急性肾损伤是顺铂的主要副作用,约1/3接受顺铂治疗的病人可发生急性肾损伤[1-4]。顺铂致急性肾损伤的特征性病理改变是肾小管-间质急性损伤,近端肾小管上皮细胞损伤及其所诱发的炎症反应是顺铂致急性肾损伤的重要病理生理机制。pannexin-1通道蛋白作为一种位于细胞膜上的非选择性通道蛋白,在全身各组织中广泛表达,离子及大分子物质可通过pannexin-1通道跨膜流动,导致细胞功能变化,应激状态下的细胞膜表面pannexin-1通道蛋白表达上调,可使得胞内ATP释放至细胞外[5-9]。研究证实,释放至胞外的ATP作为一种损伤相关的分子模式,可引发无菌性炎症反应,导致炎症细胞浸润及炎症因子释放而加剧组织损伤[10-14]。既往研究表明,阻断pannexin-1通道激活,可通过抑制受损细胞释放ATP,减轻组织损伤[14-17]。在肾脏中,pannexin-1通道蛋白在肾皮质近端肾小管上皮细胞有较高的基础表达[18]。那么,pannexin-1通道蛋白是否参与顺铂所致的近端肾小管上皮细胞损伤过程以及肾间质的炎症反应过程?目前未见相关研究报道。
1 材料和方法 1.1 主要试剂顺铂(Sigma-Aldrich)、Carbenoxolone(SigmaAldrich);DMEM低糖完全培养基、胎牛血清和胰蛋白酶(Gibco);兔抗pannexin-1单克隆抗体(GeneTex);兔抗鼠KIM-1单克隆抗体(R & D);兔抗鼠F4/80单克隆抗体(Proteintech);兔抗鼠CD4单克隆抗体(Proteintech);β- actin抗体(Proteintech);Alexa Fluor 488标记山羊抗兔IgG二抗(中杉金桥);碱性磷酸酶偶联亲和纯化山羊抗兔IgG二抗(Proteintech);Rhodamine Lens Culinaris Agglutinin(Vector Labs);DAPI(碧云天);DAB显色试剂盒(中杉金桥);Trizol、逆转录试剂盒TaKaRa Prime ScriptTM RT Master MiX及荧光定量试剂盒TaKaRa SYBR Premix Ex TaqTM(TaKaRa);细胞培养瓶25 cm2及细胞培养皿(Corning);OCT包埋剂(樱花)。引物由北京擎科生物公司设计合成。
1.2 实验动物SPF级雄性C57BL/6J小鼠26只,6~8周龄,约20~ 25 g,由南方医科大学动物中心提供。分笼饲养于南方医科大学南方医院SPF级实验动物房,室温保持在25± 2 ℃,湿度保持在(55±5)%,动物自由进食,自由饮水,保持12 h/d的昼夜循环。所有实验操作符合科技部与南方医科大学动物实验伦理委员会的要求。
1.3 顺铂致急性肾损伤小鼠模型的制备26只SPF级雄性C57BL/6J小鼠随机分为对照组、顺铂模型组、顺铂+Carbenoxolone处理组(Cis+CBX组)。顺铂模型组、Cis+CBX组给予顺铂溶液20 mg/kg腹腔注射,对照组以等量生理盐水腹腔注射;Cis+CBX组于顺铂处理前30 min、处理后24 h和48 h分别腹腔注射Carbenoxolone溶液20 mg/kg,其余两组以等量生理盐水腹腔注射。3组于顺铂处理72 h后处死小鼠。以水平位切取2 mm肾组织,福尔马林于4 ℃固定24 h,脱水、包埋保存;以水平位切取2 mm肾组织,4%多聚甲醛于4 ℃固定6 h,再以30%蔗糖溶液脱水2~3 h,包埋后储存于-80 ℃冰箱;剩余肾组织标本与血浆标本储存于-80 ℃冰箱。
1.4 细胞培养及分组人肾小管上皮细胞株HK-2细胞由广东医科大学肾脏疾病研究所刘华锋教授团队赠予。
于10%胎牛血清和抗生素(100 U/mL青霉素和0.1 mg/mL链霉素)的DMEM低糖完全培养基在37 ℃,5%CO2条件下培养,隔2 d传代1次,选取对数生长期细胞进行实验。细胞实验分对照组和顺铂组,顺铂模型组应用终浓度为50 μmol/L的顺铂分别处理4、6、12、18和24 h,对照组予等量10% FBS DMEM低糖完全培养基培养24 h。
