2. 暨南大学附属第一医院, 广东 广州 510632
2. 2 Department of Rehabilitation, First Affiliated Hospital of Jinan University, Guangzhou 510632, China
多发性骨髓瘤(MM)是一种浆细胞恶性增生性疾病, 常规治疗主要有化疗、放疗、造血干细胞移植(HSCT)[1], 以及新型分子靶向药物[2]。其中, 分子靶向药物中蛋白酶体抑制剂硼替佐米(Btz)是目前治疗MM最有效、最常用的药物之一。然而, Btz耐药性及复发率逐年升高, 其耐药机制尚未完全明确[3-4]。因此, 进一步探讨Btz耐药机制、寻找治疗多发性骨髓瘤的新靶点已成为亟待解决的问题。
有研究显示, 外泌体在调节细胞耐药性上发挥重要作用[5-6], 然而, 外泌体在多发性骨髓瘤对Btz的耐药机制尚无明确报道。外泌体是细胞分泌的胞外囊泡, 小于30~150 nm, 具有脂质双分子层, 脂质膜内包裹有miRNA、热休克蛋白、酶等[7-8]。由于外泌体的脂质双分子结构, 使其具有运载包裹的"货物"穿过体液、屏障的能力。同时其表面表达的特异性蛋白促使外泌体被远端细胞摄取, 从而发挥调控作用[9-10]。有研究认为, 外泌体的分泌可以作为信号转导的媒介以改变肿瘤对化疗药物的敏感性, 参与肿瘤进程, 同时, 外泌体能有把多种化合药物排出细胞, 降低肿瘤细胞对药物的敏感性, 从而导致化疗失败的作用[11-13]。外泌体在多发性骨髓瘤细胞对Btz耐药中的作用机制, 需要进一步的研究。
本研究为进一步探索外泌体在Btz耐药中的作用机制, 通过采集Btz敏感和耐受的骨髓瘤首次确诊患者外周血, 采用超速离心法分离血清中的外泌体及骨髓瘤细胞, 并用电镜技术、NTA法以及WB技术鉴定。用不同浓度外泌体预处理骨髓瘤细胞, 通过MTS法检测经外泌体处理的瘤细胞对Btz的敏感程度, 并搜索外泌体数据库exocarta, 运用STRING绘制网络图和蛋白互作表格, 寻找可能的外泌体耐药关键蛋白。
1 资料和方法 1.1 病例在2017年1月~2017年12月我院就诊的MM患者中, 选择15例给予Btz 9个疗程规范治疗后, 但病情仍有进展的复发难治型患者, 疾病进展及复发符合以下一项:免疫固定电泳和蛋白电泳显示血清和尿M蛋白的改变、骨髓浆细胞数目的改变、出现新的浆细胞瘤、溶骨性病变、高钙血症; 其中男性7例, 女性8例, 平均年龄54岁。诊断及疗效标准参照《中国多发性骨髓瘤诊治指南(2015年修订)》。
1.2 仪器与试剂超速离心管(Beckman, 355618); 30 000 D超滤管(Millipore, UFC903024); 0.22 µm滤器(MILLEX GP, SLGP033RB); 大型离心机(eppendorf, 5430R); 超速离心机(Beckman, Optima XE-100); NS-300 NTA检测仪(NanoSight); 基础培养基; 0.25% Trypsin-EDTA(1X) (GIBCO 25200-072); FBS(GIBCO 16000-044); PBS pH7.4 basic(1X) (GIBCO C10010500BT); MTS; 细胞培养箱(Thermo); 酶标仪(Diatek); 电泳仪(Tanon EPS 200);电泳槽(北京百晶生物技术有限公司101-510-001);抗体:CD63; Tsg 101 (Abcam); Alix (CST)。
1.3 分离骨髓患者外周血外泌体收集骨髓瘤患者外周血, Btz耐受患者15例, 并分离血清, 2000 g, 4℃离心30 min后收集上清。12 000 g, 4℃离心45 min, 收集上清。110 000 g, 4℃离心2 h除去上清, 大体积(2~3 mL) PBS重悬沉淀。0.22 μm膜过滤, 110 000 g, 4℃离心70 min。1 mL PBS重悬沉淀物, 110 000 g, 4℃离心70 min (2次)。小体积(50~200 μL)重悬-80℃保存。
1.4 外泌体特性鉴定① NTA法检测外泌体颗粒的大小于1.5 mL EP管中取适量外泌体, 溶于1 mL 1×PBS, 涡旋震荡混匀。以超声破碎仪对外泌体进行超声以使外泌体充分重悬。用1 mL一次性注射器吸取外泌体重悬液, 注射进NS- 300 NTA检测仪样品室。NS-300采集颗粒粒径大小信号, 并进行颗粒大小分析。②Western blot技术检测外泌体的表面标志物:外泌体蛋白悬液用BCA法测定蛋白浓度, 经SDS-PAGE电泳后将蛋白转至PVDF膜上, 5%脱脂牛奶封闭后, 将CD63、Alix和Tsg 101的抗体4℃孵育过夜后, 清洗多余抗体, 室温下孵育辣根过氧化物酶标记抗体1~2 h, TBST清洗PVDF膜后, 进行化学发光显影。
