2. 宁夏优势特色作物现代分子育种重点实验室,宁夏 银川 750021
2. Key Laboratory of Modern Molecular Breeding for Dominant and Special Crops in Ningxia, Yinchuan 750021, China
血栓性疾病严重威胁着人类生命健康,溶栓疗法是临床上治疗此类疾病的常用方法[1]。重组人纤溶酶原激活剂(rhPA)是溶栓治疗中的常用药物[2]。商品化的rhPA(瑞替普酶,Reteplase)由大肠杆菌(Escherich coli)表达系统生产,产物非糖基化,包涵体产物的体外复性纯化收率低,价格昂贵[3]。探索新型的表达系统是解决问题的关键。研究人员曾尝试利用中国仓鼠卵巢细胞(CHO)[4]、酵母[5]、转基因动物乳腺[6]、昆虫细胞[7]、转基因植物[8]、植物悬浮细胞[9]、利什曼原虫[10]和海藻[11]等开展研究,但是仍存在表达效率低和生产成本高等问题。生产成本低是植物生物反应器最主要的优势,瞬时表达系统的快速和高效为植物基因工程制药开辟了新的途径[12]。目前,植物病毒载体表达系统被认为是一类极具潜力的外源基因瞬时新表达平台,Zera融合标签被认为可以有效地提高其融合基因在植物中的表达水平。本研究分别构建了表达rhPA的烟草花叶病毒(TMV)载体和Zera标签融合载体,探索利用这两种新技术在烟草植物中瞬时表达rhPA的可行性,以期为解决商品化rhPA生产成本高问题提供实验依据和新的研究思路。
1 材料和方法 1.1 材料 1.1.1 菌株和质粒大肠杆菌(Escherich coli)DH5α和农杆菌(Agrobacterium tumefaciens)GV3101菌株由本实验室保存;植物二元载体pJJJ161由加拿大农业与农业食品部Rima Menassa博士实验室惠赠;TMV表达载体pJL TRBO-G[13]由美国俄亥俄州立大学John A. Lindbo博士实验室惠赠;RNA沉默抑制子表达载体pCBNoX p19由本实验室前期构建。
1.1.2 植物材料Nicotiana benthamiana种子由中国检验检疫科学研究院张永江博士提供;N. excelsiana种子购自美国ATCC。
1.1.3 试剂DNA Marker、蛋白质Marker和质粒小提试剂盒等购自全式金(北京)有限公司;T4 DNA连接酶、琼脂糖凝胶DNA回收试剂盒购自Promega(北京)生物技术有限公司;限制性内切酶购自NEB生物技术北京有限公司;利福平(Rif)、卡那霉素(Kan)、庆大霉素(Gen)、氨苄青霉素(Amp)、乙酰丁香酮(AS)和2(- N-吗啡啉)乙磺酸(MES)等购自Sigma-Aldrich(上海)贸易有限公司;Miracloth滤膜(22~24 μm)购自Merck Millipore;NC膜购自TaKaRa工程(大连)有限公司;rhPA标准样品购自爱德药业(北京)有限公司;人组织型纤溶酶原激活剂(t-PA)兔多克隆抗体购自Abcam(上海)贸易有限公司;ECL Western blotting Kit购自Thermo Fisher Scientific科技(中国)有限公司;Zera标签(R8)兔抗血清由华大基因制备;其他试剂购自天根生化科技(北京)有限公司。
1.2 方法 1.2.1 烟草植物的培养在温室中培养受体植物N. benthamiana和N. excelsiana。培养条件为24 ℃,14 h光照/10 h黑暗,相对湿度70%~80%。培育至5~6叶期用于农杆菌渗滤接种实验。
1.2.2 表达载体的构建采用基因体外合成和亚克隆技术进行表达载体的构建。(1)pTMV rhPA-NSK表达载体构建。首先使用GeneArtTM软件,以N. benthamiana密码子使用频率对rhPA编码基因序列进行密码子优化,然后合成两端分别具有PacⅠ和NotⅠ酶切位点的rhPA-NSK基因片段(金斯瑞,Genscript),获得重组克隆质粒pUC57-TMV rhPA-NSK。PacⅠ和NotⅠ分别双酶切质粒pUC57-TMV rhPA-NSK和pJL TRBO-G,酶切产物经回收、连接、转化,菌落PCR筛选等步骤获得表达载体pTMVrhPA-NSK的重组质粒;(2)pJ Zera-rhPA表达载体的构建。合成两端分别具有XhoⅠ和SacⅠ酶切位点的Zera-rhPA基因片段(金斯瑞,Genscript),获得重组克隆质粒pUC57-Zera-rhPA;XhoⅠ和SacⅠ分别双酶切植物二元载体pJJJ161和克隆质粒pUC57-ZerarhPA,回收目的片段并经连接、转化和菌落PCR筛选等步骤获得载体pJ Zera-rhPA的重组质粒。
1.2.3 重组质粒的农杆菌转化取表达载体阳性重组质粒5.0 µL,与95.0 µL农杆菌GV3101感受态细胞混合,置于冰上1 min后,进行电击转化。涂板培养后,挑取单菌落进行菌落PCR鉴定。选择阳性转化菌落保存备用。
1.2.4 农杆菌渗滤接种法将重组农杆菌菌液在28 ℃下于LB液体培养基(含50 μg/mL Rif, 50 μg/mL Gen和50 μg/mL Kan)中振荡培养16~18 h,A600nm约为0.6。离心收集菌体,加入IM液体培养基(10 mmol/Lol/L NaH2SO4,10 mmol/Lol/L MES,200 μmol/mL AS,0.5%葡萄糖)重悬至菌液A600 nm约为0.4,室温下于暗室中静置2~3 h。将pTMV rhPA-NSK和pJ Zera-rhPA农杆菌菌悬液分别与pCBNoX p19农杆菌菌悬液按1:1等体积混合。用无针头的1 mL无菌注射器轻轻压入5~6叶期烟草叶片背面,每个接种点的接菌量为0.1 mL。每组处理接种3株受体植物,每株受体植物接种3个不同叶位的叶片。
1.2.5 烟草叶片接种部位的表型观察于接种后不同天数(dpi)在可见光下观察叶片接种部位的表型变化,并用数码相机记录观察结果。
1.2.6 烟草叶片可溶性总蛋白(TSPs)的提取在液氮下研磨烟草叶片,粉末以2.5 mL/g比例加入蛋白提取缓冲液(100 mmol/L Tris-HCl, pH 8.0, 100 mmol/L NaCl, 0.5% SDS,200 mmol/Lol/L DTT,100 μmol/L PMSF);于4 ℃下放置1 h,12 000×g离心15 min,取上清-20 ℃保存。
