2. 广东医科大学附属医院血液科,广东 湛江 524000
2. Department of Hematology, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524000, China
酪氨酸激酶抑制剂(TKIs)的问世,标志着慢性髓性白血病(CML)进入靶向治疗新纪元,如今CML已成为一种可控制的慢性疾病[1]。伊马替尼高效的缓解率使80%以上的CML患者在治疗后可以取得完全细胞遗传学反应(CCyR),10年总生存率(OS)82%,大大改善了CML患者的预后[2]。然而TKI耐药性的出现,使部分患者丧失了已获得的最佳反应。研究发现BCR-ABL基因突变是TKI耐药及治疗失败的主要机制[3],ABL激酶区的点突变使得20%~30%的患者出现IM耐药并导致疾病进展[4-6],其中大部分ABL激酶区突变类型可以通过转换二代TKI能获得覆盖[7-8],但是T315I突变对现有的一、二代TKIs广泛耐药,成为CML耐药患者治疗难点[9]。CML-T315I+患者,疾病进展较快,预后差,往往在数月内死亡[10],据文献报道CML-T315I+的患者从T315I的出现到死亡/期限的中位时间仅3月[11]。泊那替尼是一种多靶点酪氨酸激酶抑制剂,属于第3代TKI抑制剂,在2013年EMA和FDA先后批准用于治疗CML或Ph+ALL的BCR-ABL1 T315I突变的患者[12-13],对CML-T315I+患者有明确疗效[14],已在我国香港上市。目前针对CML-T315I+患者的有效治疗方法不多,为了明确不同方法之间的疗效差异,改善此类患者的预后,本研究回顾性分析19例CML-T315I+患者的临床特征及泊那替尼治疗的疗效,为临床工作提供帮助。
1 资料和方法 1.1 病例资料南方医科大学南方医院、广东医科大学附属医院2013年3月~2018年9月,19例对TKI耐药的CML患者经BCR-ABL KD突变检测T315I突变(+),其中初诊时慢性期14、加速期2例、急变期3例。
1.2 诊断、TKI耐药前治疗及疗效标准所有患者初诊时均行血常规、细胞形态学、细胞遗传学、分子生物学等实验室检查明确诊断,按CML诊治指南每隔3~6月进行复查。耐药前TKI应用剂量为伊马替尼300~400 mg/d,达沙替尼100~140 mg/d,尼罗替尼剂量600~800 mg/d。治疗过程中发现TKI耐药时,检测ABL激酶区突变。CML的诊断、治疗及疗效标准参考文献[15]。TKI耐药定义参照文献[15] IM治疗CML慢性期患者治疗反应评估中的"治疗失败"。
1.3 染色体分析、BCR-ABL转录本及ABL1激酶区突变的检测染色体标本行G显带分析,核型异常识别和描述参照《人类细胞遗传学国际命名体制(ISCN2016)》;TRIzol法提取标本中总RNA,经逆转录酶逆转成cDNA,应用RTQ-PCR检测BCR-ABL1转录本,检测敏感度为10-4~10-5;利用巢式PCR对ABL1激酶区进行突变检测,扩增产物经琼脂糖凝胶电泳鉴定后,利用Sanger测序仪对PCR产物进行测序,检测BCR-ABL KD突变情况。
1.4 临床分组A组:泊那替尼治疗组,12人;B组:达沙替尼+干扰素、羟基脲、高三尖杉酯碱、及其他化疗方案治疗组,7人。泊纳替尼,Iclusig,ARIAD,患者自购于中国香港。预处理方案:BU+CY(白消安+环磷酰胺)或者改良(阿糖胞苷+白消安+环磷酰胺)。
1.5 随访通过门诊等方式进行随访,随访截止时间为2018年9月,随访内容包括疾病缓解状态、是否复发及存活情况等。
1.6 统计学处理采用SPSS17.0软件进行统计学分析。生存分析采用Kaplan-Meier法。P < 0.05为差异有统计学意义。
2 结果 2.1 19例CML-T315I患者临床特征及用药19例患者,男性15例,女性4例,中位年龄40(19~ 67)岁,初诊时CML-CP(CP)14例,CML-AP(AP)2例,CML-BP(BP)3例,73.7%(14/19例)患者处于慢性期(表 1)。Sokal评分高危组7例、中危组6例,低危组3例,中高危患者占81.2%(13/16例)。其中78.9%(15/19例)患者有附加染色体杂畸变,+der(22)、+8是最常见的染色体畸变,52.6%(10/19例)患者有多重突变,4例存在3种突变,6例存在2种突变,其中1例有CYP3A5突变。从初诊到检测出T315I突变的中位期为40 m(5~ 120 m),31.6%(6/19例)患者在慢性期检测出T315I突变,68.4%(13/19例)患者在疾病进展后(1例加速期,12例急变期)检测出T315I突变,CML-进展期的T315I检出率明显比慢性期高(表 2)。
