2. 海南医学院科学实验中心,海南 海口 570102
2. Scientific Experimental Center of Hainan Medical College, Haikou 570102, China
喉鳞状细胞癌是临床常见恶性肿瘤之一,居于耳鼻喉恶性肿瘤的第3位,晚期患者治疗效果不佳,5年生存率仅50%左右[1-2]。研究发现喉鳞癌的发生发展与机体免疫功能关系密切,患者均存在不同程度抗肿瘤免疫缺陷[3],寻找有效手段增强机体对肿瘤细胞的免疫杀伤作用对改善患者预后有重要意义。E3泛素连接酶(Cbl-b)是调控T淋巴细胞活化及机体免疫耐受的重要酶类,对调节肿瘤诱导的免疫抑制至关重要。已有研究证实[4],沉默Cbl-b基因时小鼠脾脏T细胞其免疫活性增强,且抑制cbl-b的表达可增强T淋巴细胞在体外对rm-1前列腺癌细胞的免疫激活[5]。目前,cbl-b在调节T淋巴细胞活动对喉鳞状细胞癌的影响仍未明确。为此,本研究应用小干扰RNA(siRNA)技术,探索靶向沉默人T细胞H9的Cbl-b基因对T细胞免疫杀伤人喉鳞癌细胞株Hep-2的影响,并初步探讨其相关机制。
1 资料和方法 1.1 病例2019年海南医学院附属第一医院耳鼻咽喉科确诊并行手术治疗的初诊喉鳞癌12例为喉鳞癌组,其中男性10例,女性2例,年龄43~76岁。所有患者均未接受过放疗,化疗和免疫治疗。另选健康志愿者12例作为健康对照组,男性9例,女性3例,年龄43~60岁。所有入组患者和健康志愿者均签署知情同意书。
1.2 主要试剂和仪器人T淋巴细胞H9(上海极威)、人喉鳞癌细胞株Hep-2(上海歌凡生物细胞库)。1640、Opti-MEM培养基(Gibco),全蛋白提取试剂盒(北京索莱宝),lipofectamineTM2000试剂盒(Invitrogen),逆转录试剂盒(Takara),兔抗人Cbl-b单抗一抗、山羊抗兔IgG二抗(Epitomics),抗人IL-2中和抗体(500-M02,北京盛科博源),抗人CD3-APC、CD25-PE、CD69-PE(BD),IL-2、INF-γ ELISA试剂盒(上海通蔚);TS100型倒置荧光显微镜(Nikon),StepOne Puls Real Time PCR仪(Applied Biosystems),DG5031型酶联免疫监测仪(上海珂淮仪器),FACSCantoII流式细胞仪(BD),电泳槽、电转仪、CheniDoc XRS凝胶成像分析系统(Bio-rad)。CD4+T磁珠分选试剂盒(Becton Dickinson);免疫磁珠分选仪(Mihenyi Biotech)。
1.3 外周血CD4+T细胞分离应用免疫磁性细胞分离技术分离:EDTA抗凝管采集入组患者和健康志愿者外周静脉血20 mL,2 h内应用sigma公司生产的淋巴细胞分离液,采用密度梯度离心法分离外周血单个核细胞。然后用磁珠分选缓冲液充分混悬所得的外周血单个核细胞,加入鼠抗人CD4+单克隆抗体,4 ℃反应30 min,PBS洗涤细胞后加入T细胞富集磁珠,混匀后置于8~15 ℃,反应10~15 min,将分离柱置于磁场中,加入0.5 mL分选缓冲液,在重力作用下待缓冲液自然流尽后,将分离柱移出磁场。用2 mL PBS缓冲液稍用力冲洗,1000 r/min离心5 min收集分离获得的CD4+T细胞,调整细胞数至107/管。
1.4 人T淋巴细胞H9细胞培养及Cbl-b-siRNA转染取对数期生长人淋巴细胞H9,以1.5×105/mL接种至培养基(含10%胎牛血清的1640培养基+0.197 g碳酸氢钠+0.001 g刀豆蛋白),于37 ℃ 5%二氧化碳、饱和湿度培养箱中培养,待细胞融合至85%时,用无菌生理盐水溶液洗涤2次,用Opti-MEM吹打为单细胞悬液,接种至96孔板。Cbl-b-siRNA重组序列、随机阴性对照序列由上海吉凯基因技术有限公司设计及合成。Cbl-bsiRNA重组序列:正义链5'-CCAACCACUUGGACU UGUATGCTT-3';反义链:5'-AUACUGUCAUGUAC UGUTATGATT-3';以无关通用序列为阴性对照。应用lipofectamineTM 2000试剂盒进行转染:将脂质体、siRNA溶于Opti-MEM,室温静置0.5 h后加入96孔板常规培养6 h,设为Cbl-b-siRNA组。在Cbl-b-siRNA重组序列转染T细胞成功后,在其培养液中加入抗人IL-2中和抗体(8.0 μg/mL),作为anti- IL-2 + Cbl-b-siRNA组。另设加入NC-siRNA重组序列转染的T淋巴细胞为阴性组。应用倒置荧光显微镜观察转染效率。