1.2.7 pJ Zera-rhPA表达载体接种叶片的蛋白质分级提取收取烟草叶片样品,称重后用液氮研磨成粉末。粉末按比例加入无DTT的蛋白提取缓冲液,于4 ℃下放置1 h。四层Miracloth滤膜(22~24 μm)过滤后,于12 000×g离心15 min,收集取上清(可溶部分)-20 ℃保存备用。向沉淀(蛋白质颗粒部分)中加入原体积的蛋白质提取液(含200 mmol/Lol/L DTT),重悬浮沉淀并于室温下静置0.5 h,4 ℃下12 000×g离心15 min,收集取上清备用。
1.2.8 SDS-PAGE凝胶电泳和Western blot检测样品经12.0% SDS-PAGE电泳后,以Bio-Rad半干转印仪80 mA转移2 h至NC膜上。rhPA和R8一抗分别按照1:5000和1:2000的比例稀释,二抗为1:5000稀释的HRP标记山羊抗兔IgG。ECL发光试剂显色,压片并拍照记录结果。非还原性SDS-PAGE凝胶电泳,上样缓冲液中不加入还原剂DTT,其它实验条件不变。
1.2.9 rhPA体外溶栓活性的检测利用纤维蛋白琼脂糖平板法(FAPA)对rhPA的体外生物活性进行检测。按中国药典(2015版)中的方法制备琼脂糖凝胶纤维蛋白平板并打孔备用。提取pTMV rhPA-NSK载体接种叶片的可溶性总蛋白。以生理盐水为空白对照;以加入纤溶酶原激活物抑制剂(PAI-Ⅰ)的可溶性总蛋白样品和原核表达的rhPA标准样品分别为阴性和阳性对照。平板每孔加样10.0 μL,37 ℃湿盒放置反应24 h后,用考马斯亮蓝G-250对平板进行染色和脱色并观察记录溶圈情况。
1.2.10 统计学分析结果用均数±标准差表示。使用SPSS13.0软件进行定量数据的统计学分析,组间比较采用单因素方差分析,以P < 0.05表示差异有统计学意义。
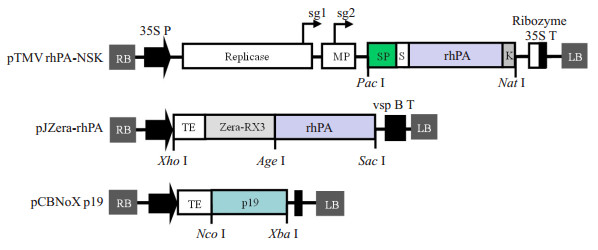
2 结果 2.1 表达载体的设计、构建及其结构特征rhPA病毒表达载体的表达框依次由烟草病程相关蛋白(PRs)1b的信号肽、Strep Ⅱ亲和标签序列、密码子优化后的rhPA基因序列和KDEL内质网截留信号肽(Endoplasmic reticulum retention sequence)序列组成;Zera-rhPA融合基因表达框是在rhPA基因序列5'端依次添加烟草蚀纹病毒(TEV)的翻译增强序列和Zera-RX3序列设计获得(图 1)。体外分别合成表达框序列,利用合成序列两侧的酶切位点,亚克隆构建相应的植物瞬时表达载体。两个载体的表达单元均位于农杆菌二元载体左右边界之间,可以通过农杆菌介导的渗滤法接种受体植物并在受体细胞内质网中表达rhPA。在后续实验中,载体将与表达番茄丛矮病毒(TBSV)编码的RNA沉默抑制子基因p19的载体pCBNoX p19(图 1)共接种,以规避转录后RNA沉默造成的外源基因表达抑制。
|
图 1 表达载体的结构 Fig.1 Structures of the expression vectors. pTMV rhPA-NSK: Tobacco mosaic virus (TMV)-based vectors used to express rhPA in the endoplasmic reticulum (ER); RB: Right borders of the T-DNA; 35S P: Enhanced Cauliflower mosaic virus 35S promoter; Replicase: RNA-dependent RNA polymerase of TMV; sg1/sg2: Subgenomic mRNA1/mRNA2 promotors of the TMV; MP: Movement-protein of TMV; SP: Pr1b tobacco secretory signal peptide; S: StrepⅡ tag; rhPA: A truncated human tissue plasminogen activator; K: ER retention signal; Ribozyme: Ribozyme; 35S T: CaMV transcription terminator; LB: Left borders of the T-DNA. pJ Zera-rhPA: Plant binary vector used for expression of recombinant Zera-rhPA fusion; TE: Translational enhancer of Tobacco etch virus (TEV); Zera- RX3: γ-Zein ER-accumulating domain; vspB T: Soybean vegetative storage protein terminator. pCBNoX p19: Plant binary vector expressing the RNA silencing suppressor of p19 protein; P19: 19 kD RNA silencing suppressor from Tomato bushy stunt virus (TBSV). All the vectors contain nptⅡ genes within their T-DNAregions (not shown in the maps). |
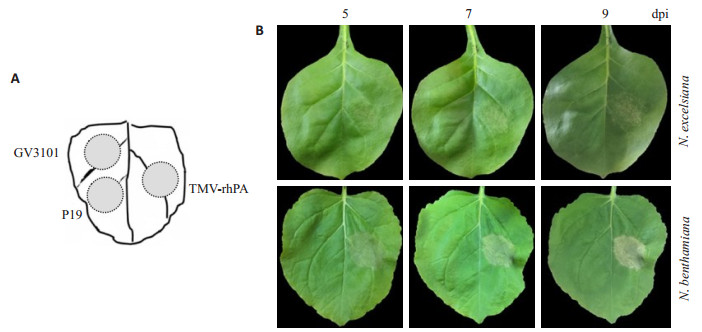
将各处理的农杆菌菌液分别接种受体植物N. benthamiana和N. excelsiana同一叶片的不同部位(图 2A)。接种后第5天,pTMV rhPA-NSK病毒载体在N. benthamiana叶片接种部位上出现明显坏死症状,在N. excelsiana叶片上出现轻微的坏死斑。随着时间的推移,坏死症状在两种烟草叶片上逐渐加强。在整个观察期内,接种农杆菌GV3101和pCBNoX p19表达载体的叶片接种部位未观察到坏死症状(图 2B)。
