2. 海南省人民医院内分泌代谢科,海南 海口 570311
2. Department of Endocrinology and Metabolism, Hainan Provincial People's Hospital, Haikou 570311, China
目前中国已成为糖尿病患者人数最多的国家,且预计在未来的30年中国糖尿病患者人数将达到1.198亿[1]。2型糖尿病(T2DM)与超重及肥胖的关系非常密切,脂肪组织过多及内脏脂肪堆积加剧糖尿病的发生和发展[2]。近年来,胰高血糖素样肽-1受体激动剂(GLP-1RAs)以其良好的改善血糖、调节血脂、降低体质量的效应受到了广泛关注[3-5]。动物研究发现GLP-1类似物可通过减少能量摄入、降低血脂[6]、促进白色脂肪棕色化[7]、提高脂肪细胞的胰岛素敏感性[8]从而改善肥胖。国外有研究报道GLP-1RAs可显著改善早期糖尿病合并肥胖患者的胰岛β细胞功能、葡萄糖稳态及内脏肥胖情况[9]。但目前对GLP-1RAs治疗T2DM患者过程中,对机体各部位脂肪分布与肌肉含量等的影响及其具体机制尚不十分清楚。本研究通过辅助应用人体成分分析仪(BCM),对比分析GLP-1RAs治疗前后超重及肥胖的T2DM患者糖脂代谢、各部位脂肪分布及肌肉率等指标,探讨不同体质人群的临床获益,为T2DM合并超重/肥胖患者的个体化治疗提供更加合理的依据。
1 资料和方法 1.1 研究对象和分组回顾分析2014年12月~2015年9月就诊于南方医科大学南方医院内分泌代谢科的T2DM合并超重/肥胖的76例患者,按体质量指数(BMI)分为超重组(BMI:24~27.9 kg/m2)、肥胖组(BMI:28~31.9 kg/m2)及严重肥胖组(BMI≥32 kg/m2),予GLP-1RAs治疗,平均随访8.9周(3.0~29.0周)。纳入标准:性别不限;年龄19~70岁;明确诊断为T2DM[10](符合1999年WHO诊断标准);超重或肥胖[11](BMI≥24 kg/m2);既往未使用GLP-1、GLP-1RA及二肽基肽酶4(DPP4)抑制剂类药物治疗。排除标准:计划妊娠或妊娠、哺乳期妇女;有甘油三酯明显升高、严重心脑血管、胃肠道、肾脏损害、胰腺炎病史、外伤等情况;精神疾病;治疗禁忌者;近3月使用减肥药物者。本研究方案获本院伦理委员会批准,签署知情同意书。
1.2 研究方法所有研究对象均在基线时采集一般资料,包括年龄、性别、身高、体质量、腰围、臀围、计算BMI及腰臀比(WHR);过夜空腹采静脉血,全自动生化分析仪酶法检测空腹血糖(FPG)、甘油三脂(TG)、总胆固醇(TC)、低密度脂蛋白胆固醇(LDL- c)、高密度脂蛋白胆固醇(HDL-c)、极低密度脂蛋白胆固醇(VLDL-c);高压液相色谱法测定糖化血红蛋白(HbA1C);化学发光法检测空腹胰岛素(FINS);采用稳态模型公式计算胰岛素抵抗指数(HOMA-IR)[12]。
1.3 BCM检测方法采用移动式多频电阻抗人体成分分析仪(JAWONIOI353,韩国)对研究对象的体质成分进行分析。被测者于空腹、排空大小便、无剧烈运动下,脱去外套、金属物品、鞋袜,将双足放置于足电极上,双手握住手电极。由测试者依次输入研究对象的年龄、身高、性别后,检测并计算其体质量、身体脂肪率(BF%)、基础代谢、BMI、各部位(上肢、躯干、下肢)皮下脂肪率及肌肉率、内脏脂肪指数(VAI)等指标。
1.4 治疗随访研究对象在原治疗基础上给予艾塞那肽(百泌达,美国礼来,起始剂量5 μg,2次/d,皮下注射,1周后增加至10 μg,2次/d,不耐受者可再减至5 μg,2次/d,连续使用12周以上)或利拉鲁肽(诺和力,丹麦诺和诺德,1.2 mg,1次/d,皮下注射,不耐受者可减至0.6 mg,1次/d,连续使用12周以上)治疗,如出现过敏反应或消化道等经过调整后仍不能耐受的症状者退出研究,每隔4周进行随访,在治疗结束时再次收集基线时各项数据。
1.5 统计学处理采用Excel建立数据库,SPSS 20.0软件进行统计学分析,正态分布的资料以均数±标准差表示,偏态资料以中位数(四分位数间距)表示。计量资料间的比较采用单因素方差分析、t检验、Kruskal-Wallis检验,两两比较采用LSD、Dunnett's T3检验,计数资料间的比较采用卡方检验,P < 0.05为差异有统计学意义。
