非酒精性脂肪肝(NAFLD)和糖尿病联系密切, NAFLD患者通常伴有胰岛素抵抗, 而2型糖尿病(T2DM)患者β细胞功能缺陷也会促成肝脏脂质沉积进而发展为NAFLD[1]。研究发现, 除了NAFLD的因素外, T2DM本身导致肝脏纤维化的风险也显著增加[2], T2DM是肝纤维化的重要预测因子[3]。而由于病毒感染、自身免疫疾病等多种其他因素引起的慢性肝炎也均可导致细胞外基质的(ECM)在肝脏的加速积累, 发展为肝纤维化[4], 而肝星状细胞(HSCs)的活化是肝纤维化发展的主要原因。
氧化应激也参与了肝纤维化的进展, 活性氧(ROS)的过多产生会导致HSCs的增殖和迁移, 从而促进肝纤维化[5]。核因子红细胞2相关因子(Nrf2)是诱导抗氧化应激基因转录的重要转录因子, 通过促进血红素氧合酶-1 (HO-1), 谷氨酸-半胱氨酸连接酶(GCLC)和醌氧化还原酶-1 (NQO-1)的表达来发挥抗氧化应激作用, 研究也表明Nrf2/HO-1信号通路可以通过抗氧化应激进而抑制肝脏的纤维化[6]。
Exendin-4是胰高血糖素样肽-1 (GLP-1)受体激动剂, 约50%氨基酸序列与哺乳动物的GLP-1序列一致, 可抵抗二肽基肽酶4(DPP-4)的降解[7]。近年来, Exendin-4已被广泛应用于临床糖尿病和肥胖等相关代谢疾病的治疗[8-9], 除了显著改善机体的糖脂代谢, Exendin-4还可以改善糖尿病状态下NAFLD相关的肝脏纤维化及肾脏纤维化[10-12], 而其具体机制尚不清楚。本研究旨在研究给予糖尿病小鼠Exendin-4治疗后, 其肝脏脂质代谢、纤维化的改变, 并从氧化应激的角度阐述其抗纤维化的机制。
1 材料和方法 1.1 实验动物24只SPF级雄性C57BL/6小鼠(5~6周龄, 体质量18~22 g), 由广东省实验动物中心提供, 饲养于南方医科大学SPF级动物房。动物适应性饲养1周, 在温度22℃, 每天12 h昼夜循环的条件下饲养。所有动物实验操作均符合南方医科大学动物实验伦理委员会的要求。
1.2 实验试剂链脲佐菌素STZ(S0130, Sigma); 高脂饲料(D12492); Exendin-4(E7144, Sigma); RIPA裂解液、PMSF、BCA蛋白定量分析试剂盒(凯基生物); mouse anti-βactin(中杉金桥); mouse anti-TGF-β1(Abcam); mouse anti-Nrf2 (CST); mouse anti-HO-1 (ABclone)。
1.3 实验动物模型建立以及分组雄性C57BL/6小鼠根据体质量随机分为正常组小鼠(Con, 8只)为对照组和糖尿病组小鼠(DM, 16只), 正常组小鼠给予普通饮食, 糖尿病组小鼠给予高脂饮食联合STZ造模诱导。DM组小鼠先给予高脂饮食饲养4周, 而后120 mg/kg体质量腹腔注射STZ, 继续给予高脂饮食饲养4周。小鼠随机血糖≥ 13.9 mmol/L判定为糖尿病造模成功[13]。
造模成功的DM组小鼠再随机分为:糖尿病对照组(DM-Con)和exendin-4干预组(DM+E4)。DM+E4组给予1 nmol/kg体质量的exendin-4腹腔注射, 1次/d, 注射8周, DM-Con组给予相同剂量的生理盐水腹腔注射。实验期间监测小鼠的血糖、体质量等生理指标。干预结束时收集小鼠的血清检测血脂, 收集肝脏标本备测。