|
图 2 农杆菌渗滤接种TMV病毒表达载体后烟草叶片上的症状观察 Fig.2 Symptoms on Nicotiana leaves agroinfiltrated with TMV-based vectors. A: Filtrates of Agrobacterium GV3101 containing either expressing vectors or empty vector were agroinfiltrated or co-agroinfiltrated at different areas within a Nicotiana leaf. GV3101: Empty GV3101; P19: pCBNoX p19 alone; TMV-rhPA: Co-infiltrated with pTMV rhPA-NSK and pCBNoX p19; B: Photographs were taken at 5, 7 and 9 days post-inoculation (dpi). |
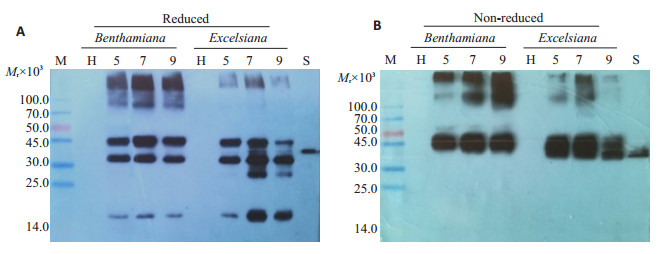
Western blot结果显示,阴性对照样品未产生特异性杂交条带,rhPA标准样品在39 000处存在特异性杂交条带(图 3)。还原条件下,pTMV rhPA-NSK病毒载体样品分别在100 000、45 000、30 000和14 000附近处呈现杂交条带(图 3A);非还原条件下,30 000和14 000附近的杂交条带消失,而45 000处的目的条带依然存在(图 3B)。
|
图 3 Western blot分析rhPA在烟叶片中的表达情况 Fig.3 Western blot analysis of the recombinant rhPA from infiltrated Nicotiana leaves. Recombinant rhPA protein was detected by Western blotting under reduced (A) and non-reduced condition (B), respectively. M: Protein molecular weight markers (in kD); H: Total soluble protein extracts from empty GV3101 infiltrated leaves; lane 5, 7 and 9: extracts from infiltrated leaves at 5, 7 and 9 dpi, respectively; S: E. coli produced rhPA reference standard. |
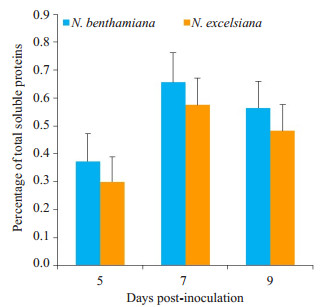
ELISA结果显示(图 4),N. benthamiana中rhPA的表达量在各采样点均高于N. excelsiana(P < 0.05),其最高表达量约占可溶性总蛋白的0.6%,相当于60.0 μg/g鲜叶左右。
|
图 4 重组rhPA蛋白的ELISA定量分析 Fig.4 Quantification of the recombinant rhPA protein in crude extracts. ELISA was used to quantify recombinant rhPA expressed by TMV-based vectors at 5, 7 and 9 dpi. Expression levels reported in percentage of total soluble proteins (n=3). P < 0.05. |
体外溶栓活性分析结果显示,在植物表达的rhPA样品和原核表达rhPA标准样品中均可以清晰地观察到纤维蛋白溶解圈;而在空白对照或加入纤溶酶原激活物抑制剂的样品中未观察到溶解圈(图 5)。此外,溶解圈的直径大小与rhPA的浓度呈现依赖效应(图 5)。

|
图 5 纤维蛋白琼脂糖平板法分析重组rhPA的体外溶栓活性 Fig.5 Bioactivity analyses of rhPA using fibrin agarose plate assay (FAPA). Samples were spotted onto a plate containing fibrin polymerized by thrombin. 1, blank control, buffer alone; 2 and 2', repeats, TSPs with PAI-1 (1 μg), inhibition of fibrinolytic activity served as negative control; 3 and 3', repeats, TSPs; 4 and 4′, repeats, two times dilution of TSPs; 5, four times dilution of TSPs; 6, 0.3 μg/mL of rhPA produced in E. coli, positive control. |
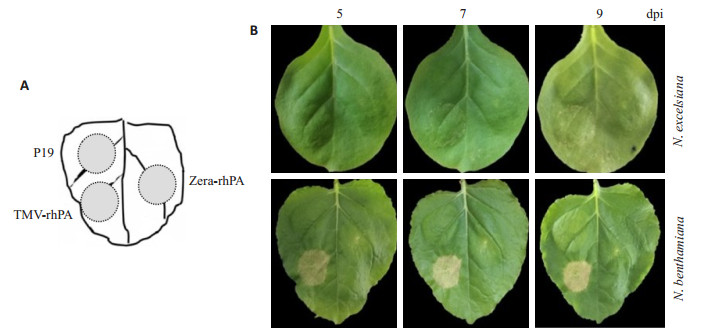
按图 6A所示,将各处理的农杆菌菌液分别接种于适龄N. benthamiana和N. excelsiana植株叶片上。观察结果显示,TMV rhPA-NSK病毒表达载体接种部位依然呈现坏死症状,坏死症状随着时间推移加剧,且N. benthamiana上的症状比N. excelsiana严重。在整个观察期内,pJ Zera-rhPA表达载体在两个烟草品种上的接种部位未观察到坏死症状(图 6B)。
|