1.5 检测外泌体对骨髓瘤细胞耐药的影响培养骨髓瘤细胞RPMI8226, Btz终浓度为0、0.01、0.1、0.25、0.5、1、5、10 nmol/L, 均加药处理24、48、72 h, 运用MTS法检测细胞活性, 设至少3复孔, 通过SPSS计算半数抑制RPMI8226的药物浓度(IC50)。0、5、10、20、40 µg Btz耐药外泌体预处理骨髓瘤细胞48 h, 随后再添加最适浓度的Btz处理骨髓瘤细胞24 h后, 运用MTS法检测细胞活性变化。
1.6 生物信息学分析人血清外泌体包含的蛋白运用外泌体数据库exocarta(http://www.exocarta.org/)搜索人血清外泌体内含物, 分析数据库收录的人血清外泌体蛋白与细胞增殖、抗凋亡和耐药的关系, 并运用STRIN数据库(https://string-db.org/)进行蛋白互作分析网络图分析。
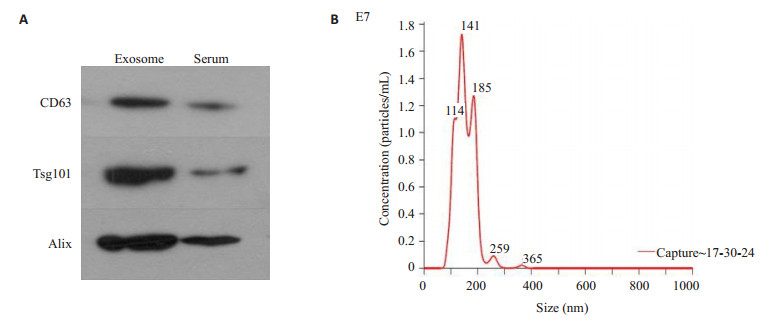
2 结果 2.1 外泌体囊泡颗粒大小及外泌体标志蛋白检测从Btz耐药患者外周血中分离出的囊泡主要分布在200 nm以下, 颗粒均值为153 nm, 众数为140.1 nm。WB检测外泌体标志蛋白CD63、Tsg101和Alix表达, 外泌体标志物呈阳性(图 1)。
|
图 1 多发性骨髓瘤患者血清外泌体标志蛋白的检测及粒径分布 Fig.1 Detection of marker proteins and particle size distribution of serum exosomed in patients with multiple myeloma. A:Western blotting was used to detect exosome marker proteins; B:NTA was used to identify the size distribution of serum exosomes. |
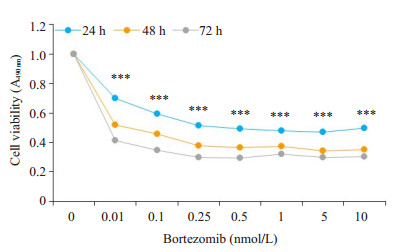
加药后24 h, 药物浓度在0~10 nmol时, 细胞活性逐渐降低。根据这一结果计算IC50, 通过SPSS统计计算, IC50为0.837 nmol (图 2)。
|
图 2 MTS检测硼替佐米浓度梯度 Fig.2 Changes in cell viability following treatments with gradient concentrations of bortezomib detected by MTS. ***P < 0.001 vs control group. |
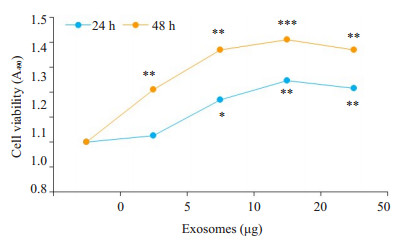
外泌体预处理骨髓瘤细胞后, 瘤细胞对Btz的敏感度下降, 耐药能力增强, 细胞的活性也增强(图 3)。
|
图 3 MTS检测浓度梯度外泌体对骨髓瘤细胞耐药的影响 Fig.3 MTS for assessing the effect of gradient concentrations of exosomes on drug resistance of myeloma cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group. |
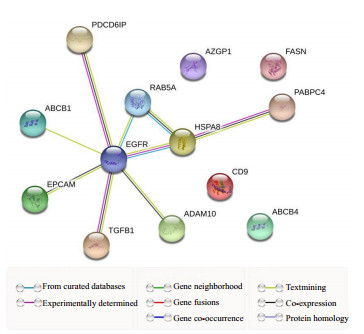
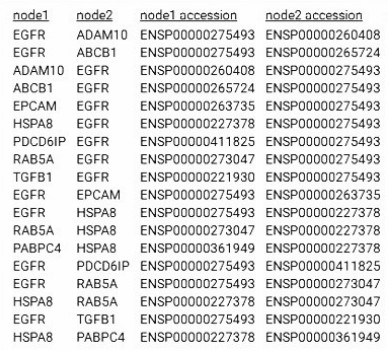
外泌体数据库exocarta搜索人血清外泌体中含有的蛋白, 有以下蛋白:ADAM10、CD9、ABCB1、CD24、AZGP1、PDCD6IP、EGFR、EPCAM、HSPA8、TGFB1、PABPC4、ABCB4、FASN、RAB5A。其中ABCB1、ABCB4与耐药相关, PDCD6IP、EGFR与细胞增殖、抗凋亡相关[14-15]。将搜索到的蛋白运用STRING绘制网络图和蛋白互作表格, 蛋白质以EGFR为节点相互连接在一起(图 4、5)。
|
图 4 外泌体数据库exocarta搜索人血清外泌体中含有的蛋白 Fig.4 Exocarta database searching for proteins in human serum exosomes. |
|
图 5 STRING网络图和蛋白互作表格 Fig.5 STRING network diagram and protein interaction table. |