2 结果 2.1 各组一般资料及临床数据比较入组受试者76例,超重组14例,肥胖组35例;严重肥胖组27例。疗程结束后,超重组、肥胖组及严重肥胖组各有13例(92.9%)、28例(80.0%)和22例(81.5%)完成此次观察,共有13例患者由于胃肠道不良反应及药物价格因素退出研究。治疗前各组的性别、年龄、身高、FPG、HbA1c、FINS、HOMA-IR、血脂、合用的其他降糖药物均无显著统计学差异(P>0.05,表 1)。
| 表 1 各组一般资料及临床数据比较 Tab.1 Comparison of baseline data among the groups [Mean±SD, M(QL, QU)] |
治疗后,超重组与肥胖组体质量均明显降低(P < 0.05),严重肥胖组的体质量虽降低,但差异无统计学意义;各组患者治疗后BMI、VAI均较前降低(P < 0.05);超重组WHR较前降低(P < 0.05),肢体皮下脂肪率稍升高、肌肉率稍下降,但均无统计学差异(均P>0.05);肥胖组及严重肥胖组治疗后BF%、各部位脂肪率均较前明显下降,肌肉率显著上升(均P < 0.05,表 2~4)。
| 表 2 各组治疗前后肥胖相关指标变化比较 Tab.2 Comparison of obesity-related indexes before and after treatment (Mean±SD, n=13-28) |
| 表 3 各组治疗前后各部位脂肪率的变化比较 Tab.3 Comparison of fat percentages before and after treatment (Mean±SD, n=13-28) |
| 表 4 各组治疗前后各部位肌肉率的变化比较 Tab.4 Comparison of muscle ratio before and after treatment (Mean±SD, n=13-28) |
各组患者体质量、BMI、WHR治疗前后的差值均无统计学差异(P>0.05);BF%、各部位脂肪率及肌肉率差值均有统计学差异(P < 0.05)。治疗后,肥胖组BF%、VAI与超重组相比,下降更为明显(P < 0.05);肥胖组与严重肥胖组治疗后肢体皮下脂肪率的降低及肌肉率的升高均较超重组明显(均P < 0.05,图 1)。
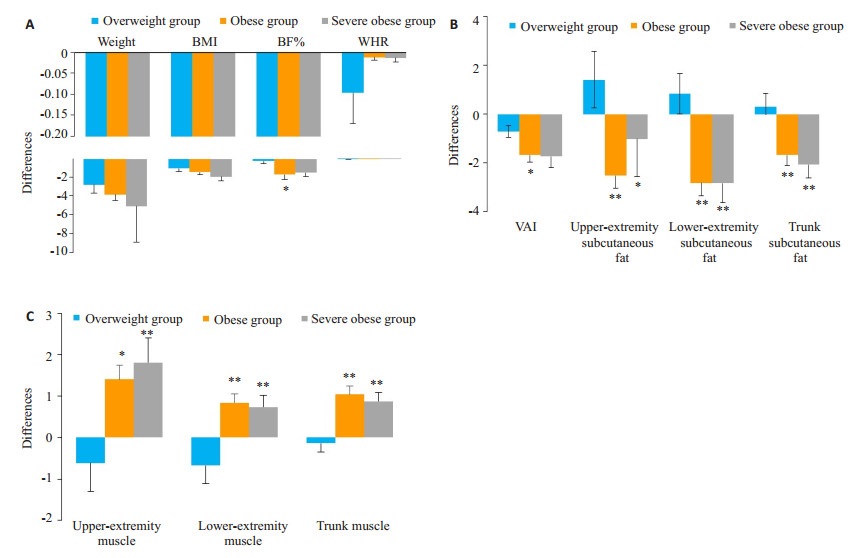
|
图 1 治疗前后三组间各项指标差值的比较 Fig.1 Comparison of the changes in body weight, BMI, BF% and WHR (A), fat percentage (B), and muscle ratio (C) among the three groups after treatment. *P < 0.05, **P < 0.01 vs overweight group. |
目前国内对于超重及肥胖的诊断标准分别为BMI≥24 kg/m2及28 kg/m2,明显低于西方人的诊断标准,但随着肥胖发病率的逐渐增加及低龄化趋势,今后严重肥胖的患者可能将明显增多,并且亚洲人在相对低的BMI水平即可出现明显的胰岛素抵抗等代谢异常[13],这与内脏脂肪的分布增加关系密切[14]。因此,对于超重/肥胖人群不仅需要控制体质量,更重要的是改善体脂分布,增加肌肉量,进而改善代谢。
近年来,代谢手术已成为治疗T2DM合并肥胖患者的重要手段之一,指南推荐BMI≥32 kg/m2的肥胖患者采取代谢手术治疗,而无论是否存在其它合并症[11]。尽管如此,由于各种原因仍有大量符合手术适应症的患者选择了药物治疗。为了更好地评估GLP-1RAs对此类人群的临床效果,提供个体化治疗依据,本研究将BMI≥32 kg/m2的患者列入严重肥胖组进行观察。
肥胖患者的脂肪组织可产生大量炎症和脂肪细胞因子,通过影响胰岛素信号通路导致糖耐量异常和高胰岛素血症[15-16],而超重及肥胖人群只需减轻5%~10%的体质量即可大幅降低糖尿病的风险[17]。BMI与BF%有良好的相关性,而WHR可较好地反映中心性肥胖[18-19]。本研究发现相对于超重组,BMI更高的患者治疗后BF%改善越明显,而体质量、WHR的差值在3组间均无显著差异,提示采用BF%可较好地反应患者体脂水平,而不同BMI患者腹型肥胖的差异并不明显。与西方国家人群相比,中国人群的BF%普遍比欧洲人高[20],这也使得糖尿病的患病及共病风险更高。