| 表 1 19例CML-T315I+患者临床特征及用药 Tab.1 Clinical characteristics and medications of 18 patients with CML-T315I+ |
| 表 2 12例行Ponatinib治疗的CML-T315I+患者的临床转归 Tab.2 Clinical outcomes of 12 patients with CML-T315I+ treated with ponatinib |
19例CML初诊后均行TKI治疗,其中16例选择一代TKI治疗,3例选择二代TKI治疗,后因耐药或不耐受,16例一代TKI治疗的患者先后转换为二代TKI治疗。检测出T315I突变后,根据治疗方案不同分为两组:A组:63.1%(12/19例)患者选择泊那替尼治疗。B组:36.8%(7/19例)患者选择达沙替尼+干扰素、羟基脲、高三尖杉酯碱、及其他化疗方案治疗。A组患者泊那替尼治疗后,91.7%(11/12例)患者重获血液学缓解,其中包括移植后复发的6例患者,复发的中位时间是9.5 m(2~48)m。50%(6/12例)患者获得完全分子生物学缓解,25%(3/12例)患者获得主要分子生物学缓解。2例因复发死亡。B组7例患者截止随访,14.3%(1/7例)存活,余6例因疾病进展,治疗无效死亡(图 1)。截止随访,8例因复发死亡,11例存活:(包括10例A组患者,1例B组患者)。
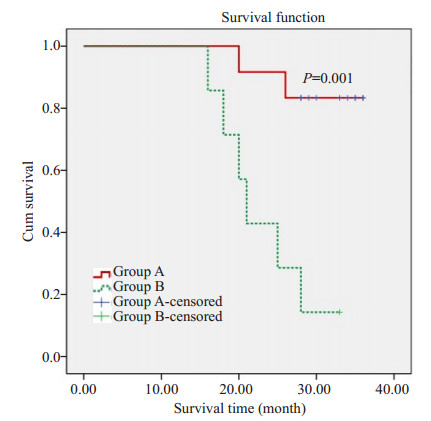
|
图 1 T315I突变后两种治疗方案的3年OS生存曲线 Fig.1 3-year overall survival probability of the patients receiving two treatments after T315I mutation. Group A was treated with Ponatinib, and group B was treated with dasatinib + interferon, hydroxyurea, homoharringtonine and other chemotherapy regimens after mutation |
CML是一种以骨髓多能造血干细胞过度克隆增殖为特征的恶性骨髓增殖性肿瘤,Ph染色体(t 9;22)(q34;q11.2)或BCR-ABL1融合基因阳性[16]。TKIs特异性抑制BCR-ABL表达,使CML进入靶向治疗新时代。BCR-ABL基因突变是导致CML对TKIs耐药和治疗失败最主要的原因。50%~90%TKI耐药是由于ABL激酶区突变导致,已被发现的BCR-ABL激酶区突变有90余种,常见的如M244V、L248V、G250E/AF、Q252H/R、Y253F/H、E255K及T315I等[17]。大部分ABL激酶突变类型可以通过现有的二代TKIs,尼罗替尼、达沙替尼、博舒替尼等得到覆盖,T315I除外[18]。T315I突变也称T315I gatekeeper突变,即ABL激酶域中315位苏氨酸突变为异亮氨酸[19]。T315I突变在ABL激酶区突变中较为常见,有研究发现T315I在CML患者耐药患者中的检出率有15% [20]。目前针对CML-T315I+患者推荐的治疗方案包括:泊那替尼、造血干细胞移植或临床研究[9],大量研究表明泊那替尼对TKI耐药或含有T315I点突变的难治性CML有确切的疗效[21-23],有I期临床试验表明,泊那替尼治疗CML-T315I+的患者,可获得高达75%的细胞遗传学缓解率[24]。