外泌体参与肿瘤发生发展的各个过程[16-18], 在血液恶性肿瘤中同样发挥重要作用。MM患者分离出的外泌体let-7b, miR-148a与MM的无进展生存时间(PFS)相关[19]。另外, Wang等[20]研究发现, 骨髓间充质干细胞(BMSC)的外泌体可诱导MM细胞的增殖, 迁移, 抗凋亡和耐药性。
我们推测MM患者发生耐药的机制可能与外泌体运送特定"货物"有关。首先分离Btz耐药MM患者的血清外泌体, 经NTA验证外泌体颗粒大小, WB检测外泌体标志蛋白CD63、Tsg101和Alix, 鉴定获得的外泌体。随后, 运用外泌体浓度梯度预处理RPMI8226, 再添加0.8 mol Btz处理RPMI8226细胞, 通过MTS检测骨髓瘤细胞的活性。结果提示, Btz浓度达到0.1~ 1 nmol时RPMI8226细胞活性逐渐下降; 而一定浓度下, 随着外泌体浓度的升高, RPMI8226的耐药性增加; 当经过10~20 µg外泌体预处理后, RPMI8226对Btz耐药性最强。这提示外泌体可能运送促进细胞增殖、耐药的相关蛋白、基因, 来调节细胞活性和耐药能力。
为进一步探寻外泌体促进MM细胞Btz耐药的原因, 我们运用外泌体数据库exocarta分析人血清外泌体内蛋白, 其中ABCB1、ABCB4与耐药相关, EGFR等与细胞增殖、抗凋亡相关。ABCB1主要定位在细胞膜上, 由两个同源的疏水跨膜域(MSD)组成, 每个区域包含6个跨膜部分和1个核巧酸结合位点(NBD)。作为一种能量依赖性药物外排泉, 位于细胞膜上的ABCB1可通过ATP供能, 将ABCB1转运体底物性化疗药物粟出细胞外, 进而导致化疗药物在师瘤细胞内的有效浓度降低, 使得临床治疗效果降低或无效, 导致多药耐药性(MDR)的产生。而肿瘤细胞耐药的主要表型为MDR, 即患者接受化疗药物治疗后, 除对原来药物产生耐药外, 还对其它结构和作用机制不同的抗癌药产生交叉耐药, 最终导致临床化疗方案疗效的降低或无效的现象[21-22]。MDR的产生与多种因素有关, 其中最为重要的原因就是ABCB1转运体(P-gp, P糖蛋白)介导的"经典的MDR"。因此, ABCB1的髙表达引起肿瘤的化疗失败及患者的预后不良, 也是外泌体促使受体细胞耐药的机制之一[21, 23]。
而EGFR是一种与表皮生长因子结合的细胞表面蛋白, 蛋白质与配体的结合诱导受体二聚化和酪氨酸自磷酸化并导致细胞增殖[24-25]。研究表明, 在许多实体肿瘤中存在EGFR的高表达或异常表达[26]。EGFR与肿瘤细胞的增殖、血管生成、肿瘤侵袭、转移及细胞凋亡抑制有关, 其可能机制有[27-30]:EGFR的高表达引起下游信号传导的增强; 突变型EGFR受体或配体表达的增加导致EGFR的持续活化; 自分泌环的作用增强; 受体下调机制的破坏; 异常信号传导通路的激活等。EGFR的过表达在恶性肿瘤的演进中起重要作用。蛋白质互作分析网站STRING结果显示EGFR也是外泌体内蛋白互作的节点, 可能是引起外泌体促进骨髓瘤细胞Btz耐药的关键蛋白。
本研究结果表明, 外泌体可以做为介质传递耐药性, 且Btz耐药的机制与外泌体内包裹的蛋白密切相关。生物信息学分析筛选出的EGFR可能是促进外泌体促进骨髓瘤细胞Btz耐药的关键蛋白。由于本实验只是生物信息学预测, 因此有必要进一步系统的研究外泌体与MM患者给予Btz治疗后, 产生耐药的发生、发展关系, 以及评估外泌体内容物作为标志物和药物靶点的可行性。
| [1] |
Cavenagh J, Oakervee H, Baetiong-Caguioa P, et al. A phase Ⅰ/Ⅱ study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma[J].
Br J Cancer, 2017, 117(9): 1295-302.
DOI: 10.1038/bjc.2017.302. |
| [2] |
Liu JR, Li J, Chen ML, et al. Bortezomib followed by autologous stem cell transplantation in a patient with rheumatoid arthritis A case report and review of the literature[J].
Medicine, 2016, 95(52): 0000000000005760.
DOI: 10.1097/MD.0000000000005760. |
| [3] |