图 6 农杆菌渗滤接种pJ Zera-rhPA载体后烟草叶片上的症状观察 Fig.6 Symptoms on Nicotiana leaves after agroinfiltrated with pJ Zera-rhPA expression vectors. A: Filtrates of Agrobacterium GV3101 containing either expressing vectors were agroinfiltrated or co-agroinfiltrated at different areas within a Nicotiana leaf. P19: pCBNoX p19 alone; TMV-rhPA: co-infiltrated with pTMV rhPA-NSK and pCBNoX p19; Zera-rhPA: co-infiltrated with pJ Zera-rhPA and pCBNoX p19; B: Photographs were taken at 5, 7 and 9 dpi. |
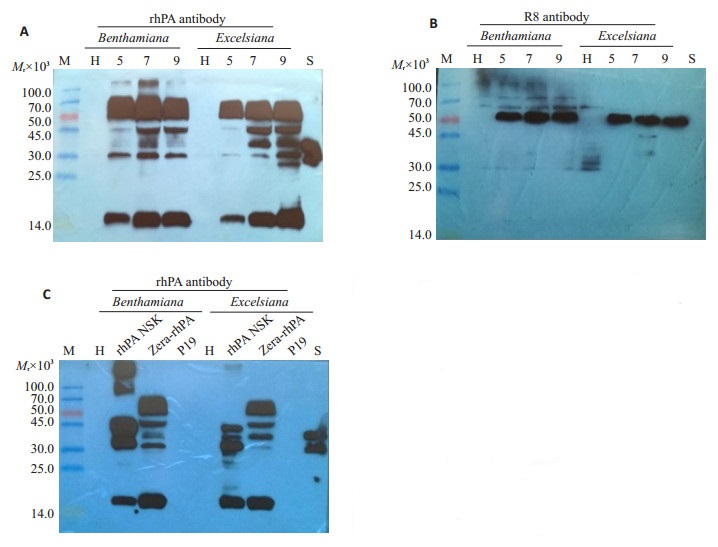
可溶性总蛋白的Western blot结果显示,阴性对照样品(H)未产生特异性杂交条带,rhPA标准样品(S)在39 000处存在特异性杂交条带。rhPA抗体的杂交结果显示,两种烟草样品中均出现了55 000左右的目的杂交条带,在14 000~45 000之间存在其它杂交条带(图 7A);用Zera标签R8抗体杂交,仅在55 000处存在目的杂交条带(图 7B);rhPA比较分析发现,Zera-rhPA重组产物在14 000~45 000之间的杂交条带的带型和条带相对分子质量与TMV病毒载体表达的rhPA基本一致(图 7C)。
|
图 7 Western blot分析重组Zera-rhPA在烟叶片中的表达情况 Fig.7 Western blot analysis of the recombinant Zera-rhPA from infiltrated Nicotiana leaves. A: Recombinant rhPA protein was detected by Western blot with rhPA antibody; B: Recombinant rhPA protein was detected by Western blot with R8 antibody; C: Recombinant rhPA protein expressing by TMV-based vectors or pJ Zera-rhPA vectors at 7 dpi was detected by Western blot with rhPA antibody. M: Protein molecular weight markers in kD; H: Total soluble protein extracts from empty GV3101 infiltrated leaves; lane 5, 7 and 9: extracts from infiltrated leaves at 5, 7 and 9 dpi, respectively; S: E. coli produced rhPA reference standard; rhPA-NSK: extracts from TMV-based vectors infiltrated leaves at 7 dpi; ZerarhPA: extracts from pJ Zera-rhPA vectors infiltrated leaves at 7 dpi; P19: extracts from pCBNoX p19 vectors infiltrated leaves at 7 dpi. |
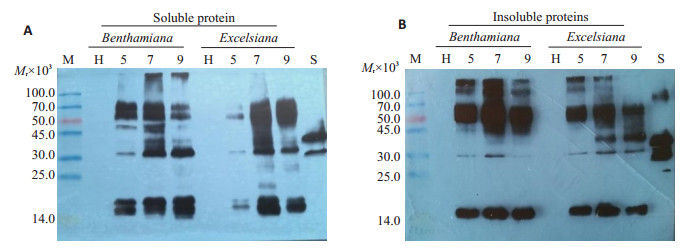
重组Zera-rhPA分级提取产物的Western blots结果显示,上清组分(可溶部分)和沉淀组分(蛋白质微粒)均存在55 000的目的杂交条带(图 8A、B)。与上清组分相比,沉淀组分14 000~45 000之间依然存在其它杂交条带,而且14 000左右的低相对分子质量多肽含量高于25 000~45 000之间的高相对分子质量多肽含量(图 8B)。
|
图 8 重组Zera-rhPA的组成分析 Fig.8 Analysis of recombinant Zera-rhPA fractions by Western blot. A: Recombinant rhPA protein in the supernatant after centrifugation was detected by Western blot; B: Recombinant rhPA protein in the pellet was detected by Western blot. M: Protein molecular weight markers in kD; H: Total soluble protein extracts from empty GV3101 infiltrated leaves; lane 5, 7 and 9: extracts from infiltrated leaves at 5, 7 and 9 dpi, respectively; S: E. coli produced rhPAreference standard. |
心血管疾病在人类致死性疾病中位列前3名,仅我国每年需要进行溶栓药物治疗的人数就超过300万。rhPA是对人组织纤溶酶原激活剂(t-PA)进行基因工程改造后的第3代溶栓药物[14]。与t-PA相比,其血浆半衰期延长了2~20倍,溶栓效率提高3~5倍,不良反应无统计学差别[15]。然而,价格昂贵已经成为限制其广泛服务于人类健康的主要“瓶颈”。植物是一个成本低廉的“绿色工厂”[16],植物瞬时表达系统为降低rhPA的生产成本提供了一种可供选择的途径。因而,我们构建了rhPA的植物病毒和非病毒瞬时表达载体,探索在植物中表达rhPA的可行性。
