with 0.5 mL sterilized physiological saline only. During the experiment, an optimal dose of rhPDCD5 (14 mg/kg) was injected intraperitoneally every other day from day 2 to day 26; as control, OVA (14 mg/kg) was injected in the same manner. RhTNFR: Fc (3.5 mg/kg, positive control) was injected every 3 days from day 2 to day 26.
Preparation of splenocytesThe spleens of the rats were harvested on day 28 after the first immunization and then pushed through a mesh to prepare the single cell suspensions. The cells were washed, transferred to red blood cell lysis buffer, centrifuged at 1200 r/min for 5 min, and counted.
Apoptosis assayThe Annexin V-FITC Apoptosis Detection Kit (Beijing Biosea Biotechnology Co., Ltd., Beijing, China) was used following the manufacturer's instructions. Briefly, the splenocytes from different rats were stimulated with CII (20 μg/mL) or with both anti-CD3 (1 μg/mL) and anti-CD28 (2 μg/mL). After 48 h and 72 h, the cells were collected and stained with anti-rat CD4-PE and Annexin V-FITC, and the percentages of CD4+ Annexin V+ double positive (CD4+AV+) cells were analyzed using the FACS Calibur flow cytometer.
Lymphocyte proliferation assayThe splenocytes collected on day 28 were seeded in triplicate in 96-well plates (5×105 cells/well) and stimulated with CII (20 μg/mL) or with anti-CD3 (1 μg/mL) + anti-CD28 (2 μg/mL) for 48 and 72 h. The cells were pulsed with 1 μCi/well [3H] thymidine (MP Biomedicals, USA) and incubated for an additional 8 h. The results were expressed as mean [3H] thymidine incorporation (cpm)±standard deviation (SD).
Detection of cytokinesThe splenocytes collected from each group were plated in triplicate wells in 96-well plates (5 × 105 cells/well) and stimulated with CII (20 μg/mL) or with anti-CD3 (1 μg/mL) + anti CD-28 CD3 (2 μg/mL). After 48 and 72 h, cytokine levels in the supernatants were detected using ELISA kits (eBioscience, San Diego, CA, USA).
Statistical analysisFor continuous variables, the data were presented as Mean±SD. The differences in cell frequency, proliferation assay and cytokine production among multiple groups were evaluated with ANOVA, and SNK-q test was used for pairwise comparison between the groups. A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS IFN-γ and IL-17A production by activated splenocytesIn a previous study we found that rhPDCD5 significantly reduced Th1 and Th17 percentages in the spleens of CIA rats[17]. IFN-γ and IL-17A are secreted mainly by Th1 and Th17, respectively. The results of ELISA showed that the concentrations of the cytokines differed significantly in the supernatants of activated splenocytes from the rats with different treatments. At both 48 and 72 h following stimulation with either CII or anti-CD3 + anti-CD28, the cells from CIA rats treated with rhPDCD5 and rhTNFR:Fc produced significantly less IFN-γ (Fig. 1A) and IL-17A (Fig. 1B) as compared with the cells from OVA-treated rats.
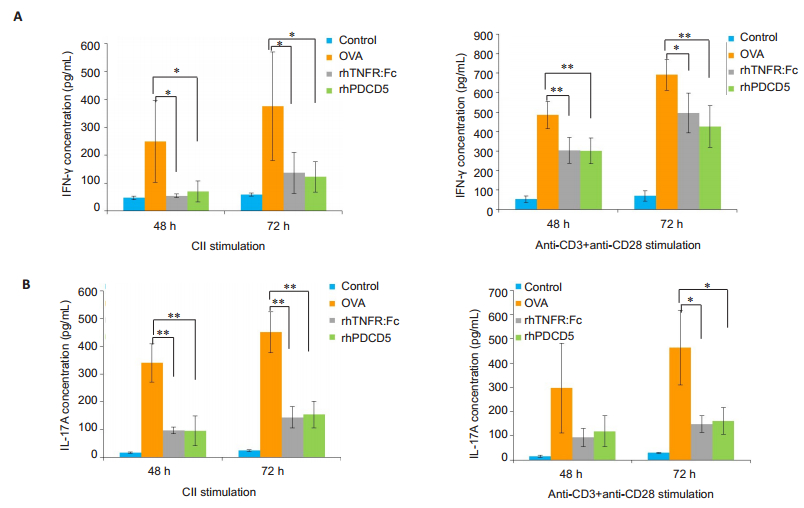
|
Fig.1 RhPDCD5 treatment of the rats inhibits secretion of IFN-γ and IL-17A by activated splenocytes. The splenocytes were obtained from the control and CIA rats on day 28 and cultured in the presence of CII or anti-CD3 + anti-CD28 for 48 h and 72 h. IFN-γ (A) and IL-17A (B) concentrations in the supernatants of the activated splenocytes were determined using ELISA, and the results are expressed as Mean±SD (*P < 0.05, **P < .005; n=6). |
The results of [3H]-thymidine incorporation assay showed that the proliferative activity differed significantly among the splenocytes from rats with different treatments. [3H]-thymidine uptake was significantly lower in the splenocytes from both rhPDCD5-treated and rhTNFR: Fc-treated CIA rats in response to stimulations with CII and anti-CD3+anti-CD28 (Fig. 2), indicating that treatment of the CIA rats with rhPDCD5 inhibits the in vitro proliferation of the activated splenocytes.
