肺癌是世界上癌症死亡的首要原因, 其中非小细胞肺癌约占85%[1]。在目前精准医疗的大环境下, 寻找有效的分子标志物及对其相应分子机制的研究有重要价值。tristetraprolin通过与mRNA 3'非翻译区上的AU富集元件结合, 诱导靶基因mRNA降解, 直接参与肿瘤相关基因的转录[2], 同时也可调控转录因子从而影响肿瘤细胞发生、发展[3-5]。tristetraprolin在多种肿瘤中低表达, 与患者疾病进展、生存时间呈负相关[6]。tristetraprolin显著抑制肿瘤细胞增殖, 其主要机制包括对细胞周期调控关键基因的转录调控, 如p21[7], cyclin D1[8], E2F1[9], LATS2[10]等, 引起细胞周期阻滞; 通过调控凋亡相关基因或通过其他分子机制诱导细胞凋亡[11-12]; 通过调控自噬相关基因诱导或抑制细胞自噬[13-16]。
目前对tristetraprolin与自噬相关性研究较少, 对二者相互关系的研究存在争议。tristetraprolin可诱导自噬, 促进肿瘤细胞增殖, 同时, tristetraprolin也可通过下调自噬相关基因, 抑制肿瘤细胞生长; 前期研究发现, tristetraprolin可能通过抑制自噬从而抑制肺癌细胞增殖, 导致细胞死亡[17], 但未对其可能的分子机制或信号通路深入研究。本文在前期研究基础上进一步探索其可能通过NF-κB发挥作用的具体分子机制, 首次在肺癌中阐明tristetraprolin可能通过NF-kappaB信号通路调控自噬的发生, 进而影响肿瘤细胞的命运, 是对tristetraprolin抑癌机制的重要探索。
1 材料和方法 1.1 实验材料人胚肺细胞系MRC-5、肺腺癌细胞系NCI-H1975及H1299 (中国科学院上海细胞库提供); 总RNA抽提试剂盒、第一链cDNA合成试剂盒、PCR试剂盒及PCR引物(Takara); tristetraprolin过表达质粒由美国圣路易斯医学院刘建国教授赠送; jetPrime DNA transfection reagent及人TNF-α生长因子(Polyplus-transfection); 质粒提取试剂盒(Omega Bio-tek); DAPI显色液、BCA蛋白定量试剂盒、RIPA裂解液、细胞核蛋白提取试剂盒(碧云天); 大肠杆菌DH5α感受态细胞(Takara); 兔抗人tristetraprolin抗体(Sigma-Aldrich)、兔抗人NF-κB p65抗体、兔抗人NF-κB p50抗体、兔抗人NF-κB c-rel抗体、兔抗人LC3Ⅱ/LC3Ⅰ抗体、兔抗人p62抗体(CST); 兔抗人Beclin1抗体、兔抗人GAPDH抗(Proteintech); 兔抗人LaminB抗体(万类生物); 鼠抗人tristetraprolin抗体、鼠抗人β-actin (Santa Cruz); 鼠抗人荧光二抗及兔抗人荧光二抗(中杉金桥生物); ECL化学发光液购(Millipore); Marker(Fermentas); RPMI 1640培养液(Hyclone); LB液体培养基、LB营养琼脂(索莱宝); 优等胎牛血清(PAN Biotech); PVDF膜(Millipore), 其余试剂均为分析纯。