病毒载体接种实验显示,TMV病毒载体可以在烟草叶片中表达rhPA,表达产物能够在纤维蛋白平板上将非活性纤溶酶原转化为活性纤溶酶进而溶解纤维蛋白形成溶解圈,表明表达产物具有体外溶栓活性。rhPA表达量约为60 μg/g鲜叶,是前人利用小西葫芦黄花叶病毒(ZYMV)在葫芦叶片中表达rhPA[17]的10倍左右,也是目前利用植物表达rhPA已报道的最高表达量,认为植物病毒表达系统为低成本rhPA的生产提供了一种富有潜力的新途径。已知TMV病毒载体是当前表达效率最高的植物病毒载体之一[18],N. benthaminana被认为是目前瞬时表达系统的最适受体植物[19],因而认为植物病毒表达载体的类型和受体植物种类是二者间rhPA表达量差异的主要原因。实验过程中观察到,TMV病毒载体的N. benthamiana叶片接种部位会呈现严重的细胞坏死现象。前人利用TMV病毒载体在N. benthamiana中表达人生长激素[20]、乙肝病毒核心抗原[21-22]、IgG单克隆抗体[23]、结核分支杆菌抗原ESAT6[24]、Actinohivin[25]、人上皮粘蛋白1[26]、人补体因子5a[27]和西尼罗病毒包膜蛋白结构域Ⅲ[28]等外源基因时也观察到类似的严重坏死症状。由于ZYMV病毒表达载体和前人转基因烟草表达rhPA未见坏死症状(表明rhPA对受体植物细胞无细胞毒性),而且TMV病毒载体超表达GFP也不会造成受体细胞死亡[13],因此推测坏死症状的产生是TMV病毒载体与rhPA共同作用的结果。同时也观察到,TMV载体在N. excelsiana叶片接种部位的坏死症状程度低于N. benthamiana。尽管N. excelsiana中rhPA的表达量略低于N. benthaminana,但是它不仅具有N. benthaminana生长快和对农杆菌敏感性高等优点,而且还具有叶片生物量高和对生长环境要求低的特点[29],综合评判认为N. excelsiana是一种更适合rhPA表达的受体植物。虽然TMV病毒载体接种烟草叶片会出现不同程度的坏死,但是在坏死组织中依然存在完整的rhPA蛋白。此外,rhPA蛋白分子第275位和第276位氨基酸之间存在蛋白酶水解位点,水解断裂后形成由第276~527位氨基酸组成的轻链以及由第1~3位氨基酸和第176~275位氨基酸组成的重链,双链间由一对二硫键连接[14]。还原与非还原条件下的实验中观察到的部分rhPA表达产物存在内部断裂现象与rhPA蛋白分子这一结构特征相关,这一现象也在其它真核表达系统表达rhPA时出现。
Zera融合标签是玉米种子γ-zein醇溶蛋白的截短突变体,由8个PPPVHL重复衔接序列和富含脯氨酸结构域构成[30]。Zera标签及其融合蛋白可以在植物叶片细胞内质网上形成蛋白质颗粒,提高了外源蛋白对受体细胞蛋白水解酶系统的抗性,有助于克服重组蛋白在细胞中积累水平低的问题[31]。人生长激素[32]、木聚糖酶[33]、HIV表面抗原Nef[34]和P24[35]、人类T细胞表面受体CD14[36]、鼠疫耶尔辛杆菌F1-V抗原[37]以及抗菌肽蛋白[38]等都先后通过与Zera标签融合的方式在植物中获得高效地表达。我们在实验中也使用Zera标签融合表达rhPA。据实验结果推测,Zera标签与其融合的rhPA之间可能存在着易断裂位点,这与前人利用HFBI标签在烟草中表达流感病毒血凝素1时发现的标签与其融合蛋白间发生断裂的情况一致[39],但是具体原因未知。Zera-rhPA重组产物的分级分离实验表明,表达产物中同时存在可溶和不溶两种形式,认为Zera标签蛋白质微粒的形成是一个从可溶到聚集的动态演变过程,推测Zera融合蛋白在细胞内质网中需要积累到一定的量才能聚集形成蛋白质微粒。聚集态蛋白质微粒对rhPA的完整性具有一定程度的保护作用,而可溶形式的ZerarhPA重组产物依然会受到细胞蛋白酶的攻击。前人对纯化的Zera标签蛋白质微粒进行蛋白质组学的研究结果显示,微粒中除了Zera融合蛋白还存在多种非预期的其它可溶蛋白质[40]。我们的结果也表明在Zera标签介导的蛋白微粒形成过程中,一些可溶性的多肽可能被包被进了蛋白质微粒中。而且,由于低分子量的多肽含量高于高分量的多肽,推测低分子量的多肽更易于被包裹进蛋白质微粒。由于Zera融合标签与rhPA序列之间存在易断裂位点,而且其形成的保护性蛋白颗粒不能对rhPA实现高效地保护,初步认为该技术目前不适合用于rhPA在整株植物的表达中。
我们利用植物病毒载体和Zera标签尝试在植物中瞬时表达rhPA,并对其表达产物的特点分别进行了分析,研究结果为解决商品化rhPA生产成本高问题提供实验依据和新的研究思路。
| [1] |
Gurman P, Miranda OR, Nathan A, et al. Recombinant tissue plasminogen activators (rtPA): A review[J]. Clin Pharmacol Ther, 2015, 97(3): 274-85. DOI:10.1002/cpt.v97.3 |
| [2] |
Bagheri RK, Keihanian F, Ahmadi M, et al. Successful treatment of prosthetic pulmonary valve thrombosis with Reteplase: a case report[J]. Int Med Case Rep J, 2018, 11(3): 205-8. |
| [3] |
Fathi-Roudsari M, Maghsoudi N, Maghsoudi A, et al. Autoinduction for high level production of biologically active Reteplase in Escherichia coli[J]. Protein Expres Purif, 2018, 151: 18-22. DOI:10.1016/j.pep.2018.05.008 |
| [4] |
Lucas BK, Giere LM, Demarco RA, et al. High-level production of recombinant proteins in CHO cells using a dicistronic DHFR intron expression vector[J]. NucleicAcids Res, 1996, 24(9): 1774-9. DOI:10.1093/nar/24.9.1774 |