分析本中心这组病人临床特征发现长期TKI耐药的CML患者易发生T315I突变,疾病进展后(加速期、急变期)检出率高于疾病慢性阶段,此类患者往往具有附加染色体畸变和多重基因突变,Sokal评分中高危者居多。T315I突变对现有的一二代TKI泛耐药,意味着CML-T315I+患者预后不良和治疗选择的有限性[25]。泊那替尼的核心分子结构碳碳三键与I315的侧链高效疏水链接,其甲基苯基后侧残基、三氟甲基苯基占用DFG外构象诱导的位点、乙炔基与T315I突变之间形成范德华力,能有效地抑制T315I及其他重要激酶结构域的突变,同时也能抑制PDGFR家族、FGFR、VEGFR和SCR [26-28]。针对长期序贯治疗复发难治的CML-T315I患者,及时选择泊那替尼治疗可能改善预后,延长生存期[29]。分析我们中心数据发现,A组患者生存预后优于B组患者(P=0.001 < 0.005),提示针对长期序惯治疗难治的CML-T315I+患者,移植后复发率极高、常规化疗手段预后很差,泊那替尼治疗的疗效优于传统化疗手段。A组患者中有6例属于移植后复发应用泊那替尼补救性治疗,且均获得不同程度的缓解,100%重新获得血液学缓解,50%的获得完全分子生物学缓解。提示泊那替尼补救性治疗对于移植后复发的患者具有显著的疗效。值得一提的是,15例在移植后+94 d,髓外复发侵及中枢,造成脑疝,给予泊那替尼(45 mg/d)治疗后,38 d后获得血液及中枢系统缓解,BCR-ABL(IS)复查阴性,连续3月获得CMR后,泊那替尼减量至30 mg/d,20 d后出现分子学复发,加量至45 mg/d后,1月后再次获得MMR,目前泊那替尼(45 mg/d)维持治疗,提示泊那替尼在有效的血药浓度下可以穿过血脑屏障起到治疗作用[30-31],以及可能诱导移植物抗白血病效应,与Petrungaro等研究结果一致[32-33]。
虽然泊那替尼作为第三代TKI,具有显著的疗效,尤其针对CML-T315I+患者。然而,泊那替尼的药物副反应,也应引起临床重视。研究指出,泊那替尼有严重致命性心肌梗死、脑卒中及血流中断或血管重度狭窄致肢体组织损伤及坏死的风险需谨慎关注[34-35]。由于本研究患者数量较少,随访期间尚未出现严重不良反应。泊那替尼作为三代TKI,对CML-T315I+患者疗效肯定,及时在TKI耐药、疾病进展时检测出T315I突变,对CML治疗方案的调整及预后判断十分重要,有待临床进一步积累研究。
| [1] |
Ali MA. Chronic myeloid leukemia in the era of tyrosine kinase inhibitors: an evolving paradigm of molecularly targeted therapy[J].
Mol Diagn Ther, 2016, 20(4): 315-33.
DOI: 10.1007/s40291-016-0208-1. |
| [2] |
Hehlmann R, Lauseker M, Saussele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia:10-year survival results of the randomized CML study IV and impact of non-CML determinants[J].
Leukemia, 2017, 31(11): 2398-406.
DOI: 10.1038/leu.2017.253. |
| [3] |
Singh VK, Coumar MS. Chronic myeloid leukemia: existing therapeutic options and strategies to overcome drug resistance[J].
Mini Rev Med Chem, 2019, 19(4): 333-45.
DOI: 10.2174/1389557518666181017124854. |
| [4] |
Branford S, Yeung DT, Parker WT, et al. Prognosis for patients with CML and>10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline[J].
Blood, 2014, 124(4): 511-8.