1.5 Western blot细胞经分组处理后,预冷的PBS清洗3遍后RAPI裂解;动物肾组织从-80 ℃冰箱取出称量,研磨后RAPI裂解。收集各组裂解物,离心后收集上清液。Bicinchoninicacid法测定样品蛋白质浓度,加入5×上样缓冲液,煮沸5 min。每孔上样50 μg,进行SDS-PAGE(10%或12%)后,恒流300 mA 60 min将蛋白电转至NC膜上。膜用含5%脱脂奶粉的TBS封闭液室温封闭1 h,以5%脱脂奶粉的TBS配制的一抗4 ℃孵育过夜。吸尽一抗,加入辣根标记二抗室温慢摇下孵育1 h,吸尽二抗,用TBST洗涤条带4次,5 min/次,使用化学试剂发光显影、拍照。
1.6 Real-time PCRTrizol试剂提取总RNA,并溶于无RNAse水中,紫外分光光度计测定A260/A280的比值1.8~2.0。按TaKaRa PrimeScriptTM RT Master Mix试剂盒说明进行cDNA合成。人Pannexin-1、鼠Pannexin-1、鼠Kim-1、鼠NGAL及人和鼠内参β-actin引物序列由北京擎科新业生物技术有限公司合成,引物序列见表 1。待测cDNA加入20 μL反应体系中,按照TaKaRa SYBRR Premix Ex TaqTM试剂盒说明在LightCycler R480型荧光定量PCR扩增仪进行定量检测。PCR反应条件:95 ℃ 30 s变性,95 ℃ 5 s,60 ℃ 30 s循环40次,95 ℃ 5 s,60 ℃ 60 s融解,50 ℃ 30 s降温。融解曲线分析表明PCR反应产物为单独的双链DNA。每一个样品目的基因扩增的循环数(Ct)都依据β-actin做校正,得出ΔCt(Ct样品目的基因-Ct样品的β-actin)。目标基因表达差异以经过处理的样本相对于未经处理的样本的倍数表示,即检测基因的差异=2-ΔΔC(ΔΔCt=ΔCt处理样品–ΔCt未处理样品)。
| 表 1 Pannexin-1、KIM-1、NGAL及β-actin引物序列 Tab.1 Primer sequence of Pannexin-1, KIM-1, NGAL and β-actin |
6 μm厚冰冻组织切片经PBS清洗后,用含2.5%牛血清白蛋白的缓冲液封闭30 min,滴加抗F4 /80抗体(1:50稀释)或抗CD4抗体(1:50稀释),4 ℃孵育过夜。次日PBS清洗3次,5 min/次,滴加Alexa Fluor 488标记山羊抗兔IgG二抗(1:50稀释)室温下避光孵育1 h后,PBS清洗3次,5 min/次,室温避光滴加Rhodamine Lens Culinaris Agglutinin(5 mg/mL)染肾小管,室温下避光滴加DAPI染核,用抗荧光淬灭剂封片。玻片干燥后,于激光共聚焦显微镜Fv1000(Olympus)上观察切片,应用Confocal Microscopy软件(Olympus,日本)拍摄及分析图片[38]。
1.8 免疫组化染色检测肾组织中KIM-1表达。3 μm厚石蜡切片经脱蜡水化后,枸橼酸修复液进行高压抗原修复,然后H2O2封闭内源性过氧化物酶;接着用含正常血清的PBS液封闭切片,再滴加抗KIM-1抗体(1:100稀释),4 ℃过夜,用PBS代替一抗作空白对照。第2天,切片清洗后,DAB显色,梯度酒精脱水、封片,显微镜下观察结果。KIM-1抗体检测在肾组织中出现棕黄色染色为阳性表达。
1.9 肾脏病理PAS染色肾组织经福尔马林常温下固定24 h后,梯度脱水、石蜡包埋,切2 μm厚石蜡切片,行PAS染色,在光学显微镜下观察组织损伤程度,并行肾小管损伤评分。每个组织随机选取10个高倍镜视野评分,采用以下评分标准:0分,无明显损伤;1分,轻度损伤,存在上皮细胞肿胀、官腔扩张;2分,严重损伤,存在扁平肾小管上皮、核染色缺失、管腔堵塞;3分,破损肾小管上皮细胞,存在细胞脱落、细胞核染色消失、大量管型。采用双盲随机分析方法,每张片子取10个视野平均值进行评估分析。
1.10 统计方法用SPSS 13.0软件处理数据,所有数据采用均数±标准差表示,两个样本均数间比较采用独立样本t检验;多个样本间均数比较采用单因素方差分析,均数间两两比较,方差齐性时用LSD法,方差不齐时用Dunnett's法分析,P < 0.05为差异有统计学意义。
2 结果 2.1 顺铂所致急性肾损伤小鼠模型肾组织pannexin-1表达升高RT-qPCR及Western blot结果均显示,与对照组相比,顺铂模型组的肾组织pannexin-1 mRNA及蛋白表达量均显著升高,差异有统计学意义(P < 0.05,图 1A~C)。
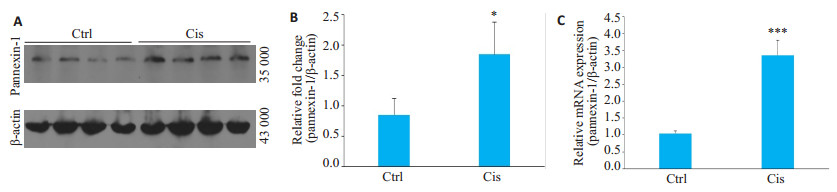