GLP-1RAs通过葡萄糖依赖性刺激胰岛素分泌、延缓胃排空、抑制食欲等降低血糖及体质量[21-22],对T2DM患者的体脂再分布特别是降低内脏脂肪沉积有明显的效果,且利拉鲁肽及艾塞那肽两种药物降低患者的BMI水平及改善脂肪分布的作用无显著差异[23],本研究发现,在治疗后,各组BMI均降低,超重组与肥胖组的体质量下降,肥胖组与严重肥胖组的BF%下降,超重组WHR降低,表明GLP-1RAs的治疗可有效降低超重及肥胖患者的体质量及体脂蓄积情况,这与既往利拉鲁肽和艾塞那肽治疗不同BMI的T2DM患者的研究结果一致[24-25]。
研究显示,与欧洲人群相比,在总脂肪相同的情况下,亚洲人有更多的内脏脂肪组织,并且更容易形成腹型肥胖[26]。在糖脂代谢过程中,内脏脂肪组织比皮下脂肪组织更活跃地分泌大量导致胰岛素抵抗的炎症细胞因子,因此VAI可更好地预测患者并发心血管等疾病的风险[27]。Kolb等[28]研究发现,GLP-1RAs降低体脂率的效果,特别是在超重患者中,优先体现于VAI的下降,患者治疗后体质量的降低主要与VAI有关,而BMI更高的患者在治疗后各部位脂肪及肌肉率的改善更大。本研究结果也显示,使用GLP-1RAs治疗后,肥胖组及严重肥胖组治疗后各部位脂肪率均得到显著改善。进一步分析发现,肥胖组VAI与超重组相比,下降更为明显,肥胖组与严重肥胖组肢体皮下脂肪率的降低均较超重组明显,提示在超重及肥胖患者中,BMI更高的人群各部位脂肪率较BMI较低的人群改善更显著。但Russo等[29]研究表明,利拉鲁肽可独立于BMI降低T2DM患者VAI,这可能与不同药物的特点、研究人群、环境及生活方式差异等有关。
研究表明,GLP-1RAs降低T2DM患者体质量的效应主要表现在降低脂肪组织的蓄积,同时增加非脂肪部分的软组织含量[30]。本研究显示,治疗后肥胖组与严重肥胖组各部位的肌肉率均明显改善,且两组升高程度均明显高于超重组,而体质量明显下降。这与Li等[31]发现利拉鲁肽在降低体质量的同时,瘦组织含量亦降低有所不同,可能与研究人群差异有关。肌肉率的升高有助于调节患者肌肉耗氧量及血管内皮功能,减轻外周胰岛素抵抗[32]。超重及肥胖的患者各部位脂肪及肌肉组织率的改善为患者的糖脂代谢提供了保护性因素,有利于降低患T2DM及相关并发症风险。
综上所述,GLP-1RA可显著改善T2DM合并超重/肥胖患者多部位脂肪的分布和肌肉含量,BMI较高患者的体质构成改善更显著。对于BMI在32 kg/m2以上需行手术治疗的患者,若因各种因素无法手术,可将GLP- 1RAs作为一种很好的备选治疗方案。
| [1] |
Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes Atlas:global estimates of diabetes prevalence for 2017 and projections for 2045[J].
Diabetes Res Clin Pract, 2018, 138(10): 271-81.
|
| [2] |
Perry RJ, Peng L, Cline GW, et al. Mechanisms by which a verylow-calorie diet reverses hyperglycemia in a rat model of type 2 diabetes[J].
Cell Metab, 2018, 27(1): 210-22.
DOI: 10.1016/j.cmet.2017.10.004. |
| [3] |
Yang WY, Min K, Zhou ZG, et al. Efficacy and safety of lixisenatide in a predominantly Asian population with type 2 diabetes insufficiently controlled with basal insulin:the getgoal-LC randomized trial[J].