1.5 CD4+T细胞和人T淋巴细胞H9的Cbl-b mRNA表达检测Trizol法获取总RNA,逆转录获得cDNA,经电泳鉴定、酶标仪定量后,采用采用实时荧光定量聚合酶链反应(RT-PCR)法进行检测,反应条件:95 ℃预变性1min;95 ℃变性30 s,59 ℃退火45 s,72 ℃延伸40 s,重复42个循环。以β-actin为管家基因,2-△△CT为目的基因的相对表达强度。Cbl-b:上游引物:5'-ACGCTAGCTA GCTACGCATGCTAG-3';下游引物:5'-TACGTAGCT AGCTAGCTGATCGAC-3';β-actin:上游引物:5'-AGC TAGCTAGTCGATCGAGCATCG-3';下游引物:5'-GC GTACGATCGTAGCTAGCTCCAC-3'。
1.6 人T淋巴细胞H9的Cbl-b蛋白表达检测各组培养至48 h后,1000 r/min离心5 min后取下层细胞,全蛋白提取试剂盒提取总蛋白,BCA法进行蛋白定量,取30 μg样品加入等量上样缓冲液混匀后,沸水浴后12 000 r/min离心10 min取上清液,进行十二烷基硫酸钠聚丙烯酰氨凝胶电泳分离,电转后,加入封闭液室温摇床孵育1 h,加入兔抗人Cbl-b单抗一抗(1: 1000),4℃摇床孵育过夜,加入山羊抗兔IgG二抗(1: 5000),常温孵育1 h,暗室中曝光。应用凝胶成像系统扫描并进行灰度值分析,以Cbl-b蛋白灰度值与内参β-actin灰度值比值表示蛋白相对表达量。
1.7 人T淋巴细胞H9表面活化分子CD69、CD25表达检测各组培养至48 h,预冷磷酸盐缓冲液(PBS)洗涤细胞3次,3000 r/min离心8 min,弃去上清后加入100 μL PBS混合均匀,分别加入抗人CD3-APC、抗人CD25-PE和抗人CD3-APC、CD69-PE,混匀后,避光孵育30 min(室温),过滤后在1 h内采用流式细胞仪分析CD69、CD25表达水平。
1.8 人T淋巴细胞分泌的细胞因子IL-2、INF-γ检测人T淋巴细胞H9培养至48 h,将细胞培养液1100 r/min离心5 min后取上清液,采用酶联免疫吸附法(ELISA)检测IL-2、INF-γ水平,严格按照试剂盒说明书要求操作,将标准品、待测样品加入酶标版,37 ℃恒温孵育2 h,加入IL-2、INF-γ抗体工作液,继续孵育1 h,然后加入亲和素过氧化酶复合物,继续孵育30 min后,TMB显色、终止液终止反应,应用酶联免疫监测仪于450 nm波长下测定A值。
1.9 人T淋巴细胞H9对Hep-2人喉鳞癌细胞杀伤率检测各组培养至48 h,将各组人淋巴细胞H9(效应细胞)分别与Hep-2人喉鳞癌细胞(靶细胞)按照效靶比10:1、20:1、40:1接种至96孔板,每孔5个复孔,其中Hep-2细胞接种密度为1×105/mL,共培养72 h后,应用细胞计数试剂盒(CCK-8)检测各组肿瘤细胞杀伤率。
肿瘤细胞杀伤率(%)=([AE+AT)-AE+T]/AT×100%
AE+T=A效应细胞+A靶细胞
1.10 统计学分析采用SPSS25.0统计工具分析数据,计量资料用均数±标准差表示,两样本间均数比较采用t检验,多样本比较采用单因素方差分析,两两样本计量资料采用SNK-q检验。P < 0.05为差异有统计学意义。
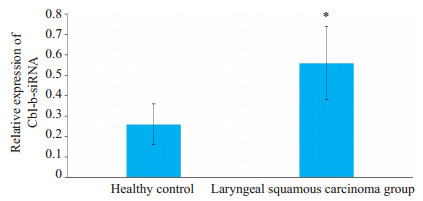
2 结果 2.1 健康人和喉鳞癌患者外周血CD4+T淋巴细胞Cblbm RNA相对表达水平的比较喉鳞癌患者外周血CD4+T淋巴细胞Cbl-bm RNA水平均较健康对照组显著升高,差异具有统计学意义(图 1)。
|
图 1 健康人和喉鳞癌患者CD4+T淋巴细胞中Cbl-b mRNA的相对表达 Fig.1 Expression of Cbl-b mRNA in CD4+ T lymphocytes from patients with laryngeal squamous carcinoma and healthy individuals detected by RT-PCR. *P < 0.05 vs healthy group |
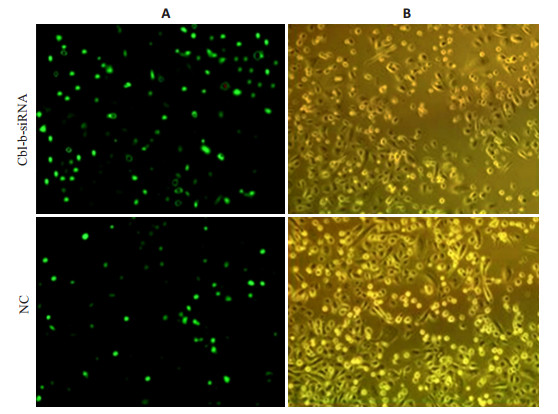
人T淋巴细胞H9转染Cbl-b-siRNA和NCsiRNA,培养至6 h后,于倒置荧光显微镜下观察转染效果,Cbl-b-siRNA组转染效率为(78.30±6.06)%、阴性组转染效率为(77.25±6.38)%,转染效果良好,可进行后续实验(图 2)。
|