| [5] |
Majidzadeh-AK, Khalaj V, Fatemeh D, et al. Cloning and expression of functional Full-Length human tissue plasminogen activator in pichia pastoris[J]. Appl Biochem Biotechnol, 2010, 162(7): 2037-48. DOI:10.1007/s12010-010-8979-z |
| [6] |
He Z, Lu R, Zhang T, et al. A novel recombinant human plasminogen activator: Efficient expression and hereditary stability in transgenic goats and in vitro thrombolytic bioactivity in the milk of transgenic goats[J]. PLoS One, 2018, 13(8): e0201788. DOI:10.1371/journal.pone.0201788 |
| [7] |
Aflakiyan S, Sadeghi HM, Shokrgozar M, et al. Expression of the recombinant plasminogen activator (Reteplase) by a non-lytic insect cell expression system[J]. Res Pharm Sci, 2013, 8(1): 9-15. |
| [8] |
Goojani HG, Javaran MJ, Nasiri J, et al. Expression and Large-Scale production of human tissue plasminogen activator (t-PA) in transgenic tobacco plants using different signal peptides[J]. Appl Biochem Biotechnol, 2013, 169(6): 1940-51. DOI:10.1007/s12010-013-0115-4 |
| [9] |
Hidalgo D, Abdoli-Nasab M, Jalali-Javaran MA, et al. Biotechnological production of recombinant tissue plasminogen activator protein (reteplase) from transplastomic tobacco cell cultures[J]. Plant Physiol Biochem, 2017, 118(9): 130-7. |
| [10] |
Hemayatkar M, Mahboudi F, Majidzadeh- A KA, et al. Increased expression of recombinant human tissue plasminogen activator in Leishmania tarentolae[J]. Biotechnol J, 2010, 5(11, SI): 1198-206. DOI:10.1002/biot.v5.11 |
| [11] |
Peng J, Gao JT, Zhang YC, et al. Recombinant expression of rt-PA gene (encoding Reteplase) in gametophytes of the seaweed Laminaria japonica (Laminariales, Phaeophyta)[J]. Sci China C Life Sci, 2008, 51(12): 1116-20. DOI:10.1007/s11427-008-0143-4 |
| [12] |
Hidalgo D, Sanchez R, Lalaleo L, et al. Biotechnological production of pharmaceuticals and biopharmaceuticals in plant cell and organ cultures[J]. Curr Med Chem, 2018, 25(30): 3577-3596. DOI:10.2174/0929867325666180309124317 |
| [13] |
Lindbo JA. TRBO: a high-efficiency Tobacco Mosaic virus RNAbased overexpression vector[J]. Plant Physiol, 2007, 145(4): 1232-40. DOI:10.1104/pp.107.106377 |
| [14] |
Mohammadi E, Seyedhosseini-Ghaheh H, Mahnam K, et al. Reteplase: Structure, Function, and Production[J]. Adv Biomed Res, 2019, 8(1): 19. DOI:10.4103/abr.abr_169_18 |
| [15] |
Zamanlu M, Farhoudi M, Eskandani M, et al. Recent advances in targeted delivery of tissue plasminogen activator for enhanced thrombolysis in ischaemic stroke[J]. J Drug Target, 2018, 26(2): 95-109. DOI:10.1080/1061186X.2017.1365874 |