Diabetes Obes Metab, 2018, 20(2): 335-43.
DOI: 10.1111/dom.2018.20.issue-2. |
| [4] |
Abd E, Kahle M, Meier J, et al. A meta analysis comparing clinical effects of short-or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetes patients[J].
Diabetes Obes Metab, 2017, 19(2): 216-27.
DOI: 10.1111/dom.12804. |
| [5] |
Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion[J].
J Diabetes, 2016, 8(6): 753-65.
DOI: 10.1111/1753-0407.12439. |
| [6] |
Wan Y, Bao X, Huang JB, et al. Novel GLP-1 analog supaglutide reduces HFD-Induced obesity associated with increased Ucp-1 in white adipose tissuein mice[J].
Front Physiol, 2017, 8(3): 294-304.
|
| [7] |
Heppner KM, Perez-Tilve D. GLP-1 based therapeutics:simultaneously combating T2DM and obesity[J].
Front Neurosci, 2015, 9(5): 92-102.
|
| [8] |
Jiang YJ, Wang ZH, Ma B, et al. GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress[J].
Front Pharmacol, 2018, 9(12): 1168-78.
|
| [9] |
Santilli F, Simeone PG, Guagnano MT, et al. Effects of liraglutide on weight loss, fat distribution, and β-Cell function in obese subjects with prediabetes or early type 2 diabetes[J].
Diabetes Care, 2017, 11(40): 1556-64.
|
| [10] |
American Diabetes Association. Diagnosis and classification of diabetes mellitus[J].
Diabetes Care, 2014, 37(Suppl 1): S81-90.
|
| [11] |
中华医学会内分泌学分会. 中国2型糖尿病合并肥胖综合管理专家共识[J].
药品评价, 2016, 13(17): 5-10, 12.
DOI: 10.3969/j.issn.1672-2809.2016.17.001. |
| [12] |
Zhang FF, Yu LC, Lin XF, et al. Minireview:roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases[J].
Mol Endocrinol, 2015, 29(10): 1400-13.
DOI: 10.1210/me.2015-1155. |
| [13] |
Yip WC, Sequeira IR, Plank LD. Prevalence of Pre-Diabetes across ethnicities:a review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia[J].
Nutrients, 2017, 9(11): 1273-90.