Landis-Piwowar KR. Proteasome inhibitors in cancer therapy:a novel approach to a ubiquitous problem[J].
Clin Lab Sci, 2012, 25(1): 38-44.
DOI: 10.29074/ascls.25.1.38. |
| [4] |
Raninga PV, Di Trapani G, Vuckovic SA. TrxR1 inhibition overcomes both hypoxia-induced and acquired bortezomib resistance in multiple myeloma through NF-k beta inhibition[J].
Cell Cycle, 2016, 15(4): 559-72.
DOI: 10.1080/15384101.2015.1136038. |
| [5] |
Huynh M, Pak C, Markovina S, et al. Hyaluronan and proteoglycan Link protein 1 (HAPLN1) activates bortezomib-resistant NF-kappa B activity and increases drug resistance in multiple myeloma[J].
J Biol Chem, 2018, 293(7): 2452-65.
DOI: 10.1074/jbc.RA117.000667. |
| [6] |
Xu X, Liu J, Huang B, et al. Reduced response of IRE1α/Xbp-1 signaling pathway to bortezomib contributes to drug resistance in multiple myeloma cells[J].
Tumori, 2017, 103(3): 261-7.
DOI: 10.5301/tj.5000554. |
| [7] |
Song M, Wang Y, Shang ZF, et al. Bystander autophagy mediated by radiation-induced exosomal miR-7-5p in non-targeted human bronchial epithelial cells[J].
Sci Rep, 2016, 6: 10.
DOI: 10.1038/s41598-016-0003-6. |
| [8] |
Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes[J].
Proc Natl Acad Sci USA, 2016, 113(8): E968-77.
DOI: 10.1073/pnas.1521230113. |
| [9] |
Cocucci E, Meldolesi J. Ectosomes and exosomes:shedding the confusion between extracellular vesicles[J].
Trends Cell Biol, 2015, 25(6): 364-72.
DOI: 10.1016/j.tcb.2015.01.004. |
| [10] |
Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer:Cell-to-Cell mediators of metastasis[J].
Cancer Cell, 2016, 30(6): 836-48.
DOI: 10.1016/j.ccell.2016.10.009. |
| [11] |
Zhang FF, Yf Z, Zhao QN, et al. Microvesicles mediate transfer of P-glycoprotein to paclitaxe-l sensitive A2 7 80 human ovarian cancer cells, conferring paclitaxe-l resistance[J].
Eur J Pharmacol, 2014, 738: 83-90.
DOI: 10.1016/j.ejphar.2014.05.026. |
| [12] |
Harada T, Yamamoto H, Kishida S, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation[J].