| [16] |
Ganapathy M. Plants as bioreactors-A review[J]. Adv Tech Biol Med, 2016, 4(161): 2379-1764. |
| [17] |
Javaran VJ, Shafeinia A, Javaran MJ, et al. Transient expression of recombinant tissue plasminogen activator (rt-PA) gene in cucurbit plants using viral vector[J]. Biotechnol Lett, 2017, 39(4): 607-12. DOI:10.1007/s10529-017-2285-6 |
| [18] |
Hefferon K. Plant virus expression vectors: a powerhouse for global health[J]. Biomedicines, 2017, 5(3): 44. |
| [19] |
Bally J, Jung H, Mortimer C, et al. The rise and rise of Nicotiana benthamiana: a plant for all reasons[J]. Annu Rev Phytopathol, 2018, 56: 405-426. DOI:10.1146/annurev-phyto-080417-050141 |
| [20] |
Gils M, Kandzia R, Marillonnet S, et al. High-yield production of authentic human growth hormone using a plant virus-based expression system[J]. Plant Biotechnol J, 2005, 3(6): 613-20. DOI:10.1111/pbi.2005.3.issue-6 |
| [21] |
Huang Z, Santi L, Lepore K, et al. Rapid, high- level production of hepatitis B core antigen in plant leaf and its immol/Lunogenicity in mice[J]. Vaccine, 2006, 24(14): 2506-13. DOI:10.1016/j.vaccine.2005.12.024 |
| [22] |
Huang Z, Lepore K, Elkin G, et al. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system[J]. Plant Biotechnol J, 2008, 6(2): 202-9. DOI:10.1111/pbi.2008.6.issue-2 |
| [23] |
Giritch A, Marillonnet S, Engler C, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors[J]. Proc NatlAcad Sci USA, 2006, 103(40): 14701-6. DOI:10.1073/pnas.0606631103 |
| [24] |
Dorokhov YL, Sheveleva AA, Frolova OY, et al. Superexpression of tuberculosis antigens in plant leaves[J]. Tuberculosis, 2007, 87(3): 218-24. DOI:10.1016/j.tube.2006.10.001 |
| [25] |
Matoba N, Husk AS, Barnett BW, et al. HIV-1 neutralization profile and plant-based recombinant expression of actinohivin, an Env glycan-specific lectin devoid of T-cell mitogenic activity[J]. PLoS One, 2010, 5(6): e11143. DOI:10.1371/journal.pone.0011143 |
| [26] |
Pinkhasov J, Alvarez ML, Rigano MM, et al. Recombinant plantexpressed tumour-associated MUC1 peptide is immol/Lunogenic and capable of breaking tolerance in muc1.tg mice[J]. Plant Biotechnol J, 2011, 9(9): 991-1001. DOI:10.1111/pbi.2011.9.issue-9 |
| [27] |
Nausch H, Mikschofsky H, Koslowski R, et al. Expression and subcellular targeting of human complement factor c5a in Nicotiana species[J]. PLoS One, 2012, 7(12): e53023. DOI:10.1371/journal.pone.0053023 |
| [28] |
He J, Li P, Lai H, et al. A plant-produced antigen elicits potent immol/ Lune responses against West Nile Virus in mice[J]. Biomed Res Int, 2014(1): 952865. |
| [29] |