图 2 人T淋巴细胞H9转染效率评价 Fig.2 Evaluation of transfection efficiency in H9 T lymphocytes detected by fluorescence microscopy (Original magnification: ×100).A: Fluorescence microscopy; B: Light microscopy |
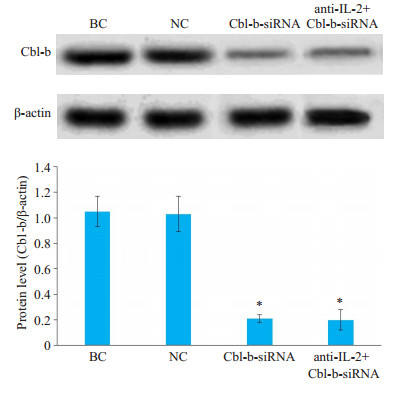
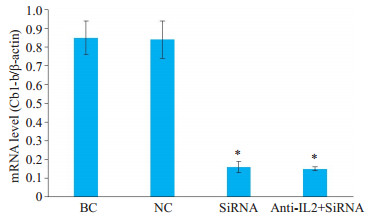
Cbl-b-siRNA组人T淋巴细胞H9的Cbl-b mRNA和蛋白相对表达量与anti-IL-2+Cbl-b-siRNA组无显著差异(P>0.05),但显著低于空白组和阴性组(P < 0.05);空白组与阴性组比较,差异无统计学意义(P>0.05,图 3、图 4)。
|
图 3 Western blot检测Cbl-b蛋白在人T淋巴细胞H9中的表达 Fig.3 Expression of Cbl-b protein in H9 T lymphocytes in different groups detected by Western blotting. *P < 0.05 vs blank control (BC) group and negative control (NC) group |
|
图 4 RT-PCR检测Cbl-b mRNA在人T淋巴细胞H9中的表达 Fig.4 Expression of Cbl-b mRNA in H9 T lymphocytes in different groups detected by RT-PCR. *P < 0.05 vs blank control (BC) group and negative control (NC) group |
不同效靶比时,Cbl-b-siRNA组Hep-2肿瘤细胞杀伤率均显著高于anti-IL-2+Cbl-b-siRNA组、阴性组和空白组(P < 0.05)。anti-IL-2+Cbl-b-siRNA组、空白组与阴性组比较,肿瘤细胞杀伤率差异无统计学意义(P> 0.05,图 5)。
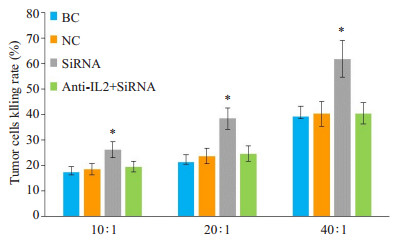
|
图 5 人T淋巴细胞H9的肿瘤细胞杀伤率 Fig.5 Tumor cell killing rate of T lymphocyte H9 at differen target-effector ratios in each group. *P < 0.05 vs blank control (BC) group, negative control (NC) group and anti-IL-2+Cbl-bsiRNAgroup |
Cbl-b-siRNA组人T淋巴细胞H9表面活化分子CD69、CD25阳性表达率均显著高于anti-IL-2+Cbl-bsiRNA组、空白组和阴性组(P < 0.05);anti-IL-2+Cbl-bsiRNA组、空白组与阴性组比较,差异无统计学意义(P> 0.05,图 6~8)。
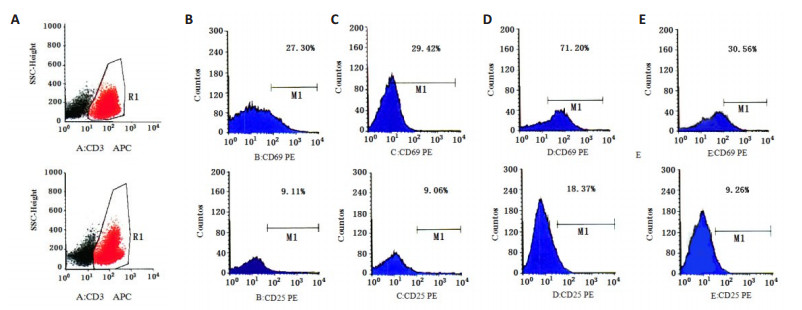
|
图 6 H9T淋巴细胞表面活化分子CD69、CD25的表达 Fig.6 Expression of surface activation molecules CD69 and CD25 on H9 T lymphocytes. A: CD3 APC; B: Blank control group; C: Negative control group; D: Cbl-b-siRNAgroup; E: Anti-IL-2+Cbl-b-siRNAgroup |
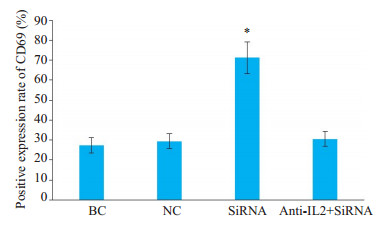
|
图 7 人T淋巴细胞H9表面活化分子CD69阳性表达率 Fig.7 Expression of H9 T lymphocyte surface activation molecule CD69. *P < 0.05 vs blank control (BC) group, negative control (NC) group and anti-IL-2+Cbl-b-siRNA group |
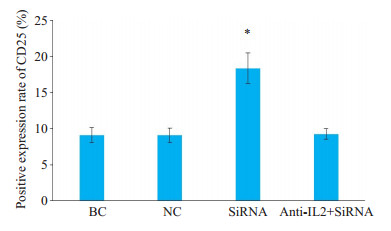
|