DOI: 10.1182/blood-2014-03-566323. |
| [5] |
Soverini S, De Benedittis C, Polakova KM, et al. Next-generation sequencing for sensitive detection of BCR-ABL1 mutations relevant to tyrosine kinase inhibitor choice in imatinib-resistant patients[J].
Oncotarget, 2016, 7(16): 21982-90.
|
| [6] |
Qin YZ, Chen SS, Jiang B, et al. Characteristics of BCR-ABL kinase domain point mutations in Chinese imatinib-resistant chronic myeloid leukemia patients[J].
Ann Hematol, 2011, 90(1): 47-52.
DOI: 10.1007/s00277-010-1039-5. |
| [7] |
O'brien S, Berman E, Moore JO, et al. NCCN task force report: tyrosine kinase inhibitor therapy selection in the management of patients with chronic myelogenous leukemia[J].
J Natl Compr Canc Netw, 2011, 9(Suppl 2): S1-25.
|
| [8] |
Marce S, Zamora L, Cabezon M, et al. Frequency of ABL gene mutations in chronic myeloid leukemia patients resistant to imatinib and results of treatment Switch to second-generation tyrosine kinase inhibitors[J].
Med Clin (Barc), 2013, 141(3): 95-9.
DOI: 10.1016/j.medcli.2012.10.028. |
| [9] |
Xu L, Xu Z, Zhang X, et al. Allogeneic stem cell transplantation for patients with T315I BCR-ABL mutated chronic myeloid leukemia[J].
Biol Blood Marrow Transplant, 2016, 51(1): S158-9.
|
| [10] |
Nicolini FE, Mauro MJ, Martinelli GA, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph + acute lymphoblastic leukemia patients with BCR-ABL T315I mutation[J].
Blood, 2009, 114(26): 5271-8.
DOI: 10.1182/blood-2009-04-219410. |
| [11] |
Wang J, Zhang Y, Zu Y, et al. Characteristics and clinical outcome of T315I mutation in Philadelphia chromosome-positive acute lymphoblastic leukemia and chronic myeloid leukemia[J].
Zhonghua Xue ye Xue Za Zhi, 2016, 37(2): 110-4.
|
| [12] |
Musumeci F, Greco C, Grossi GA, et al. Recent studies on ponatinib in cancers other than chronic myeloid leukemia[J].
Cancers (Basel), 2018, 10(11): 36.
|
| [13] |
Fda OK. s iclusig's return to market[J].
Cancer Discov, 2014, 4(2): 138.
|
| [14] |
Wehrle J, von BN. Ponatinib: a Third-generation inhibitor for the treatment of CML[J].
Recent Results Cancer Res, 2018, 212(2): 109-18.
|
| [15] |
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring[J].
Am J Hematol, 2018, 93(3): 442-59.
DOI: 10.1002/ajh.v93.3. |
| [16] |
Foley SB, Hildenbrand ZL, Soyombo AA, et al. Expression of BCR/ ABL p210 from a knockin allele enhances bone marrow engraftment without inducing neoplasia[J].
Cell Rep, 2013, 5(1): 51-60.
DOI: 10.1016/j.celrep.2013.08.037. |
| [17] |
Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet[J].
Blood, 2011, 118(5): 1208-15.
DOI: 10.1182/blood-2010-12-326405. |
| [18] |
Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter[J].
Blood, 2009, 114(27): 5426-35.
DOI: 10.1182/blood-2009-08-215939. |
| [19] |
Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 Cancer therapy caused by BCR-ABL gene mutation or amplification[J].
Science, 2001, 293(5531): 876-80.
DOI: 10.1126/science.1062538. |
| [20] |
Kim H, Kim SH, Kim HJ, et al. Comparison of frequency and sensitivity of BCR-ABL1 kinase domain mutations in Asian and white patients with imatinib-resistant chronice phase chronic myeloid leukemia[J].
Clin Lymphoma Myeloma Leuk, 2018, 18(10): e391-9.
DOI: 10.1016/j.clml.2018.06.031. |
| [21] |
Breccia M, Efficace F, Iurlo A, et al. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: the possible role of ponatinib[J].
Expert Opin Drug Saf, 2018, 17(6): 623-8.