Cancer Sci, 2017, 108(1, 1): 42-52.
|
| [13] |
Takeuchi T, Suzuki M, Fujikake N, et al. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level[J].
Proc Natl Acad Sci USA, 2015, 112(19): E2497-24506.
DOI: 10.1073/pnas.1412651112. |
| [14] |
Fanelli M, Hattinger CM, Vella S, et al. Targeting ABCB1 and ABCC1 with their specific inhibitor CBT-1®can overcome drug resistance in osteosarcoma[J].
Curr Cancer Drug Targets, 2016, 16(3): 261-74.
DOI: 10.2174/1568009616666151106120434. |
| [15] |
Duan ZF, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells[J].
Mol Cancer Ther, 2004, 3(7): 833-8.
|
| [16] |
Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J].
Nat Cell Biol, 2007, 9(6): 654-9.
DOI: 10.1038/ncb1596. |
| [17] |
Greening DW, Gopal SK, Xu R, et al. Exosomes and their roles in immune regulation and cancer[J].
Semin Cell Dev Biol, 2015, 40: 72-81.
DOI: 10.1016/j.semcdb.2015.02.009. |
| [18] |
Sandfeld-Paulsen B, Aggerholm-Pedersen N, Boek R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer[J].
Mol Oncol, 2016, 10(10): 1595-602.
DOI: 10.1016/j.molonc.2016.10.003. |
| [19] |
Zhao HY, Achreja A, Iessi E, et al. The key role of extracellular vesicles in the metastatic process[J].
Biochim Biophys Acta Rev Cancer, 2018, 1869(1): 64-77.
DOI: 10.1016/j.bbcan.2017.11.005. |
| [20] |
Manier S, Liu CJ, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma[J].
Blood, 2017, 129(17): 2429-36.
DOI: 10.1182/blood-2016-09-742296. |
| [21] |
Campa D, Mueller P, Edler L, et al. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis[J].
Int J Cancer, 2012, 131(12): 2920-8.
DOI: 10.1002/ijc.27567. |
| [22] |
Zhu YZ, Liu CF, Nadiminty N, et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer[J].
Mol Cancer Ther, 2013, 12(9): 1829-36.
DOI: 10.1158/1535-7163.MCT-13-0208. |
| [23] |
Yang K, Chen YF, To KK, et al. Alectinib (CH5424802) antagonizes ABCB1-and ABCG2-mediated multidrug resistance in vitro, in vivo and ex vivo[J].
Exp Mol Med, 2017, 49(3): 168.
|
| [24] |
Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR (C797S) resistance with mutant-selective allosteric inhibitors[J].
Nature, 2016, 534(765): 129-32.
|
| [25] |
Bae JH, Schlessinger J. Asymmetric tyrosine kinase arrangements in activation or autophosphorylation of receptor tyrosine kinases[J].
Mol Cells, 2010, 29(5): 443-8.
DOI: 10.1007/s10059-010-0080-5. |
| [26] |
Gotoh K, Kariya R, Matsuda K, et al. A novel EGFP-expressing nude mice with complete loss of lymphocytes and NK cells to study tumor-host interactions[J].
Biosci Trends, 2014, 8(4): 202-5.
DOI: 10.5582/bst.2014.01049. |
| [27] |
Ju HL, Calvisi DF, Moon H, et al. Transgenic mouse model expressing P53(R172H), luciferase, EGFP, and KRAS(G12D) in a single open reading frame for live imaging of tumor[J].
Sci Rep, 2015, 5: 10.
|
| [28] |
Moen I, Jevne C, Wang J, et al. Gene expression in tumor cells and stroma in dsRed 4T1 tumors in eGFP-expressing mice with and without enhanced oxygenation[J].
BMC Cancer, 2012, 12: 10.
DOI: 10.1186/1471-2407-12-10. |
| [29] |
Zhang B, Jiang T, Ling L, et al. Enhanced antitumor activity of EGFP-EGF1-Conjugated nanoparticles by a multitargeting strategy[J].
ACS Appl Mater Interfaces, 2016, 8(14): 8918-27.
DOI: 10.1021/acsami.6b00036. |
| [30] |
Shi W, Yin YX, Wang Y, et al. A tissue factor-cascade-targeted strategy to tumor vasculature:a combination of EGFP-EGF1 conjugation nanoparticles with photodynamic therapy[J].
Oncotarget, 2017, 8(19): 32212-27.
|
 2019, Vol. 39
2019, Vol. 39