1.4 RT-PCR检测肝脏各脂质代谢基因mRNA表达Trizol法提取小鼠肝脏组织的总RNA, 应用ND-1000分光光度计(USA)测定RNA的纯度和浓度。根据反转录试剂盒说明书将总RNA反转录成cDNA, 再以cDNA为模板进行目的基因的扩增。反应条件:预变性95℃ 30 s; PCR反应95℃ 5 s, 60℃ 34 s, 总共40个循环。结果以2-△△Ct方法分析基因的表达差异。以Actin为内参, 计算目的基因的mRNA相对表达量。引物序列如表 1, 包括脂质代谢相关基因:过氧化物酶体增殖物激活受体γ (PPARγ)、脂肪酸合成酶(FAS)、乙酰辅酶A羧化酶α(ACCα)、甾醇调节元件结合转录因子1 (SREBP-1c)、过氧化物酶体增殖物激活受体α (PPARα)、硬脂酰辅酶A去饱和酶1 (Scd-1), 纤维化相关基因:转化生长因子β1 (TGF-β1)、α-平滑肌肌动蛋白(α-SMA)、胶原蛋白Ⅰ(collagen-Ⅰ, Col-Ⅰ), 氧化应激相关基因:NADPH氧化酶4 (NOX4)、超氧化物歧化酶1 (SOD1)、谷胱甘肽过氧化物酶4 (GPX4)、血红素加氧酶-1 (HO-1)和核因子红细胞2相关因子2 (Nrf2)。
| 表 1 引物序列 Tab.1 Primer sequence |
剪取适量肝脏组织加入含有磷酸酶抑制剂以及蛋白酶抑制剂的RIPA裂解液中, 冰上研磨后离心提取蛋白。BCA法测蛋白浓度后, 取20~30 μg蛋白加样, 经聚丙烯酰胺凝胶电泳分离蛋白并将其转移至PVDF膜上, 于5%脱脂奶粉中封闭1~2 h, 然后在4℃将PVDF膜置于一抗TGF-β1(1:1000; Abcam)、一抗Nrf2(1:500; CST)、一抗HO-1(1:1000; Abclone)和一抗β-actin(1:1000;中杉金桥)中孵育过夜, 用Tris缓冲盐水加吐温洗膜3次, 再用二抗(1:5000)室温孵育1 h, Tris缓冲盐水加吐温洗膜3次后, 凝胶成像系统显色曝光, 对结果进行灰度分析。
1.6 病理学分析肝脏组织经多聚甲醛固定后, 一部分直接油红O染色, 一部分进行石蜡包埋, 然后切片, HE染色和Sirus Red染色观察肝脏组织形态变化。
1.7 统计分析实验数据以均数±标准差表示, 采用SPSS 20.0软件进行统计分析。单因素方差分析统计结果。P < 0.05认为差异有统计学意义。
2 结果 2.1 糖尿病小鼠的造模在造模期间, 高脂饮食饲养的DM组小鼠体质量较对照组显著增加。STZ注射后, DM小鼠的体质量也有显著增加, 血糖水平与对照组小鼠相比有显著升高(图 1)。造模成功的小鼠给予exendin-4干预8周后, 其血糖水平相比于DM-con组小鼠有显著下降, 还显著降低了血清甘油三酯(TG)水平, 但低密度脂蛋白(LDL)和高密度脂蛋白(HDL)无明显变化(图 2)。
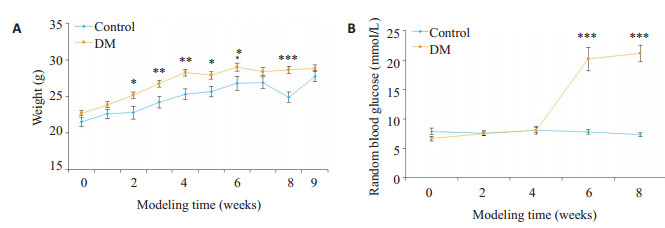
|
图 1 造模期间小鼠体质量和随机血糖的变化 Fig.1 Changes in body weight (A) and random blood glucose (B) of the mice in control (n=8) and diabetic (DM, n=16) groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs DM group. |