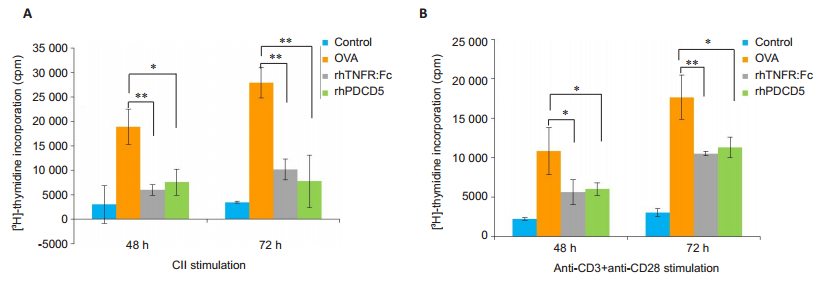
|
Fig.2 RhPDCD5 treatment of the rats suppresses in vitro proliferation of activated splenocytes. The proliferation of the activated splenocytes was quantified by [3H] thymidine uptake. The results are expressed as Mean±SD (*P < 0.05, **P < 0.005; n=6). |
Given the pro-apoptotic function of rhPDCD5, we next investigated AICD of CD4 + T cells using FITC-Annexin V staining and flow cytometry. We noted a significant increase in the frequency of CD4+AV+ cells in the spleens from rhPDCD5-treated CIA rats compared with OVA-treated rats (Fig. 3). Treatment of the CIA rats with rhTNFR: Fc also induced apoptosis of CII-activated CD4 + T cells (Fig. 3A), but this finding was not statistically significant, possibly due to experimental errors or the stronger apoptosis-inducing ability of PDCD5 than rhTNFR: Fc. The activated lymphocytes from rhPDCD5-treated CIA rats more easily underwent AICD, which may explain the reduced severity of CIA and decreased Th1/Th17 frequency in CIA rats receiving rhPDCD5treatment [17].
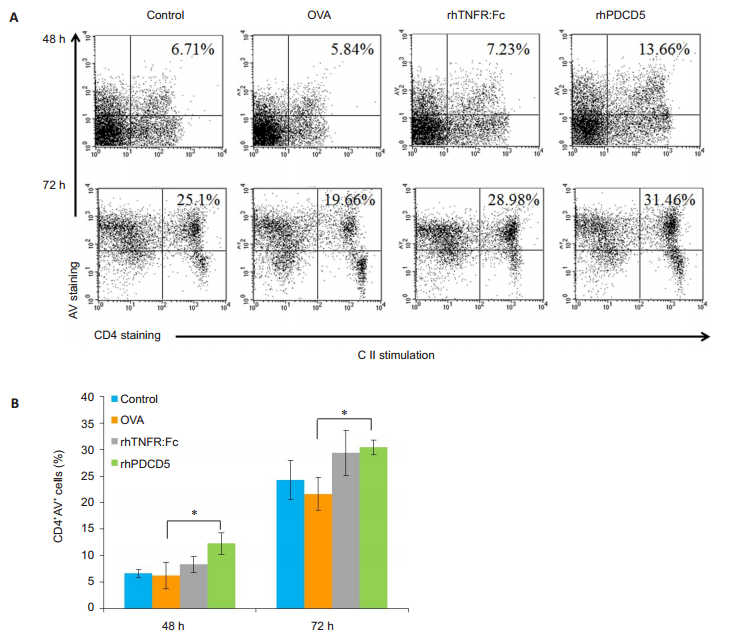
|
Fig.3 RhPDCD5 treatment of CIA rats promotes AICD of CD4 + T cells activated by CII. A: Representative data of CD4 + AV + cells in the spleen analyzed by flow cytometry; B: Quantification of the proportion of CD4+AV+ cells in the spleen. Results are expressed as Mean ± SD (*P < 0.05, n=6). |
Flow cytometry was used to detect the frequency of CD4+ AV+ cells at 48 h and 72 h following anti-CD3+ anti-CD28 stimulation for evaluating apoptosis of the activated splenocytes. As shown in Fig. 4, the splenocytes from rhPDCD5-treated CIA rats were more sensitive to AICD following stimulation by anti-CD3+ anti-CD28. CD4 + T cells from rhTNFR: Fc-treated CIA rats also showed obviously increased apoptosis at 48 h following activation by anti-CD3 + anti-CD28 but not at 72 h, possibly as a result of potential experimental errors.