|
图 1 顺铂诱导急性肾损伤小鼠模型的肾组织内pannexin-1表达水平变化 Fig.1 Expression of pannexin-1 in renal tissue in cisplatin-induced AKI mice model (Mean±SD, n=8-9). A: Western blotting for detecting pannexin-1 and β-actin expressions; B: Densitometric analysis of Western results; C: RT-qPCR showing pannexin-1 expressions; Ctrl: control group; Cis: Cis model group. *P < 0.05, ***P < 0.001 vs Ctrl. |
RT-qPCR检测结果显示,与对照组相比,体外培人肾小管上皮细胞株(HK-2细胞)经顺铂(50 μmol/L)刺激后,pannexin-1 mRNA表达自12 h后开始升高(P < 0.05,图 2C),且在24 h观察时间内,pannexin-1 mRNA表达升高呈时间依赖性(P < 0.005,图 2C)。Western blot结果显示,顺铂刺激HK-2细胞后可致pannexin-1通道蛋白表达水平上调(P < 0.005,图 2A、B)。
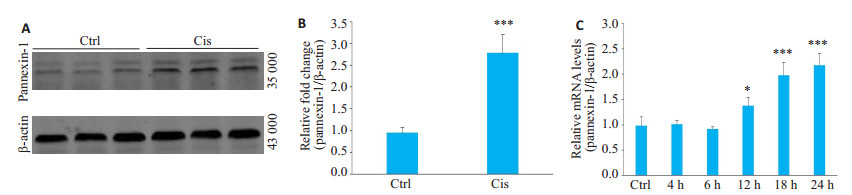
|
图 2 顺铂刺激HK-2细胞后pannexin-1表达水平变化 Fig.2 Changes of pannexin-1 expression after cisplatin treatment in HK-2 cells (Mean±SD, n=3). A: Western blotting showing pannexin-1 and β-actin expressions; B: Densitometric analysis of Western results. C: RT-qPCR showing pannexin-1 expressions. Ctrl: control group; Cis: Cis model group. *P < 0.05, ***P < 0.001 vs Ctrl. |
经pannexin-1阻断剂Carbenoxolone阻断pannexin-1后,与顺铂模型组相比,Cis+CBX组血浆BUN、Scr水平降低(P < 0.01,图 3A、B)。
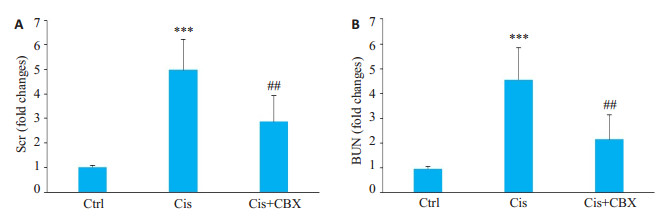
|
图 3 Carbenoxolone预处理对顺铂所致急性肾损伤小鼠模型血浆BUN、Scr水平影响 Fig.3 Effect of carbenoxolone pretreatment on plasma BUN (A) and Scr (B) levels in mice with cisplatininduced AKI (Mean±SD, n=8-9). Ctrl: control group; Cis: Cis model group; Cis+CBX: Cis+CBX treatment group. ***P < 0.001 vs Ctrl; ##P < 0.01 vs Cis. |
PAS染色结果显示,与顺铂模型组相比,经Carbenoxolone阻断pannexin-1后,Cis+CBX组肾小管损伤减轻(P < 0.001,图 4A、B)。RT-qPCR检测显示,与顺铂模型组相比,经Carbenoxolone阻断pannexin-1后,Cis+CBX组肾组织内KIM-1及NGAL mRNA表达水平回降(P < 0.05,图 4C)。通过免疫组化检测肾小管损伤标记物KIM-1的表达,进一步明确各组小鼠肾小管损伤情况,结果显示,与顺铂模型组相比,Cis+CBX组肾组织KIM-1阳性肾小管数量显著减少(图 4D)。
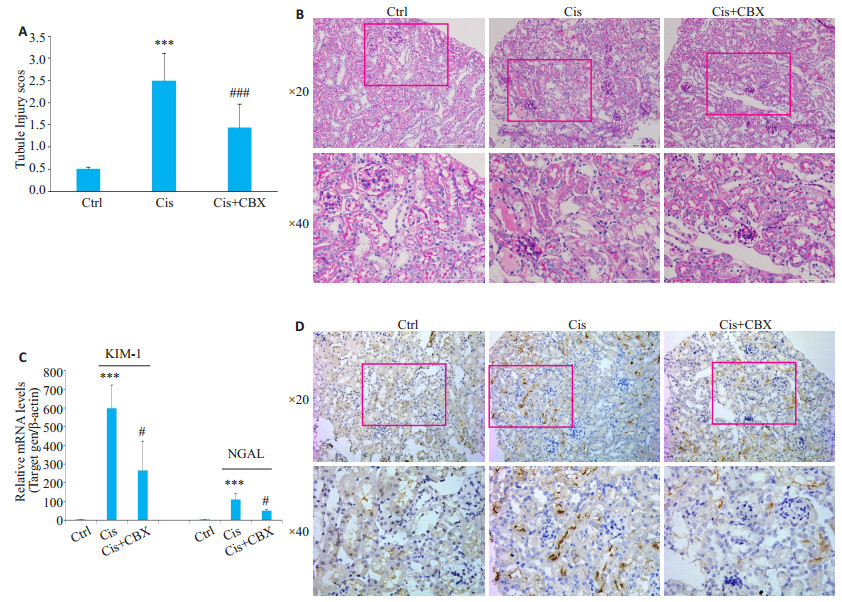
|