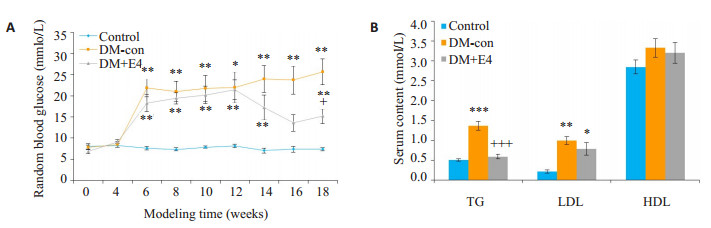
|
图 2 各组小鼠随机血糖和血脂的变化 Fig.2 Changes in random blood glucose (A) and blood lipid content (B) of the mice in the 3 groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; +P < 0.05, +++P < 0.001 vs DM-con group. |
HE染色观察到对照组小鼠的肝细胞排列较规则, 以中央静脉为中心向外辐射状排列。DM组小鼠的肝脏组织可以观察到空泡样变、局部细胞坏死等。天狼猩红染色提示DM小鼠的肝脏肝窦周围有显著的纤维化改变, 而在Con组无此改变。油红O染色显示DM组肝脏脂质沉积明显多于对照组。Exendin-4干预后, DM+ E4组小鼠的肝脏结构较DM-con组小鼠有所改善, 天狼猩红染色也可见肝脏的纤维组织增生减少, 但油红O染色结果显示肝脏脂质沉积并无明显改善(图 3)。
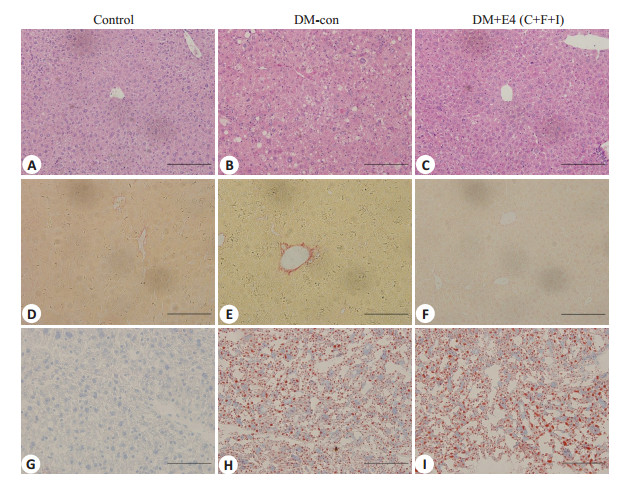
|
图 3 小鼠肝脏组织HE染色、天狼猩红染色和油红O染色 Fig.3 HE staining, Sirius red staining and oil red staining of the liver tissue in the 3 groups (Original magnification:×200). |
DM+E4组小鼠肝脏PPARγ、FAS、ACCα、SREBP-1c、PPARα以及Scd-1的mRNA表达没有明显变化(图 4), 但纤维化指标TGF-β1、α-SMA以及Col-Ⅰ的表达均显著降低(图 4)。
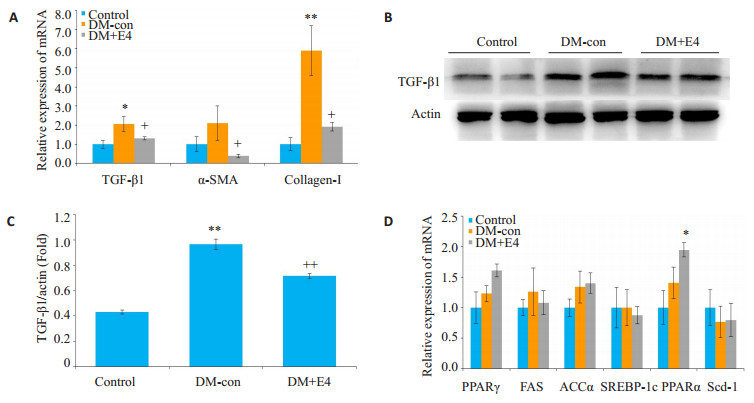
|
图 4 各组小鼠肝脏组织纤维化和脂质代谢相关基因的表达 Fig.4 Expression of fibrosis-related genes in the 3 groups. A:mRNA expression of TGF-β1, α-SMA and collagen-Ⅰ; B:Protein level of TGF-β1; C:Densitometric analysis of Western blotting results; D:mRNA expressions of PPARγ, FAS, ACCα, SREBP-1c, PPARα and Scd-1. *P < 0.05, **P < 0.01 vs control group; +P < 0.05 vs DM-con group. ++P < 0.01 vs DM-con group. |
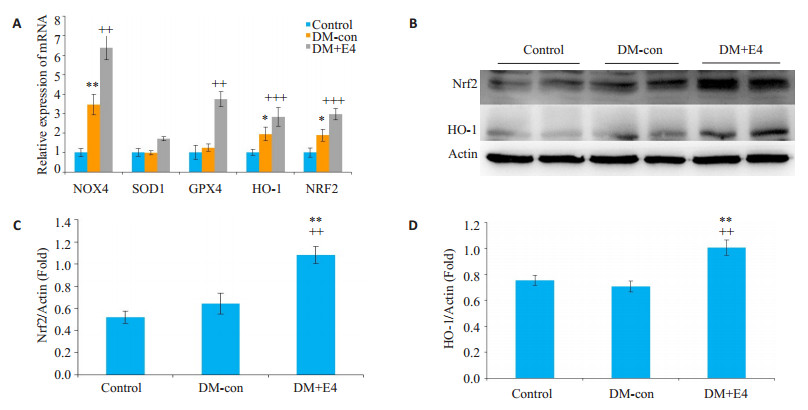
DM-con小鼠肝脏Nrf2、HO-1及NOX4的mRNA表达显著升高, 而GPX4无明显变化, 提示DM导致肝脏氧化应激, 同时抗氧化应激水平也代偿性增加。Exendin-4干预后小鼠肝脏Nrf2和HO-1的mRNA和蛋白表达都有进一步升高, GPX4的mRNA水平也显著高于DM-con组(图 5)。