图 8 人T淋巴细胞H9表面活化分子CD25阳性表达率 Fig.8 Expression of H9 T lymphocytes surface activation molecule CD25. *P < 0.05 vs blank control (BC) group, negative control (NC) group and anti-IL-2+Cbl-bsiRNAgroup |
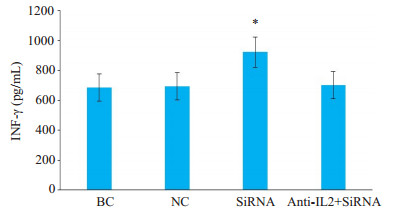
Cbl-b-siRNA组人T淋巴细胞H9上清液中INF-γ水平均显著高于anti-IL-2+Cbl-b-siRNA组、空白组和阴性组(P < 0.05),而anti-IL-2+Cbl-b-siRNA组、空白组与阴性组比较,差异无统计学意义(P>0.05,图 9)。
|
图 9 人T淋巴细胞H9上清液中INF-γ水平 Fig.9 Levels of interferon-gamma (INF- γ) in the supernatants of H9 T lymphocytes detected by ELISA. *P < 0.05 vs blank control (BC) group, negative control (NC) group and anti-IL-2+Cbl-b-siRNAgroup |
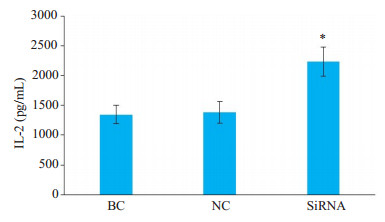
Cbl-b-siRNA组人T淋巴细胞H9上清液中IL-2水平均显著高于空白组和阴性组(P < 0.05);空白组与阴性组比较,差异无统计学意义(P>0.05,图 10)。
|
图 10 人T淋巴细胞H9上清液中IL-2水平 Fig.10 Levels of interleukin-2 (IL-2) in the supernatants of H9 T lymphocytes detected by ELISA. *P < 0.05 vs blank control (BC) group and negative control (NC) group |
目前临床多种治疗后,喉鳞癌死亡率仍居高不下[6]。喉鳞癌发病过程涉及多因素、多阶段共同参与,其发病的危险因素除环境暴露等传统危险因素外,机体免疫抑制导致免疫力低下与喉鳞癌的发生发展关系紧密[7-8]。既往研究表明[9],鳞状癌细胞表面人类白细胞抗原表达障碍及免疫抑制因子的异常分泌,均可导致机体对鳞状癌细胞产生免疫耐受, 且我国既往研究发现在喉鳞癌组织中普遍存在着T淋巴细胞浸润,但多数为静止状态,喉癌局部细胞免疫处于抑制状态[10]。目前认为,CD4+T细胞不仅有调控免疫的功能,甚至具有不依赖于CD8+CTL肿瘤杀伤作用[11]。
Cbl-b在机体多种正常组织和细胞中均有表达,其基因定位于第3号染色体,属于T淋巴细胞的负调控基因,过表达可导致T细胞无能,形成免疫耐受[12]。本研究发现Cbl-b mRNA在喉鳞癌患者CD4+T细胞中表达升高,与健康对照差异有统计学意义(P < 0.05)。因此,降低CD4+T细胞Cbl-b基因及蛋白的表达,激活T细胞免疫杀伤作用进而治疗喉鳞癌是一种新的可能治疗手段。
人T淋巴细胞H9是一种人源的CD4+T淋巴细胞,本研究中Cbl-b-siRNA组和阴性组转染人T淋巴细胞H9效率为(78.30±6.06)%、(77.25±6.38)%,而Cbl-bsiRNA组人T淋巴细胞H9的Cbl-b mRNA和蛋白相对表达量显著低于空白组和阴性组(P < 0.05)。这表明本研究应用体外合成重组Cbl-b-siRNA后,借助脂质体将小片段的干扰核酸转入T淋巴细胞内,通过降解或抑制mRNA转录等过程沉默Cbl-b基因,转染效率高,且对Cbl-b mRNA和蛋白的干扰效率约80%,有效达到基因沉默效果。本研究中,不同效靶比时,Cbl-b-siRNA组Hep-2肿瘤细胞杀伤率均显著高于阴性组和空白组(P < 0.05),而中和IL-2以后,anti-IL-2+Cbl-b-siRNA组的Hep-2肿瘤细胞杀伤率明显下降。Lee A等应用靶向siRNA技术干扰Cbl-b基因表达,可有效增强T739小鼠脾脏T淋巴细胞对肺腺癌细胞LA795的杀伤率,与本研究结果相似[13]。本研究提示沉默T细胞Cbl-b基因可增强T细胞对Hep-2体外免疫杀伤作用,而且这种作用是和IL-2相关的。