图 4 Carbenoxolone预处理减轻顺铂所致急性肾损伤小鼠模型的肾小管损伤 Fig.4 Carbenoxolone pretreatment alleviated renal tubular injury in mice with cisplatin-induced AKI. A: Tubule injury score of the kiney issue. ***P < 0.001 vs Ctrl; ###P < 0.05 vs Cis; B: PAS staining showing kidney tubule injury; C: RT-qPCR showing KIM-1 and NGAL expression (Mean±SD, n=8-9); D: Immunohistochemistry showing KIM-1 expression. Ctrl: control group; Cis: Cis model group; Cis+ CBX: Cis+CBX treatment group. ***P < 0.001 vs Ctrl; #P < 0.05 vs Cis. |
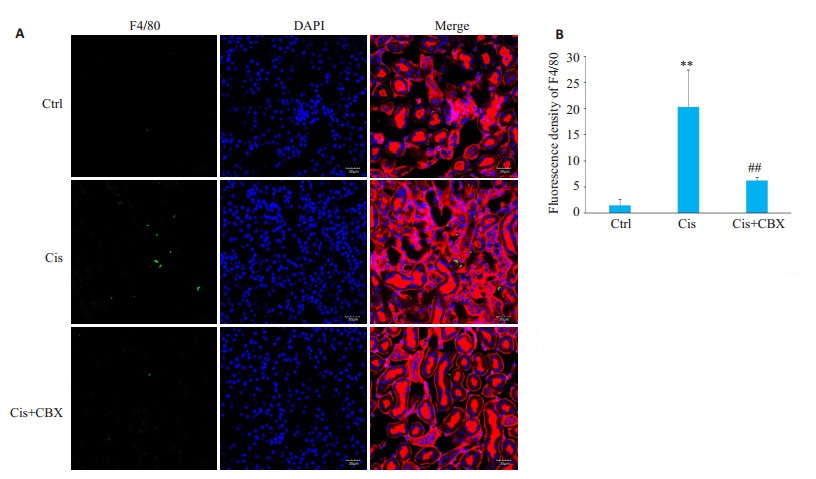
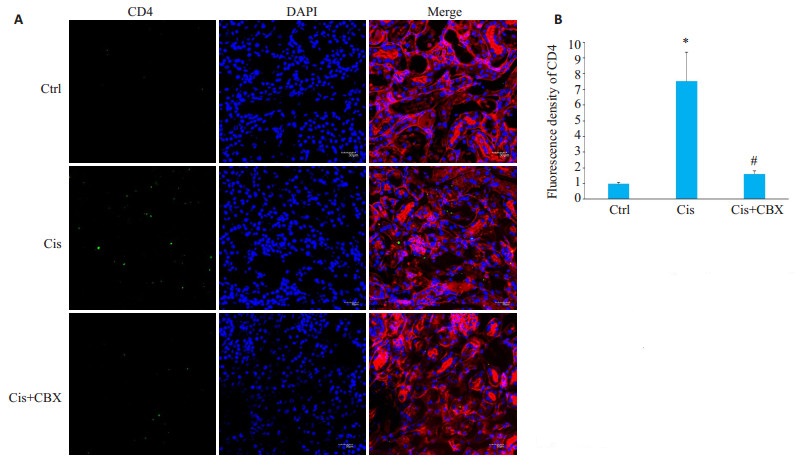
通过免疫荧光标记F4/80表达反应肾组织巨噬细胞浸润水平、CD4表达反应肾组织T细胞浸润水平,结果显示,与顺铂模型组比较,Cis+CBX组肾组织内巨噬细胞(P < 0.01,图 5)及T细胞(P < 0.05,图 6)等炎症细胞浸润减少。
|
图 5 Carbenoxolone预处理对顺铂所致急性肾损伤小鼠模型肾组织内F4/80+巨噬细胞浸润的影响 Fig.5 Effect of carbenoxolone pretreatment on F4/80+macrophage infiltration in kidney tissue of mice with cisplatin induced AKI (Original magnification:×400). Ctrl: Control group; Cis: Cis model group; Cis+CBX: Cis+ CBX treatment group. **P < 0.01 vs Ctrl; ##P < 0.01 vs Cis. |
|
图 6 Carbenoxolone预处理对顺铂所致急性肾损伤小鼠模型肾组织内CD4+细胞浸润的影响 Fig.6 Effect of carbenoxolone pretreatment on CD4+ T cell infiltration in kidney tissue of mice with cisplatin-induced AKI (× 400). Ctrl: control group; Cis: Cis model group; Cis + CBX: Cis + CBX treatment group. *P < 0.05 vs Ctrl; #P < 0.5 vs Cis. |
本研究在顺铂致急性肾损伤小鼠模型及细胞培养模型上观察pannexin-1通道蛋白的表达情况,结果显示在体内及体外模型中pannexin-1通道蛋白表达均显著上调。在顺铂所致急性肾损伤小鼠模型中应用Carbenoxolone阻断pannexin-1通道后发现:血浆BUN、Scr水平下降;肾小管损伤损伤减轻;Kim-1及NGAL等肾小管损伤标记物表达均下调;肾脏组织内的巨噬细胞及T细胞浸润减少。结果提示pannexin-1可能在顺铂所致急性肾损伤中发挥重要作用。
Pannexin-1通道蛋白是一种可释放ATP的非选择性大孔通道,在黑色素瘤、气道炎症性疾病、内毒素休克、缺血性脑病及癫痫等多种疾病模型,均可检测到pannexin-1表达上调和ATP释放[9, 19-24]。在肾脏疾病上,有研究通过基因测序发现,在肾缺血-再灌注急性肾损伤模型中,肾组织内pannexin-1的基因表达明显上调[25]。本研究在顺铂所致急性肾损伤体内及体外模型中均证实,肾小管上皮细胞pannexin-1 mRNA及蛋白表达升高,提示pannexin-1通道蛋白可能在顺铂所致急性肾损伤中发挥作用。