1.2 实验方法 1.2.1 细胞培养人胚肺细胞系MRC-5、肺腺癌细胞系NCI-H1975细胞及H1299细胞均生长于含10%胎牛血清及1%青霉素/链霉素的RPMI 1640培养基, 培养于37℃、5%CO2体积分数的恒温无菌培养箱, 0.25%的胰蛋白酶传代, 取对数期细胞进行试验。
1.2.2 基因过表达将tristetraprolin过表达及IKBα-mut质粒转化后进行测序, 测序成功后进行单克隆扩增, 按照质粒提取试剂盒说明书提取DNA并测浓度、将对数生长期细胞按照质粒转染试剂说明书转染tristetraprolin及IκBα-mut过表达质粒, 同时转入相应空载作为对照组, 转染后5 h进行换液, 每隔24 h提取蛋白、RNA验证相关目的基因表达及进行下一步实验, 人TNF-α生长因子于提取蛋白及RNA前2 h加入细胞培养瓶。
1.2.3 Real-time PCR检测RNA水平按照Takara总RNA提取试剂盒提取纯化RNA并测RNA浓度, 用Takara逆转录试剂盒将RNA转为cDNA, 经过染色后进行荧光实时定量PCR反应。PCR反应条件为: 95℃预变性30 s, PCR反应(95℃5 s, 60℃30 s, 40个循环)。tristetraprolin上游引物: 5'-GGAGTGTCTTCCGAGG TTCTTG-3', 下游引物: 5'-AACGGCTTTGGCTACTT GCTT-3'; Beclin1上游引物: 5'-TACCACAGCCCAGG CGAAAC-3', 下游引物: 5'-CCAGTGACCTTCAGTC TTCGGC-3; P62上游引物: 5'-TTCCAGCACAGAGG AGAAGAGC-3', 下游引物: 5'-GATTCTGGCATCTGT AGGGACTG-3';GAPDH上游引物: 5'-TGAAGGTC GGAGTCAACGGAT-3', 下游引物: 5'-CCTGGAAGA TGGTGATGGGAT-3'。目的基因表达量以GAPDH作为参照, 采用相对定量法, 以2-△△Ct表示实验组目的基因mRNA的相对表达量。每组设置3个副孔, 实验重复3次。
1.2.4 Western blot检测蛋白水平将各组细胞按照RIPA裂解液及细胞核提取试剂盒说明书于冰上裂解后提取各细胞总蛋白、核蛋白及细胞浆蛋白。用BCA法测定蛋白浓度, 蛋白样品与5x蛋白上样缓冲液按照4: 1混合, 100℃加热10 min, 取一部分进行试验, 其余放置- 80℃冰箱保存。分别取30 μg总蛋白、60 μg核蛋白及细胞浆蛋白样品上样, 后于10%及12%SDS-PAGE胶中进行电泳分离, 电泳后将蛋白转移至PVDF膜上, 快速封闭液封闭13 min, 于TBST中洗2 min, 加入相应特异性一抗, 于4℃房间孵育过夜(约12 h), 于TBST洗3次, 10 min/次, 加入相对应二抗, 于37℃中孵育1 h, 于TBST中洗30 min后进行ECL发光显影。采用Quantity one 4.6.2软件对条带进行分析, 总蛋白以GAPDH为内参, 胞浆蛋白以β-actin为内参, 核蛋白以LaminB为内参, 以目的蛋白与内参蛋白之比表示蛋白的相对表达水平, 实验重复3次。