静息状态下,T淋巴细胞表面分子仅少量表达或不表达,而处于激活状态T淋巴细胞可大量表达CD69、CD25等表面分子,分泌大量IL-2、INF-γ,发挥免疫杀伤作用。一方面,CD69、CD25可作为T细胞活化标志物,另一方面,CD25可作为IL-2受体γ链,与IL-2集合后选择性支持活化T细胞的增殖,进一步增强T细胞免疫杀伤作用[14-26]。CD69属于C型凝集素,当细胞接收到刺激信号短时间(2~4 h)内即可大量表达,属于T细胞早期活化标志性抗原,CD25属于活化中期标志性抗原,促进T细胞增殖[27-30]。IL-2、INF-γ是免疫细胞合成并分泌的一种小分子多肽,可激活免疫细胞对肿瘤细胞的免疫杀伤效果[31-37]。本研究结果中,Cbl-b-siRNA组人T淋巴细胞H9表面活化分子CD69、CD25阳性表达率和上清液中INF-γ、IL-2水平均显著高于空白组和阴性组(P < 0.05);而anti-IL-2+Cbl-b-siRNA组用中和抗体中和IL-2后,沉默T细胞的CD69、CD25和分泌INF-γ下降,对肿瘤细胞的杀伤率也下降。由此,本研究提示:沉默T细胞Cbl-b基因增强对Hep-2体外免疫杀伤作用与促进T细胞表面活化分子CD69、CD25表达及T细胞活化后分泌大量细胞因子IL-2、INF-γ相关,其中IL-2起关键的作用。
综上所述,应用siRNA技术沉默T细胞H9的Cbl-b基因通过IL-2途径可有效激活T细胞分泌细胞因子对人喉鳞癌细胞株Hep-2体外免疫杀伤作用,为临床中应用沉默T细胞Cbl-b基因治疗喉鳞癌提供了新的思路。
| [1] |
陶磊, 周梁, 吴海涛, 等. 760例喉鳞状细胞癌开放性喉功能保全手术的回顾性分析[J]. 临床耳鼻咽喉头颈外科杂志, 2018, 32(10): 466-7. |
| [2] |
Tassone P, Savard C, Topf MC, et al. Association of positive initial margins with survival among patients with squamous cell carcinoma treated with total laryngectomy[J]. JAMA Otolaryngol Head Neck Surg, 2018, 144(11): 1030-6. |
| [3] |
Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma[J]. AIDS, 2016, 30(8): 1257-66. DOI:10.1097/QAD.0000000000001030 |
| [4] |
Goetz B, An W, Mohapatra B, et al. A novel CBL-Bflox/flox mouse model allows tissue-selective fully conditional CBL/CBL-B doubleknockout: CD4-Cre mediated CBL/CBL-B deletion occurs in both Tcells and hematopoietic stem cells[J]. Oncotarget, 2016, 7(32): 51107-23. |
| [5] |
Zhou SK, Chen WH, Shi ZD, et al. Silencing the expression of Cbl-b enhances the immune activation of T lymphocytes against RM-1 prostate cancer cells in vitro[J]. J Chin Med Assoc, 2014, 77(12): 630-6. DOI:10.1016/j.jcma.2014.03.008 |
| [6] |
Wang Q, Wu ZX, Zhan TW, et al. Long-term outcomes of 530 esophageal squamous cell carcinoma patients with minimally invasive Ivor Lewis esophagectomy[J]. J Surg Oncol, 2018, 117(5, SI): 957-69. DOI:10.1002/jso.v117.5 |
| [7] |
刘亚超, 魏洁, 李燕萍, 等. 吸烟相关喉鳞状细胞癌氧化应激机制的探讨[J]. 临床耳鼻咽喉头颈外科杂志, 2016, 30(9): 733-6. |
| [8] |