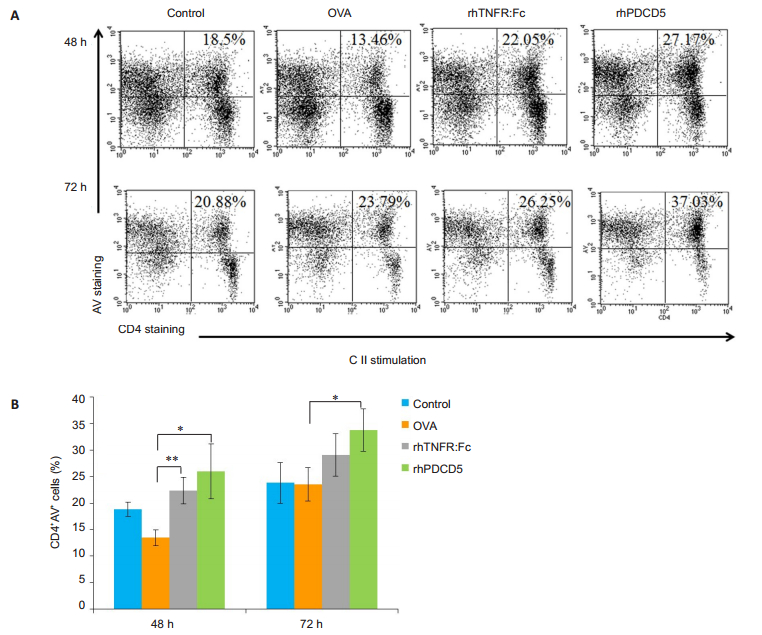
|
Fig.4 CD4 + T cells from rhPDCD5-treated rats undergo AICD following activation by anti-CD3 + anti-CD28 stimulation. A: Representative data of CD4+AV+ cells in the spleen analyzed by flow cytometry; B: Quantification of the proportion of CD4+AV+ cells in the spleen. Results are expressed as Mean±SD (*P < 0.05, **P < 0.005, n=6). |
The results presented in this study demonstrate that rhPDCD5 treatment of CIA rats resulted in a decrease of IFN-γ and IL-17A secretion by the activated lymphocytes, which also showed a much lowered proliferative activity and obviously enhanced AICD.
Th1 cell activation accompanied by secretion of inflammatory cytokines such as IFN-γ contributes to inflammatory reactions and immunological destruction in RA[18, 19]. Th17 cells also participate in joint inflammation and erosion in RA[20, 21], and IL-17A, which substantially contributes to synovial inflammation and bone erosion, is predominantly secreted by Th17 cells [21]. The elevation of IL-17A in the synovium tissue and serum is positively correlated with the disease activity in RA patients [22], and in both animal models of RA and clinical trials, inhibition of IL-17A secretion has proved to enhance the efficacy of new treatment agents[23]. Herein we demonstrated that rhPDCD5 treatment of CIA rats significantly decreased IFN-γ and IL-17A secretion by activated splenocytes. As IFN-γ and IL-17A are primarily secreted by Th1 and Th17 cells, respectively, the results indicate that rhPDCD5 treatment of the rats promotes AICD of activated Th1/Th17 cells and down-regulates the inflammatory Th1/Th17 response, which are consistent with our previous findings [17].