|
图 5 各组小鼠肝脏组织氧化应激相关基因的表达 Fig.5 Expressions of oxidative stress-related genes in the liver tissue of the mice in each group. A:mRNA expressions of NOX4, SOD1, GPX4, HO-1 and Nrf2; B:Protein levels of Nrf2 and HO-1. C, D:Densitometric analysis of Western blotting results. *P < 0.05, **P < 0.01 vs control group; ++P < 0.01, +++P < 0.001 vs DM-con. |
糖尿病与肝脏代谢密切相关, 糖尿病可与NAFLD伴随或先后发生, NAFLD可进展至非酒精性脂肪性肝炎(NASH), 甚至发生肝硬化。在这一过程中, 细胞外基质在肝脏持续积累, 而TGF-β被认为是最有效的纤维化因子[14], TGF-β与Ⅰ型受体结合引起下游蛋白的磷酸化, 其磷酸化进一步促进了肝脏星状细胞活化期间Ⅰ型和Ⅲ型胶原的转录[15]。我们的研究结果也表明糖尿病小鼠的肝脏脂质沉积并伴随TGF-β1表达显著增加, 而后者可能进一步激活下游信号, 从而促进了肝脏纤维化。
GLP-1是由回结肠L细胞分泌的一种肠促胰素, 可通过葡萄糖依赖的方式促进胰岛素分泌, 同时抑制胰高血糖素分泌, 并减缓胃排空[16-17]。近年来, Exendin-4作为一种GLP-1受体激动剂已广泛应用于临床治疗糖尿病以及肥胖症等相关代谢疾病的治疗。在不同的肥胖小鼠模型中, Exendin-4均可显著改善肝脏的脂肪变性以及纤维化改变[18]。而除了改善肝脏纤维化之外, Exendin-4还可以显著降低心脏、肺以及胰腺等器官的纤维化。研究表明exendin-4显著降低了单核细胞趋化蛋白-1 (MCP-1)过表达小鼠心脏的纤维化[11], 同样给予exendin-4的糖尿病小鼠在肺血管周围表现出显著TGF-β和纤连蛋白的表达下降[19], 以及通过减少高糖介导下小鼠胰岛的活性氧(ROS)和血管紧张素Ⅱ (AT-Ⅱ)的产生直接抑制TGF-β1的表达[20]。我们的研究结果表明, 糖尿病小鼠的肝脏纤维化指标TGF-β1、α-SMA以及collagen-Ⅰ表达显著升高, Exendin-4治疗后显著降低了上述纤维化指标表达, 但是Exendin-4并未改善肝脏脂质沉积, 提示Exendin-4可能并不依赖于脂质代谢而改善肝脏纤维化。
氧化应激是由于活性氧的产生与机体抗氧化防御之间的不平衡导致的, 高水平的氧化应激可引起细胞凋亡和损伤, 这一过程也贯穿不同疾病的发展过程。NADPH氧化酶(NOXs)是ROS的重要来源之一, 有助于肝纤维化过程中ROS的产生, 促进了肝纤维化的发展[21-22]。本研究显示DM小鼠的肝脏NOX4表达显著增加, 提示肝脏存在明显的氧化应激。研究表明在STZ诱导的糖尿病小鼠模型中, Exendin-4治疗可显著降低ROS的水平[12]并提高抗氧化应激的能力[23], 并改善肥胖小鼠线粒体的功能, 恢复线粒体氧化呼吸链的作用[24]。