Hidalgo-Tenorio C, Gil-Anguita C, Ramirez-Taboada J, et al. Risk factors for infection by oncogenic human papillomaviruses in HIVpositive MSM patients in the ART era (2010-2016)[J]. Medicine (Baltimore), 2017, 96(39): 810-9. |
| [9] |
Agnihotri V, Gupta A, Kumar R, et al. Promising Link of HLA-G polymorphism, tobacco consumption and risk of head and neck squamous cell carcinoma (HNSCC) in north indian population[J]. Hum Immunol, 2017, 78(2): 172-8. |
| [10] |
褚汉启, 谭慎微, 王奇, 等. 喉鳞癌组织淋巴细胞亚群分布的免疫组化研究[J]. 临床耳鼻咽喉科杂志, 1994, 4(1): 37-9. |
| [11] |
张梦瑶, 孙晓莉, 唐丽, 等. Cbl-b调控T细胞的免疫耐受[J]. 细胞与分子免疫学杂志, 2012, 28(4): 445-7. |
| [12] |
Gao SF, Zhong B, Lin D. Regulation of T helper cell differentiation by E3 ubiquitin ligases and deubiquitinating enzymes[J]. Int Immunopharmacol, 2017, 42(1): 150-6. |
| [13] |
Lee A, Rayner SL, De Luca A, et al. Casein kinase II phosphorylation of cyclin F at serine 621 regulates the Lys48-ubiquitylation E3 ligase activity of the SCF(cyclin F) complex[J]. Open Biol, 2017, 7(10): 125-9. |
| [14] |
黄海涛, 张华. Cbl-b基因介导T细胞免疫杀伤小鼠LA795肺腺癌细胞的实验研究[J]. 临床肺科杂志, 2017, 22(3): 538-41. DOI:10.3969/j.issn.1009-6663.2017.03.043 |
| [15] |
Lei L, Cui L, Mao Y, et al. Augmented CD25 and CD69 expression on circulating CD8+ T cells in type 2 diabetes mellitus with albuminuria[J]. Diabetes Metab, 2017, 43(4): 382-4. |
| [16] |
Karimi G, Mahmoudi M, Balali-Mood M, et al. Decreased levels of spleen tissue CD4(+)CD25(+)Foxp3(+) regulatory T lymphocytes in mice exposed to berberine[J]. J Acupunct Meridian Stud, 2017, 10(2): 109-13. DOI:10.1016/j.jams.2016.10.003 |
| [17] |
Woods M, Guy R, Waldmann H, et al. A humanised therapeutic CD4 mAb inhibits TCR-induced IL-2, IL-4, and I-10 secretion and expression of CD25, CD40L, and CD69[J]. Cell Immunol, 1998, 185(2): 101-13. |
| [18] |
Vitales- Noyola M, Oceguera-Maldonado B, Niño-Moreno P, et al. Patients with systemic lupus erythematosus show increased levels and defective function of CD69+ T regulatory cells[J]. Mediators Inflamm, 2017, 2513829. |
| [19] |