Shamloul M, Trusa J, Mett V, et al. Optimization and utilization of agrobacterium-mediated transient protein production in nicotiana[J]. J Vis Exp, 2014, 86(86): e51204. |
| [30] |
Llompart B, Lloptous I, Marzabal P, et al. Protein production from recombinant protein bodies[J]. Process Biochem, 2010, 45(11): 1816-20. DOI:10.1016/j.procbio.2010.01.016 |
| [31] |
Llop I, Torrent M, Marzabal P, et al. Zera®, a novel technology for stable accumulation and easy recovery of recombinant proteins in eukaryotic protein-production hosts[J]. Microb Cell Fact, 2006, 5(1): S42. |
| [32] |
Torrent M, Llompart B, Lasserre- Ramassamy S, et al. Eukaryotic protein production in designed storage organelles[J]. BMC Biol, 2009, 7(1): 5. DOI:10.1186/1741-7007-7-5 |
| [33] |
Lloptous I, Ortiz M, Torrent M, et al. The expression of a xylanase targeted to ER-protein bodies provides a simple strategy to produce active insoluble enzyme polymers in tobacco plants[J]. PLoS One, 2011, 6(4): e19474. DOI:10.1371/journal.pone.0019474 |
| [34] |
Virgilio MD, Marchis FD, Bellucci M, et al. The human immol/ Lunodeficiency virus antigen Nef forms protein bodies in leaves of transgenic tobacco when fused to zeolin[J]. J Exp Bot, 2008, 59(10): 2815. DOI:10.1093/jxb/ern143 |
| [35] |
Virgili-López G, Langhans M, Bubeck J, et al. Comparison of membrane targeting strategies for the accumulation of the human immol/Lunodeficiency virus p24 protein in transgenic tobacco[J]. Int J Mol Sci, 2012, 14(7): 13241-65. |
| [36] |
Liu XX, Sun L, Li CL, et al. Enhanced expression of the human CD14 protein in tobacco using a 22- kDa alpha-zein signal peptide[J]. Plant Cell Tissue Organ Cult, 2013, 112(1): 9-18. DOI:10.1007/s11240-012-0206-x |
| [37] |
Alvarez ML, Topal E, Martin F, et al. Higher accumulation of F1-V fusion recombinant protein in plants after induction of protein body formation[J]. Plant Mol Biol, 2009, 45(1): S36-7. |
| [38] |
Company N, Nadal A, La PL, et al. The production of recombinant cationic α-helical antimicrobial peptides in plant cells induces the formation of protein bodies derived from the endoplasmic reticulum[J]. New Biotech, 2014, 12(1): 81-92. |
| [39] |
Jacquet N, Navarre C, Desmecht D, et al. Hydrophobin fusion of an influenza virus hemagglutinin allows high transient expression in Nicotiana benthamiana, easy purification and immol/Lune response with neutralizing activity[J]. PLoS One, 2014, 9(12): e115944. DOI:10.1371/journal.pone.0115944 |
| [40] |
Joseph M. Proteomic characterisation of endoplasmic reticulumderived protein bodies in tobacco leaves[J]. BMC Plant Biol, 2012, 12(1): 36-36. DOI:10.1186/1471-2229-12-36 |
 2019, Vol. 39
2019, Vol. 39