1.2.5 细胞免疫荧光检测核移位将对数期生长细胞接种于铺好无菌载玻片的6孔板中, 继续培养40 h, 按照说明书加入转染试剂及tristetraprolin过表达质粒, 5 h后换液, 继续培养48 h。4%多聚甲醛固定10 min, PBS洗3次, 5 min/次, 加入0.1%TritonX-100破膜10 min, PBS洗3次, 5 min/次。山羊血清封闭, 37℃恒温箱中敷育1 h, 吸去多余血清, 加入anti-NF-κB p65、p50、c-rel及anti-tristetraprolin抗体4℃孵育过夜, PBS洗3次, 分别用FTTC及PE荧光二抗避光孵育1 h, PBS洗3次, DAPI染细胞核5 min, PBS洗3次, 用抗荧光淬灭剂封片后于显微共聚焦镜下拍照。每组样品为2, 实验重复3次。
1.3 统计学分析所有资料采用SPSS 17.0和GraphPad Prism 6软件统计并进行处理, 数据以均数±标准差表示, 多样本均数比较用单因素方差分析, 两独立样本间比较采用t检验, P < 0.05为差异有统计学意义。
2 结果 2.1 tristetraprolin在肺腺癌细胞中低表达采用Western blot及RT-qPCR检测人胚肺细胞系MRC-5、肺腺癌细胞系NCI-H1975细胞及H1299细胞表达水平。结果显示, tristetraprolin蛋白水平在肺腺癌H1975及H1299中的表达低(图 1A), 几乎检测不出其表达量, 同样在mRNA水平, 其表达量也极低(图 1B)。
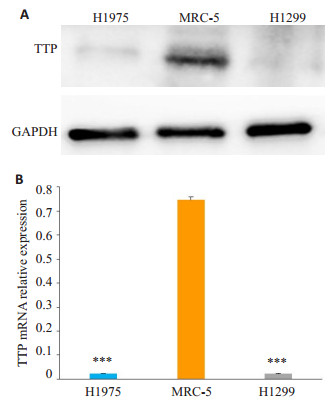
|
图 1 Tristetraprolin在肺腺癌细胞H1975及H1299中低表达 Fig.1 Low expression of tristetraprolin in lung adenocarcinoma cell lines H1975 and H1299. A: Protein expression of tristetraprolin in lung adenocarcinoma cells detected by Western blotting; B: mRNA expression level of tristetraprolin in lung adenocarcinoma cells detected by RT-qPCR. ***P < 0.001 vs MRC- 5 cells (a normal lung epithelial cell line). |
将肺腺癌细胞系H1975细胞及H1299细胞瞬时转染tristetraprolin后检测自噬相关基因Beclin1、LC3Ⅱ/ LC3Ⅰ及P62在mRNA及蛋白水平表达变化。结果显示, 在肺腺癌H1975细胞中, 转染48 h后tristetraprolin mRNA及蛋白表达水平最高(图 2A、B、F、H), 同时发现Beclin1表达量随tristetraprolin增高而降低, 与空载组相比较, 其在mRNA及蛋白水平表达均明显降低, 差异具有统计学意义(图 2A、C、D、F、I、J, P < 0.05), 同样, LC3 Ⅱ/LC3Ⅰ比值明显降低, 差异具有统计学意义(图 2A、E、F、K, P < 0.01)。p62在H975及H1299肺腺癌细胞中表达变化不稳定, 差异没有统计学意义(图 2A、F)。