本研究应用小剂量Carbenoxolone相对特异地阻断pannexin-1通道,观察是否能够减轻顺铂所致的肾脏损伤。结果显示,多次小剂量腹腔注射Carbenoxolone(20 mg/kg)阻断pannexin-1通道后,顺铂所致的肾小管损伤显著减轻,对肾脏有明显保护作用。Carbenoxolone是一种以琥珀酸替代甘草甜素的葡萄糖醛酸的化学药物,高浓度Carbenoxolone除阻断pannexin-1通道外,还可阻断Connexin、FFA等通道,而低浓度Carbenoxolone(IC50 5 μmol/L)可相对特异性地抑制pannexin-1通道[26-30]。已有研究报道,在黑色素瘤、气道炎症性疾病、动脉粥样硬化和缺血性脑病等多种病理生理环境中,阻断pannexin-1通道可能是有益的治疗方案[20-25]。肾缺血-再灌注急性肾损伤小鼠模型中,应用Carbenoxolone药物阻断或敲除pannexin-1基因亦对肾脏有保护作用[31]。可见在药源性及缺血性等急性肾损伤模型中pannexin-1通道普遍上调,阻断pannexin-1通道对肾脏存在保护作用。
那么,pannexin-1通道活化是通过什么机制发挥作用呢?本研究在顺铂致急性肾损伤小鼠模型中检测肾组织炎症细胞浸润状况,结果显示应用Carbenoxolone可显著减少肾组织内巨噬细胞及T细胞浸润。既往研究证实,ATP释放至胞外可引发无菌性炎症反应,因为ATP可作为一种损伤相关的分子模式诱导炎症细胞浸润至受损组织[27, 32]。有研究报道,香烟烟雾刺激支气管上皮细胞pannexin-1通道蛋白高表达可致ATP释放增多[22],说明pannexin-1通道活化可介导ATP释放至胞外。既往研究也证实损伤应激可致肾小管上皮细胞释放ATP,进而趋化巨噬细胞及中性粒细胞等炎症细胞浸润至肾组织,引发肾脏损伤加重[32-38]。因而顺铂致肾小管损伤后pannexin-1通道活化促进ATP释放,趋化炎症细胞浸润增加,可能是顺铂致急性急性肾损伤的重要机制。
综上所述,顺铂损伤的肾小管上皮细胞pannexin-1通道蛋白表达上调及其引发的无菌性炎症反应可能是急性肾损伤的重要机制,干预pannexin-1通道蛋白活化可能是减轻顺铂所致急性肾损伤的一新策略。
致谢: 广东医科大学肾脏疾病研究所刘华锋教授课题组给本课题赠予人肾小管上皮细胞株HK-2细胞,感谢刘华锋教授在实验设计及稿件撰写中给予的帮助!| [1] |
Raimann JG, Riella MC, Levin NW, et al. International society of nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): focus on diagnosis of acute kidney injury in low-income countries[J]. Clin Kidney J, 2017, 11(1): 12-9. |
| [2] |
Zuk A, Bonventre JV. Acute kidney injury[J]. Annu Rev Med, 2016, 67(1): 293-307. DOI:10.1146/annurev-med-050214-013407 |
| [3] |
Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury[J]. Biomed Res Int, 2014, 49(10): 967826-38. |
| [4] |
Herrera-Perez Z, Gretz N, Dweep H. A comprehensive review on the genetic regulation of cisplatin-induced nephrotoxicity[J]. Curr Genomics, 2016, 17(3): 279-93. DOI:10.2174/1389202917666160202220555 |
| [5] |
Panchin Y, Kelmanson I, Matz M, et al. A ubiquitous family of putative gap junction molecules[J]. Curr Biol, 2000, 10(13): R473-4. DOI:10.1016/S0960-9822(00)00576-5 |
| [6] |
Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels[J]. Biochim Biophys Acta, 2013, 1828(1, SI): 15-22. DOI:10.1016/j.bbamem.2012.01.017 |
| [7] |
Sandilos JK, Bayliss DA. Physiological mechanisms for the modulation of pannexin 1 channel activity[J]. J Physiol, 2012, 590(24): 6257-66. DOI:10.1113/jphysiol.2012.240911 |
| [8] |
Bruzzone R, Hormuzdi SG, Barbe MT, et al. Pannexins, a family of gap junction proteins expressed in brain[J]. Proc Natl Acad Sci USA, 2003, 100(23): 13644-9. DOI:10.1073/pnas.2233464100 |
| [9] |
Chiu YH, Ravichandran KS, Bayliss DA. Intrinsic properties and regulation of Pannexin 1 channel[J]. Channels, 2014, 8(2, SI): 1-7. |
| [10] |
Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation[J]. Front Immunol, 2013, 3(11): 1308-19. |
| [11] |
Boyce AJ, Swayne LA. P2X7 receptor cross-talk regulates ATPinduced pannexin 1 internalization[J]. Biochem J, 2017, 474(13): 2133-44. DOI:10.1042/BCJ20170257 |
| [12] |
Di Virgilio F, Dal Ben D, Sarti AC, et al. The P2X7 receptor in infection and inflammation[J]. Immunity, 2017, 47(1): 15-31. DOI:10.1016/j.immuni.2017.06.020 |
| [13] |
Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance[J]. Nature, 2009, 461(7261): 282-U165. DOI:10.1038/nature08296 |
| [14] |
Chekeni FB, Elliott MR, Sandilos JK, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis[J]. Nature, 2010, 467(7317): 863-U136. DOI:10.1038/nature09413 |
| [15] |
Yanguas SC, Willebrords J, Johnstone SR, et al. Pannexin1 as mediator of inflammation and cell death[J]. Biochim Biophys Acta, 2017, 1864(1): 51-61. DOI:10.1016/j.bbamcr.2016.10.006 |
| [16] |
Boyd-Tressler A, Penuela S, Laird DW, et al. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism[J]. J Biol Chem, 2014, 289(39): 27246-63. DOI:10.1074/jbc.M114.590240 |
| [17] |
Chiu YH, Jin XE, Medina CB, et al. A quantized mechanism for activation of pannexin channels[J]. Nat Commun, 2017, 8(13): 14324-33. |