Zhao XS, Wang XH, Zhao XY, et al. Non-traditional CD4+CD25- CD69+ regulatory T cells are correlated to leukemia relapse after allogeneic hematopoietic stem cell transplantation[J]. J Transl Med, 2014, 12: 187. DOI:10.1186/1479-5876-12-187 |
| [20] |
Xu LQ, Tanaka S, Bonno M, et al. Cord blood CD4(+)CD25(+) regulatory T cells fail to inhibit cord blood NK cell functions due to insufficient production and expression of TGF-beta1[J]. Cell Immunol, 2014, 290(1): 89-95. |
| [21] |
Li J, Andreyev O, Chen M, et al. Human T cells upregulate CD69 after coculture with xenogeneic genetically-modified pig mesenchymal stromal cells[J]. Cell Immunol, 2013, 285(1/2): 23-30. |
| [22] |
Sarhan D, Palma M, Mao Y, et al. Dendritic cell regulation of NKcell responses involves lymphotoxin-α, I-12, and TGF-β[J]. Eur J Immunol, 2015, 45(6): 1783-93. |
| [23] |
Qin Q, Luo D, Shi YJ, et al. CD25 siRNA induces Treg/Th1 cytokine expression in rat corneal transplantation models[J]. Exp Eye Res, 2016, 151(1): 134-41. |
| [24] |
买文丽, 韩清娟, 曹文轩, 等. 人源化NOD小鼠中CD8-+ CD25-+ Tregs的频率和功能研究[J]. 免疫学杂志, 2016, 32(1): 7-12. |
| [25] |
Rudnicka K, Matusiak A, Chmiela M. CD25(IL-2R) expression correlates with the target cell induced cytotoxic activity and cytokine secretion in human natural killer cells[J]. Acta Biochim Pol, 2015, 62(4): 885-94. DOI:10.18388/abp.2015_1152 |
| [26] |
Peters JH, Koenen HJ, Fasse EA, et al. Human secondary lymphoid organs typically contain polyclonally-activated proliferating regulatory T cells[J]. Blood, 2013, 122(13): 2213-23. |
| [27] |
ignali D, Gürth CM, Pellegrini S, et al. IL- 7 mediated homeostatic expansion of human CD4+CD25+FOXP3+regulatory T cells after depletion with Anti-CD25 monoclonal antibody[J]. Transplantation, 2016, 100(9): 1853-61. DOI:10.1097/TP.0000000000001276 |
| [28] |
Gomez-Eerland R, Nuijen B, Heemskerk B, et al. Manufacture of gene-modified human T-cells with a memory stem/central memory phenotype[J]. Hum Gene Ther Methods, 2014, 25(5): 277-87. DOI:10.1089/hgtb.2014.004 |