在不同器官的氧化应激病变中, Exendin-4治疗均可提高抗氧化应激能力, 发挥抗氧化应激和抗细胞凋亡作用, 明显改善心肌细胞功能[25], 还能改善急性肾损伤小鼠的肾脏氧化应激[26]。肝脏氧化应激可能是导致糖尿病小鼠肝脏纤维化的一个重要机制, 本研究中DM小鼠肝脏氧化应激指标显著升高, 而Exendin-4干预后HO-1、GPX4以及Nrf2等抗氧化应激指标也显著增加, 提示Exendin-4可明显提高糖尿病小鼠的肝脏抗氧化应激水平, 这可能是其改善糖尿病肝脏纤维化的重要机制之一。
Nrf2是一种转录因子, 是调节细胞抗氧化应激的重要因子, 正常情况下, Nrf2依赖Kelch的ECH相关蛋白1 (Keap1)的泛素化而降解, 但在氧化应激的条件下, ROS的产生导致Keap1失活以及Nrf2的磷酸化, 磷酸化的Nrf2转移至细胞核中并与抗氧化反应元件(ARE)结合, 诱导下游抗氧化基因HO-1等的表达[27-28]。Nrf2/HO-1信号通路的激活可以保护机体多器官免于氧化应激的损伤。研究表明, 多种药物都可以上调肝脏Nrf2/HO-1信号通路的表达来改善肝纤维化和肝损伤[5, 29-30], 本研究也发现给予Exendin-4后肝脏Nrf2和HO-1的mRNA和蛋白的表达均显著增加, 提示Nrf2/HO-1通路的激活, 并可能通过此通路改善了肝脏纤维化。Exendin-4治疗显著降低了H2O2所诱导的胰腺β细胞系(INS-1)的氧化应激水平, 而此作用主要通过蛋白激酶C (PKCδ)激活的Nrf2/HO-1通路实现[31]。Exendin-4还可以通过激活Nrf2/HO-1来改善血管紧张素Ⅱ(Ang Ⅱ)诱导的血管平滑肌细胞(VSMC)衰老改变[32]和高糖(HG)诱导的心肌细胞凋亡[33]。而Liraglutide作为一种GLP-1长效合成类似物, 也可以通过激活Nrf2/HO-1信号通路发挥保护糖尿病大鼠脑神经细胞的作用[34]。上述研究均表明Exendin-4可以激活Nrf2/HO-1通路的表达, 与本研究中Exendin-4激活糖尿病小鼠肝脏Nrf2/HO-1通路的结果一致, 进一步表明Exendin-4可以通过激活Nrf2/HO-1来提高肝脏的抗氧化应激并改善肝脏纤维化。
综上, 我们发现在STZ联合高脂饮食诱导的糖尿病小鼠中, 肝脏脂质沉积、纤维化及氧化应激水平显著增加, 而exendin-4治疗后尽管未显著改善肝脏脂质沉积, 但可能通过激活Nrf2/HO-1信号通路提高了肝脏抗氧化应激的水平并改善了糖尿病小鼠的肝脏纤维化。
| [1] |
Stefan N, Haering HU, Cusi K. Non-alcoholic fatty liver disease:causes, diagnosis, cardiometabolic Consequences, and treatment strategies[J].
Lancet Diabetes Endo, 2019, 7(4): 313-24.
DOI: 10.1016/S2213-8587(18)30154-2. |
| [2] |
Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH:Cause or consequence[J].
? J Hepatol, 2018, 68(2): 335-52.