| [18] |
Hanner F, Lam L, Nguyen MX, et al. Intrarenal localization of the plasma membrane ATP Channel pannexin1[J]. AJP Renal Physiologyl, 2012, 303(12): F1454-9. |
| [19] |
Ransford GA, Fregien N, Qiu F, et al. Pannexin 1 contributes to ATP release in airway epithelia[J]. Am J Respir Cell Mol Biol, 2009, 41(5): 525-34. DOI:10.1165/rcmb.2008-0367OC |
| [20] |
Bargiotas P, Krenz A, Hormuzdi SG, et al. Pannexins in ischemiainduced neurodegeneration[J]. Proc Natl Acad Sci USA, 2011, 108(51): 20772-7. DOI:10.1073/pnas.1018262108 |
| [21] |
Baxter M, Eltom S, Dekkak B, et al. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung[J]. Thorax, 2014, 69(12): 1080-9. DOI:10.1136/thoraxjnl-2014-205467 |
| [22] |
Penuela S, Gyenis L, Ablack A, et al. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype[J]. J Biol Chem, 2012, 287(34): 29184-93. DOI:10.1074/jbc.M112.377176 |
| [23] |
Yang DH, He Y, Munoz-Planillo R, et al. Caspase-11 requires the pannexin-1 Channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock[J]. Immunity, 2015, 43(5): 923-32. DOI:10.1016/j.immuni.2015.10.009 |
| [24] |
Seminario-Vidal L, Okada SF, Sesma JI, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia[J]. J Biol Chem, 2011, 286(30): 26277-86. DOI:10.1074/jbc.M111.260562 |
| [25] |
Xu K, Rosenstiel P, Paragas N, et al. Unique transcriptional programs identify subtypes of AKI[J]. J Am Soc Nephrol, 2017, 28(6): 1729-40. DOI:10.1681/ASN.2016090974 |
| [26] |
Li W, Li J, Sama AE, et al. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release[J]. Mol Med, 2013, 19(1): 203-11. DOI:10.2119/molmed.2013.00064 |
| [27] |
Riteau N, Baron L, Villeret B, et al. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation[J]. Cell Death Dis, 2012, 3(10): e403-14. DOI:10.1038/cddis.2012.144 |
| [28] |