| [29] |
Ben Merzoug L, Marie S, Satoh-Takayama NA, et al. Conditional ablation of NKp46(+) cells using a novel Ncr1(greenCre) mouse strain: NK cells are essential for protection against pulmonary B16 metastases[J]. Eur J Immunol, 2014, 44(11): 3380-91. |
| [30] |
Yan ZF, Liu NX, Mao XX, et al. Activation effects of polysaccharides of Flammulina velutipes mycorrhizae on the T lymphocyte immune function[J]. J Immunol Research, 2014, 285421. |
| [31] |
Baens M, Bonsignore L, Somers R, et al. MALT1 Auto-Proteolysis is essential for NF-kappa B-Dependent gene transcription in activated lymphocytes[J]. PLoS One, 2014, 9(8): e103774. DOI:10.1371/journal.pone.0103774 |
| [32] |
Tilahun AY, Chowdhary VR, David CS. Systemic inflammatory response elicited by superantigen destabilizes T regulatory cells, rendering them ineffective during toxic shock syndrome[J]. J Immunol, 2014, 193(6): 2919-30. |
| [33] |
Lin KH, Lin KC, Lu WJ, et al. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-gamma and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo[J]. Int J Mol Sci, 2016, 17(1): 148-54. |
| [34] |
Ni J, Miller M, Stojanovic A, et al. Sustained effector function of IL- 12/15/18-preactivated NK cells against established tumors[J]. J Exp Med, 2012, 209(13): 2351-65. DOI:10.1084/jem.20120944 |
| [35] |
Lippitz BE. Cytokine patterns in patients with cancer: a systematic review[J]. Lancet Oncol, 2013, 14(6): e218-28. DOI:10.1016/S1470-2045(12)70582-X |
| [36] |
Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-a, iNOS, COX-2, I-1β, IL-6, IFN-γ, IL-2, GM-CSF)and proangiogenic growth factor-VEGF[J]. Asian Pac J Cancer Prev, 2013, 14(6): 3909-19. DOI:10.7314/APJCP.2013.14.6.3909 |
| [37] |
Parackova Z, Kayserova J, Danova K, et al. T regulatory lymphocytes in type 1 diabetes: Impaired CD25 expression and IL-2 induced STAT5 phosphorylation in pediatric patients[J]. Autoimmunity, 2016, 49(8): 523-31. |
 2019, Vol. 39
2019, Vol. 39