DOI: 10.1016/j.jhep.2017.09.021. |
| [3] |
Mcpherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies:Implications for prognosis and clinical management[J].
J Hepatol, 2015, 62(5): 1148-55.
DOI: 10.1016/j.jhep.2014.11.034. |
| [4] |
Nishikawa K, Osawa Y, Kimura K. Wnt/β-Catenin signaling as a potential target for the treatment of liver cirrhosis using antifibrotic drugs[J].
Int J Mol Sci, 2018, 19(10): 3103.
DOI: 10.3390/ijms19103103. |
| [5] |
Wang R, Wang J, Song FX, et al. Tanshinol ameliorates CCL4- induced liver fibrosis in rats through the regulation of Nrf2/HO-I and NF-kappa B/I kappa B alpha signaling pathway[J].
Drug Des Devel Ther, 2018, 12(5): 1281-92.
|
| [6] |
Li J, Hu R, Xu SF, et al. Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats[J].
Biomed Pharmacot, 2017, 96(1): 847-53.
|
| [7] |
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus[J].
Nat Rev Endocrinol, 2012, 8(12): 728-42.
DOI: 10.1038/nrendo.2012.140. |
| [8] |
Verspohl EJ. Novel therapeutics for type 2 diabetes:incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors[J].
Pharmacol Ther, 2009, 124(1): 113-38.
|
| [9] |
Bloomgarden ZT. Gut-derived incretin hormones and new therapeutic approaches[J].
Diabetes Care, 2004, 27(10): 2554-9.
DOI: 10.2337/diacare.27.10.2554. |
| [10] |
Kim S, Jung J, Kim H, et al. Exendin-4 improves nonalcoholic fatty liver disease by regulating glucose transporter 4 expression in ob/ob mice[J].
Korean Journal of Physiology & Pharmacology, 2014, 18(4): 333-9.
|
| [11] |
Younce CW, Niu JL, Ayala J, et al. Exendin-4 improves cardiac function in mice overexpressing monocyte chemoattractant protein- 1 in cardiomyocytes[J].
J Mol Cell Cardiol, 2014, 76: 172-6.
DOI: 10.1016/j.yjmcc.2014.08.022. |
| [12] |
Sancar-Bas S, Gezginci-Oktayoglu S, Bolkent S. Exendin-4 attenuates renal tubular injury by decreasing oxidative stress and inflammation in streptozotocin-induced diabetic mice[J].
Growth Factors, 2015, 33(5/6): 419-29.
|
| [13] |
Kusakabe T, Tanioka H, Ebihara K, et al. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet[J].
Diabetologia, 2009, 52(4): 675-83.
DOI: 10.1007/s00125-009-1258-2. |
| [14] |
Chen Q, Yang W, Wang X, et al. TGF-β1 induces EMT in bovine mammary epithelial cells through the TGFβ1/Smad signaling pathway[J].
Cell Physiol Biochem, 2017, 43(1): 82-93.
DOI: 10.1159/000480321. |
| [15] |
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation[J].
Nat Rev Gastroenterol Hepatol, 2017, 14(7): 397-411.
DOI: 10.1038/nrgastro.2017.38. |
| [16] |
Drucker DJ, Nauck MA. The incretin system:glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes[J].
Lancet, 2006, 368(9548): 1696-705.
DOI: 10.1016/S0140-6736(06)69705-5. |
| [17] |
Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus[J].
Nat Rev Endocrinol, 2016, 12(10): 566-92.
DOI: 10.1038/nrendo.2016.86. |
| [18] |
Patel V, Joharapurkar A, Kshirsagar S, et al. Coagonist of GLP-1 and glucagon receptor ameliorates development of Non-Alcoholic fatty liver disease[J].
Cardiovasc Hematol Agents Med Chem, 2018, 16(1): 35-43.
DOI: 10.2174/1871525716666180118152158. |
| [19] |
Oztay F, Sancar-Bas S, Gezginci-Oktayoglu SA, et al. Exendin-4 partly ameliorates-hyperglycemia-mediated tissue damage in lungs of streptozotocin-induced diabetic mice[J].
Peptides, 2018, 99: 99-107.