Abnormal immune responses mediated by activated T cells and sustained inflammation fueled by dysregulated cytokines from lymphocytes play an important role in the pathogenesis and progression of autoimmune arthritis [24, 25]. The development of new anti-inflammatory agents targeting activated T cells can prove to be an effective strategy for controlling the symptoms of RA patients[26, 27]. Activated T cells can be eliminated by AICD, which is central to maintaining immune homeostasis, and a low sensitivity to AICD is associated with immunopathologies [28]. Excessive proliferation and reduced AICD of the self-reactive effector T cells are the pathological characteristics of RA[29, 30], and the T cell clones from RA patients showed a suppressed cell apoptosis and a resistance to AICD as compared with the T cells from healthy individuals [4, 31].
We found in this study that rhPDCD5 treatment inhibited the proliferation of activated lymphocytes and promoted AICD of activated CD4 + T cells. The imbalance between proliferation and apoptosis of activated lymphocytes contributes to the pathogenesis of RA. A likely interpretation of our results is that rhPDCD5 treatment of the CIA rats promotes AICD of activated lymphocytes, thereby suppressing their proliferation. As the activated T cells in RA are resistant to AICD[31], re-sensitization of the activated T cells to AICD by enhancing PDCD5 activity might be of therapeutic values for RA.
Taken together, the results presented herein and our previous data[17]support an immunosuppressive role of rhPDCD5 in modulating the proliferation of activated T lymphocytes and as a positive regulator of AICD. Further studies are warranted to further elucidate the potential immunosuppressant mechanisms of rhPDCD5.
| [1] |
Firestein GS. Evolving concepts of rheumatoid arthritis[J]. Nature, 2003, 423(6937): 356-61. DOI:10.1038/nature01661 |
| [2] |
Firestein GS. Evolving concepts of rheumatoid arthritis[J]. Nature, 2003, 423(6937): 356-61. DOI:10.1038/nature01661 |
| [3] |
Leah E. Rheumatoid arthritis: Metabolic reprogramming of T cells in RA[J]. Nat Rev Rheumatol, 2013, 9(11): 635. DOI:10.1038/nrrheum.2013.158 |
| [4] |
Boissier MC, Semerano L, Challal S, et al. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction[J]. J Autoimmun, 2012, 39(3): 222-8. DOI:10.1016/j.jaut.2012.05.021 |
| [5] |
Salmon M, Scheel-Toellner D, Huissoon AP, et al. Inhibition of T cell apoptosis in the rheumatoid synovium[J]. J Clin Invest, 1997, 99(3): 439-46. DOI:10.1172/JCI119178 |
| [6] |
Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off[J]. Science, 1998, 280(5361): 243-8. DOI:10.1126/science.280.5361.243 |
| [7] |
Roberts AI, Devadas S, Zhang X, et al. The role of activation-induced cell death in the differentiation of T-helper-cell subsets[J]. Immunol Res, 2003, 28(3): 285-93. DOI:10.1385/IR:28:3 |
| [8] |
Kabelitz D, Janssen O. Antigen-induced death of T-lymphocytes[J]. Front Biosci, 1997, 2: d61-77. DOI:10.2741/A175 |
| [9] |
Zhang J, Xu X, Liu Y. Activation-induced cell death in T cells and autoimmunity[J]. Cell Mol Immunol, 2004, 1(3): 186-92. |
| [10] |
Liu H, Wang Y, Zhang Y, et al. TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal[J]. Biochem Biophys Res Commun, 1999, 254(1): 203-10. DOI:10.1006/bbrc.1998.9893 |
| [11] |
Han XR, Sun Y, Bai XZ. The anti-tumor role and mechanism of integrated and truncated PDCD5 proteins in osteosarcoma cells[J]. Cell Signal, 2012, 24(8): 1713-21. DOI:10.1016/j.cellsig.2012.04.011 |
| [12] |
Zhuge C, Chang Y, Li Y, et al. PDCD5-regulated cell fate decision after ultraviolet-irradiation-induced DNA damage[J]. Biophys J, 2011, 101(11): 2582-91. DOI:10.1016/j.bpj.2011.10.044 |
| [13] |