Sridharan M, Adderley SP, Bowles EA, et al. Pannexin 1 is the conduit for low Oxygen tension- induced ATP release from human erythrocytes[J]. Am J Physiol Heart Circ Physiol, 2010, 299(4): H1146-52. DOI:10.1152/ajpheart.00301.2010 |
| [29] |
Spray DC, Ye ZC, Ransom BR. Functional connexin hemichannels: a critical appraisal[J]. Glia, 2006, 54(7): 758-73. DOI:10.1002/(ISSN)1098-1136 |
| [30] |
Bruzzone R, Barbe MT, Jakob NJ, et al. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes[J]. J Neurochem, 2005, 92(5): 1033-43. DOI:10.1111/jnc.2005.92.issue-5 |
| [31] |
Jankowski J, Perry HM, Medina CB, et al. Epithelial and endothelial pannexin1 channels mediate AKI[J]. J Am Soc Nephrol, 2018, 29(7): 1887-99. DOI:10.1681/ASN.2017121306 |
| [32] |
Rabb H, Griffin MD, Mckay DB, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps[J]. J Am Soc Nephrol, 2016, 27(2): 371-9. DOI:10.1681/ASN.2015030261 |
| [33] |
Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup[J]. Nat Rev Nephrol, 2017, 13(4): 241-57. DOI:10.1038/nrneph.2017.2 |
| [34] |
Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes[J]. N Engl J Med, 2014, 371(1): 58-66. DOI:10.1056/NEJMra1214243 |
| [35] |
Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease[J]. Clin Kidney J, 2016, 9(6): 765-71. DOI:10.1093/ckj/sfw096 |
| [36] |
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis[J]. Immunity, 2016, 44(3): 450-62. DOI:10.1016/j.immuni.2016.02.015 |
| [37] |
Liu Z, Li H, Su JQ, et al. Numb depletion promotes Drp1-Mediated mitochondrial fission and exacerbates mitochondrial fragmentation and dysfunction in acute kidney injury[J]. Antioxid Redox Signal, 2018, 30(15): 1797-816. |
| [38] |
Choi EK, Jung H, Kwak KH, et al. Inhibition of oxidative stress in renal ischemia-reperfusion injury[J]. Anesth Analg, 2017, 124(1): 204-13. DOI:10.1213/ANE.0000000000001565 |
 2019, Vol. 39
2019, Vol. 39