DOI: 10.1016/j.peptides.2017.12.007. |
| [20] |
Kim J, Park S, You Y, et al. Suppression of ROS production by exendin-4 in PSC attenuates the high glucose-induced islet fibrosis[J].
PLoS One, 2016, 11(12): e163187.
|
| [21] |
Crosas-Molist E, Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis[J].
Redox Biol, 2015, 6: 106-11.
DOI: 10.1016/j.redox.2015.07.005. |
| [22] |
Richter K, Kietzmann T. Reactive Oxygen species and fibrosis: further evidence of a significant liaison[J].
Cell Tissue Res, 2016, 365(3): 591-605.
DOI: 10.1007/s00441-016-2445-3. |
| [23] |
Gezginci-Oktayoglu S, Sacan O, Yanardag R, et al. Exendin-4 improves hepatocyte injury by decreasing proliferation through blocking NGF/TrkA in diabetic mice[J].
Peptides, 2011, 32(2): 223-31.
DOI: 10.1016/j.peptides.2010.10.025. |
| [24] |
Wang ZX, Hou L, Huang LH, et al. Exenatide improves liver mitochondrial dysfunction and insulin resistance by reducing oxidative stress in high fat diet-induced obese mice[J].
Biochem Biophys Res Commun, 2017, 486(1): 116-23.
DOI: 10.1016/j.bbrc.2017.03.010. |
| [25] |
Mangmool S, Hemplueksa P, Parichatikanond W, et al. Epac is required for GLP-1R-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes[J].
Mol Endocrinol, 2015, 29(4): 583-96.
DOI: 10.1210/me.2014-1346. |
| [26] |
Chen YT, Tsai TH, Yang CC, et al. Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction[J].
J Transl Med, 2013, 11: 270.
DOI: 10.1186/1479-5876-11-270. |
| [27] |
Loboda A, Damulewicz M, Pyza EA, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases:an evolutionarily conserved mechanism[J].
Cell Mol Life Sci, 2016, 73(17): 3221-47.
DOI: 10.1007/s00018-016-2223-0. |
| [28] |
Oh YS, Jun HS. Effects of Glucagon-Like peptide-1 on oxidative stress and Nrf2 signaling[J].
Int J Mol Sci, 2018, 19(1): 26.
|
| [29] |
Mahmoud AM, Hozayen WG, Ramadan SM. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats[J].
Biomed Pharmacother, 2017, 94: 280-91.
DOI: 10.1016/j.biopha.2017.07.101. |
| [30] |
Ma X, Luo Q, Zhu H, et al. Aldehyde dehydrogenase 2 activation ameliorates CCl4-induced chronic liver fibrosis in mice by upregulating Nrf2/HO-1 antioxidant pathway[J].
J Cell Mol Med, 2018, 22(8): 3965-78.
DOI: 10.1111/jcmm.2018.22.issue-8. |
| [31] |
Kim MH, Kim EH, Jung HS, et al. EX4 stabilizes and activates Nrf2 via PKC delta, contributing to the prevention of oxidative stress-induced pancreatic beta cell damage[J].
Toxicol Appl Pharmacol, 2017, 315: 60-9.
DOI: 10.1016/j.taap.2016.12.005. |
| [32] |
Zhou TF, Zhang MQ, Zhao L, et al. Activation of Nrf2 contributes to the protective effect of Exendin-4 against angiotensin Ⅱ-induced vascular smooth muscle cell senescence[J].
Am J Physiol Cell Physiol, 2016, 311(4): C572-82.
DOI: 10.1152/ajpcell.00093.2016. |
| [33] |
Zhao SM, Gao HL, Wang YL, et al. Attenuation of high GlucoseInduced rat cardiomyocyte apoptosis by exendin-4 via intervention of HO-1/Nrf-2 and the PI3K/AKT signaling pathway[J].
Chin J Physiol, 2017, 60(2): 89-96.
DOI: 10.4077/CJP.2017.BAF434. |
| [34] |
Deng CH, Cao J, Han JQ, et al. Liraglutide activates the Nrf2/HO-1 antioxidant pathway and protects brain nerve cells against cerebral ischemia in diabetic rats[J].
Comput Intell Neurosci, 2018: 1-7.
|
 2019, Vol. 39
2019, Vol. 39