DOI: 10.1080/14740338.2018.1480719. |
| [22] |
Mueller MC, Cervantes F, Hjorth-Hansen H, et al. Ponatinib in chronic myeloid leukemia (CML): Consensus on patient treatment and management from a European expert panel[J].
Crit Rev Oncol Hematol, 2017, 120(2): 52-9.
|
| [23] |
Pavlovsky C, Chan O, Talati CA. Ponatinib in the treatment of chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia[J].
Future Oncol, 2019, 15(3): 257-69.
DOI: 10.2217/fon-2018-0371. |
| [24] |
Poch MM, Sibai H, Deotare U, et al. Ponatinib in the therapy of chronic myeloid leukemia[J].
Expert Rev Hematol, 2016, 9(10): 923-32.
DOI: 10.1080/17474086.2016.1232163. |
| [25] |
Tadokoro K, Ishikawa M, Suzuki M, et al. Comparative quantitative analysis of BCR-ABL transcripts with the T315I mutant clone by polymerase chain reaction (PCR)-Invader method[J].
Transl Res, 2011, 158(3): 169-79.
DOI: 10.1016/j.trsl.2011.02.005. |
| [26] |
Huang WS, Metcalf CA, Sundaramoorthi R, et al. Discovery of 3-[2- (imidazo[1, 2-b] pyridazin-3-yl) ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-y l) methyl]-3-(trifluoromethyl) phenyl} benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant[J].
J Med Chem, 2010, 53(12): 4701-19.
DOI: 10.1021/jm100395q. |
| [27] |
Zhou TJ, Commodore L, Huang WS, et al. Structural mechanism of the Pan-BCR-ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance[J].
Chem Biol Drug Des, 2011, 77(1): 1-11.
|
| [28] |
O'hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance[J].
Cancer Cell, 2009, 16(5): 401-12.
DOI: 10.1016/j.ccr.2009.09.028. |
| [29] |
Defilip Z, Langston AA, Chen ZJ, et al. Does Post-transplant maintenance therapy with tyrosine kinase inhibitors. improve outcomes of patients with high-risk philadelphia chromosomepositive leukemia[J].
Clin Lymphoma Myeloma Leuk, 2016, 16(8): 466-71.
DOI: 10.1016/j.clml.2016.04.017. |
| [30] |
Laramy JK, Kim M, Parrish KE, et al. Pharmacokinetic assessment of cooperative efflux of the multitargeted kinase inhibitor ponatinib across the blood-brain barrier[J].
J Pharmacol Exp Ther, 2018, 365(2): 249-61.
DOI: 10.1124/jpet.117.246116. |
| [31] |
Kort A, van HS, Sparidans RW, et al. Brain accumulation of ponatinib and its active metabolite, N-Desmethyl ponatinib, is limited by P-Glycoprotein(P-GP/ABCB1)and breast Cancer resistance protein(BCRP/ABCG2)[J].
Mol Pharm, 2017, 14(10): 3258-68.
DOI: 10.1021/acs.molpharmaceut.7b00257. |
| [32] |
Petrungaro A, Gentile M, Mazzone C, et al. Ponatinib-Induced Graftversus-host disease/graft-versus-leukemia effect in a patient with philadelphia-positive acute lymphoblastic leukemia without the T315I mutation relapsing after allogeneic transplant[J].
Chemotherapy, 2017, 62(6): 353-6.
DOI: 10.1159/000477714. |
| [33] |
Daniela R, Marchesi F, De Angelis GA, et al. Ponatinib induces a persistent molecular response and graft-versus-host disease/graftversus-leukemia effect in a patient with Philadelphia-positive acute lymphoblastic leukemia with a T315I mutation following early relapse after allogeneic transplant[J].
Chemotherapy, 2017, 62(1): 58-61.
DOI: 10.1159/000448750. |
| [34] |
Cortes JE, Kim DW, Pinilla- Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial[J].
Blood, 2018, 132(4): 393-404.
DOI: 10.1182/blood-2016-09-739086. |
| [35] |
Hoy SM. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lympho-blastic leukaemia[J].
Drugs, 2014, 74(7): 793-806.
DOI: 10.1007/s40265-014-0216-6. |
 2019, Vol. 39
2019, Vol. 39