DOI: 10.3390/nu9111273. |
| [14] |
González N, Moreno-Villegas Z, González-Bris A, et al. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes[J].
Cardiovasc Diabetol, 2017, 16(1): 44-53.
DOI: 10.1186/s12933-017-0528-4. |
| [15] |
Blueher M. Adipose tissue inflammation:a cause or consequence of obesity-related insulin resistance[J].
Clin Sci, 2016, 130(18): 1603-14.
DOI: 10.1042/CS20160005. |
| [16] |
Ghorpade DS, Ozcan L, Zheng Z, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance[J].
Nature, 2018, 555(7698): 673-7.
DOI: 10.1038/nature26138. |
| [17] |
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes[J].
JAMA, 2015, 314(7): 687-99.
DOI: 10.1001/jama.2015.9676. |
| [18] |
Abate N, Chandalia M. Risk of Obesity-Related cardiometabolic complications in special populations:a crisis in asians[J].
Gastroenterology, 2017, 152(7, SI): 1647-55.
DOI: 10.1053/j.gastro.2017.01.046. |
| [19] |
Lee DH, Keum NN, Hu FB, et al. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk:two large prospective studies in US men and women[J].
Eur J Epidemiol, 2018, 33(11): 1113-23.
DOI: 10.1007/s10654-018-0433-5. |
| [20] |
Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. Body-mass index and all-cause mortality:individual-participant-data meta-analysis of 239 prospective studies in four continents[J].
Lancet, 2016, 388(146): 776-86.
|
| [21] |
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN):a multicentre, double-blind, randomised, placebo-controlled phase 2 study[J].
Lancet, 2016, 387(119): 679-90.
|
| [22] |
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists:efficacy and safety in diabetes and beyond[J].
Drug Context, 2015, 4(10): 212283-92.
|
| [23] |
Morano S, Romagnoli E, Filardi T, et al. Short-term effects of glucagon-like peptide 1(GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus:an ultrasonography study[J].
Acta Diabetol, 2015, 52(4): 727-32.
DOI: 10.1007/s00592-014-0710-z. |
| [24] |
林坚. 利拉鲁肽治疗不同体重指数2型糖尿病患者的效果观察[J].
白求恩医学杂志, 2015, 12(2): 136-8.
|
| [25] |
Deng H, Lin S, Yang X, et al. Impact of baseline BMI on glycemic control and weight change with exenatide monotherapy in Chinese drug-na?ve type 2 diabetic patients[J].
J Diabetes, 2018, 407(9): 12883-95.
|
| [26] |
Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes:a global perspective[J].
Curr Diab Rep, 2016, 16(1): 7-18.
DOI: 10.1007/s11892-015-0699-x. |
| [27] |
Kim G, Jo K, Kim KJ, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by 18FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes[J].
Cardiovasc Diabetol, 2015, 14(1): 148-58.
DOI: 10.1186/s12933-015-0310-4. |
| [28] |
Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes[J].
BMC Med, 2017, 15(1): 131-42.
DOI: 10.1186/s12916-017-0901-x. |
| [29] |
Russo GT, Labate AM, Giandalia A, et al. Twelve-month treatment with liraglutide ameliorates visceral adiposity index and common cardiovascular risk factors in type 2 diabetes outpatients[J].
J Endocrinol Invest, 2015, 38(1): 81-9.
DOI: 10.1007/s40618-014-0163-9. |
| [30] |
Perna S, Guido D, Bologna C, et al. Liraglutide and obesity in elderly:efficacy in fat loss and safety in order to prevent sarcopenia.a perspective case series study[J].
Aging Clin Exp Res, 2016, 28(6): 1251-7.
DOI: 10.1007/s40520-015-0525-y. |
| [31] |
Li CJ, Yu Q, Yu P, et al. Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients[J].
Cardiovasc Diabetol, 2014, 13(1): 36-45.
DOI: 10.1186/1475-2840-13-36. |
| [32] |
Koska J, Lopez L, D'souza K, et al. Effect of liraglutide on dietary lipid-induced insulin resistance in humans[J].
Diabetes Obes Metab, 2018, 20(1): 69-76.
DOI: 10.1111/dom.2018.20.issue-1. |
 2019, Vol. 39
2019, Vol. 39