Li P, Fei H, Wang L, et al. PDCD5 regulates cell proliferation, cell cycle progression and apoptosis[J]. Oncol Lett, 2018, 15(1): 1177-83. |
| [14] |
Wang K, Zhang X, Wang Y, et al. PDCD5 regulates iNKT cell terminal maturation and iNKT1 fate decision[J]. Cell Mol Immunol, 2018. DOI:10.1038/s41423-018-0059-2 |
| [15] |
Perga S, Martire S, Montarolo F, et al. The footprints of poly-autoimmunity: evidence for common biological factors involved in multiple sclerosis and Hashimoto's thyroiditis[J]. Front Immunol, 2018, 9: 311. DOI:10.3389/fimmu.2018.00311 |
| [16] |
Yuan F, Wang J, Zhang K, et al. Programmed cell death 5 transgenic mice attenuates adjuvant induced arthritis by 2 modifying the T lymphocytes balance[J]. Biol Res, 2017, 50(1): 40. DOI:10.1186/s40659-017-0145-4 |
| [17] |
Xiao J, Liu W, Chen Y, et al. Recombinant human PDCD5 (rhPDCD5) protein is protective in a mouse model of multiple sclerosis[J]. J Neuroinflammation, 2015, 12(1): 117. DOI:10.1186/s12974-015-0338-0 |
| [18] |
Xiao J, Li G, Hu J, et al. Anti-inflammatory effects of recombinant human PDCD5 (rhPDCD5) in a rat collagen-induced model of arthritis[J]. Inflammation, 2015, 38(1): 70-8. |
| [19] |
Pandey A, Rizvi M, Shah BA, et al. Anti-arthritogenic effect of Saponin-1 by alteration of Th1/Th2 cytokine paradigm in arthritic mice[J]. Cytokine, 2016, 79: 103-13. DOI:10.1016/j.cyto.2016.01.004 |
| [20] |
Ahmad SF, Zoheir KM, Abdel-Hamied HE, et al. Amelioration of autoimmune arthritis by naringin through modulation of T regulatory cells and Th1/Th2 cytokines[J]. Cell Immunol, 2014, 287(2): 112-20. DOI:10.1016/j.cellimm.2014.01.001 |
| [21] |
Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity[J]. Nat Immunol, 2007, 8(4): 345-50. DOI:10.1038/ni0407-345 |
| [22] |
Yang X, Yang J, Zou H. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis[J]. Clin Dev Immunol, 2013, 2013: 268065. |
| [23] |
Kirkham BW, Lassere MN, Edmonds JP, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort)[J]. Arthritis Rheum, 2006, 54(4): 1122-31. DOI:10.1002/(ISSN)1529-0131 |
| [24] |
Lubberts E, van den Bersselaar L, Oppers-Walgreen B, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance[J]. J Immunol, 2003, 170(5): 2655-62. DOI:10.4049/jimmunol.170.5.2655 |
| [25] |
Chen M, Su W, Lin X, et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation[J]. Arthritis Rheum, 2013, 65(5): 1181-93. DOI:10.1002/art.37894 |
| [26] |
Mellado M, Martinez-Munoz L, Cascio G, et al. T cell migration in rheumatoid arthritis[J]. Front Immunol, 2015, 6: 384. |
| [27] |
Murphy KM, Reiner SL. The lineage decisions of helper T cells[J]. Nat Rev Immunol, 2002, 2(12): 933-44. DOI:10.1038/nri954 |
| [28] |
Chen W, Wang J, Xu Z, et al. Apremilast ameliorates experimental arthritis via suppression of Th1 and Th17 cells and enhancement of CD4 + Foxp3 + regulatory T cells differentiation[J]. Front Immunol, 2018, 9: 1662. DOI:10.3389/fimmu.2018.01662 |
| [29] |
Zhou Y, Leng X, He Y, et al. Loss of Perp in T cells promotes resistance to apoptosis of T helper 17 cells and exacerbates the development of experimental autoimmune encephalomyelitis in mice[J]. Front Immunol, 2018, 9: 842. DOI:10.3389/fimmu.2018.00842 |
| [30] |
Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis[J]. Proc Natl Acad Sci USA, 2000, 97(17): 9561-6. DOI:10.1073/pnas.160105897 |
| [31] |
Moodley D, Mody GM, Chuturgoon AA. Initiation but no execution - modulation of peripheral blood lymphocyte apoptosis in rheumatoid arthritis-a potential role for heat shock protein 70[J]. J Inflamm (Lond), 2011, 8(1): 30. |
| [32] |
Peroumal D, Abimannan T, Tagirasa R, et al. Inherent low Erk and p38 activity reduce Fas Ligand expression and degranulation in T helper 17 cells leading to activation induced cell death resistance[J]. Oncotarget, 2016, 7(34): 54339-59. |
 2019, Vol. 39
2019, Vol. 39