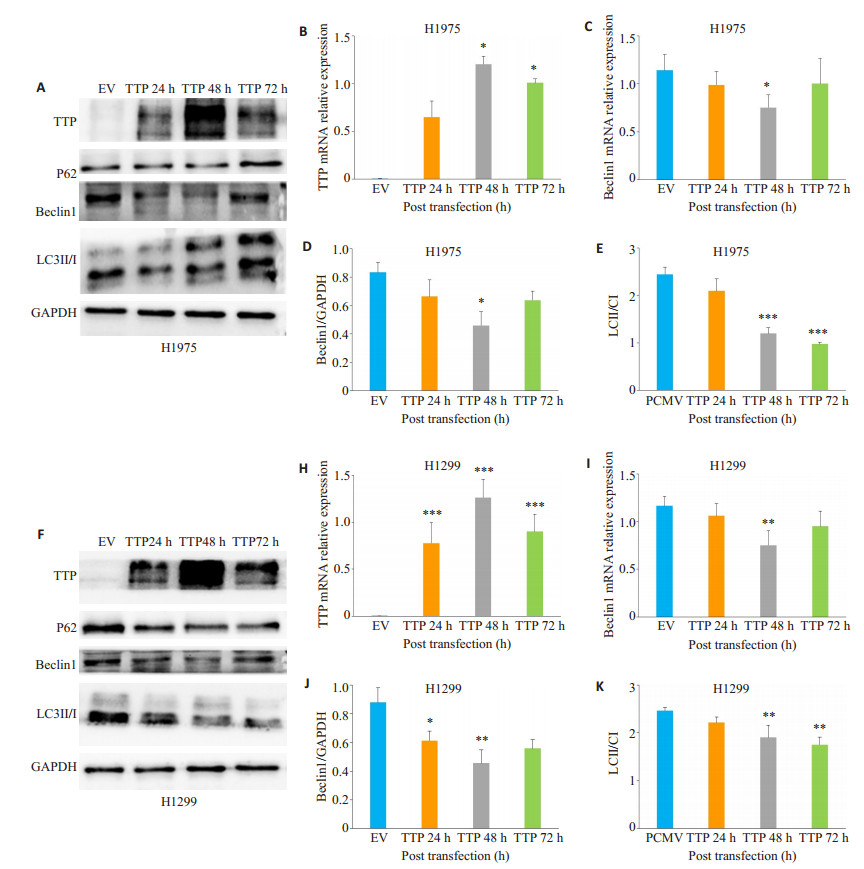
|
图 2 Tristetraprolin在肺腺癌H1975及H1299细胞中对自噬相关基因表达影响 Fig.2 Effect of tristetraprolin overexpression on the expression of autophagy-related genes in lung adenocarcinoma H1975 and H1299 cells. The relative expressions of tristetraprolin and autophagy-related mRNA in H1975 and H1299 cells (B, C, H, I) were analyzed by RT-qPCR. Western blotting was used to detect the expressions of autophagy-related proteins (A, D, E, F, J, k). The samples were detected every 24 h until 3 days after transfection. EV: H1299 and H1975 cells transfected with the empty vector; tristetraprolin: H1299 and H1975 cells transfected with tristetraprolin-overexpressing plasmid. *P < 0.05, **P < 0.01, ***P < 0.001 vs EV. |
将肺腺癌系H1975细胞瞬时转染tristetraprolin过表达质粒, 转染40 h后用人TNF-α处理细胞2 h, 采用Western blot和细胞免疫荧光检测细胞核中NF-κB p65, p50, c-rel蛋白表达水平。在细胞核中可见少量细胞浆蛋白β-actin表达, 细胞浆中无细胞核蛋白LaminB表达, 细胞浆及细胞核蛋白分离成功(图 3B)。过表达tristetraprolin后细胞核中NF-κB p65, c-rel表达量与空载组相比减少, 差异具有统计学意义(图 3B、C、D, P < 0.001), 但p50在细胞核及细胞浆中无明显变化(图 3B)。同样, 细胞免疫荧光显示, 过表达tristetraprolin组较空载组细胞核中NF-κB p65, c-rel表达减少(图 3A), 而p50无明显变化。
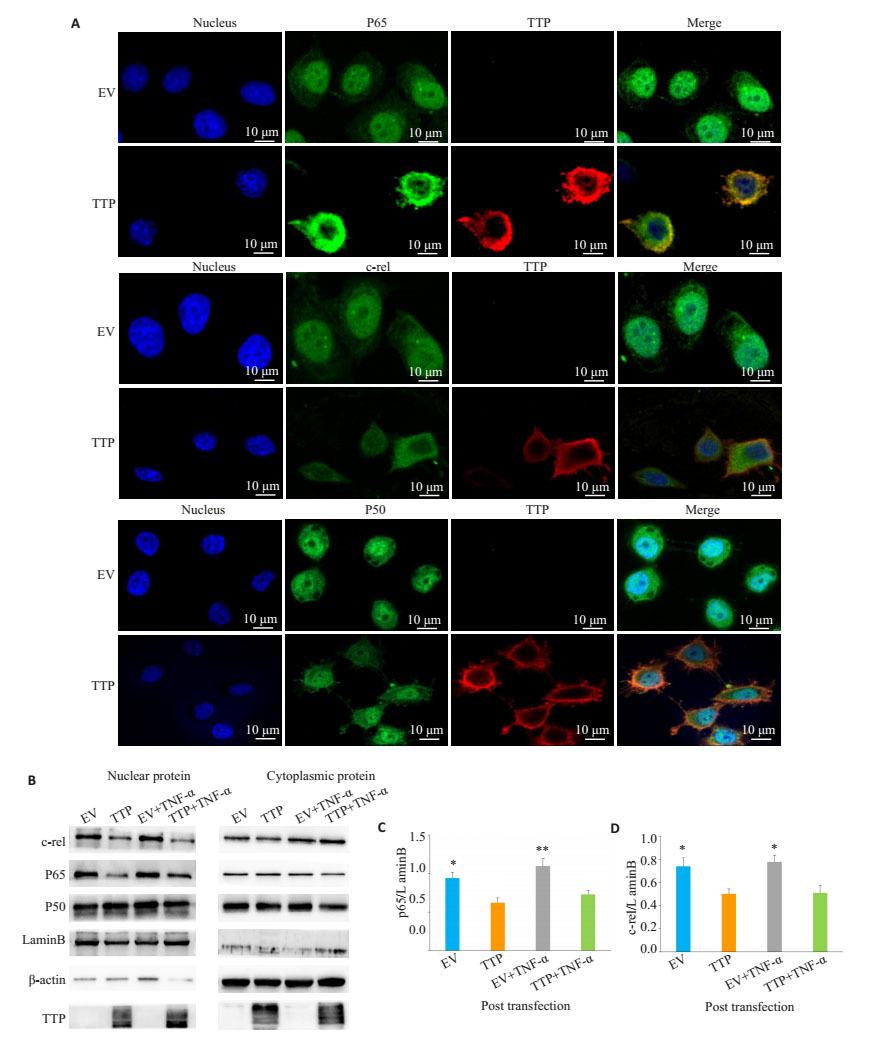
|
图 3 Tristetraprolin抑制肺腺癌H1975细胞中NF-κB p-65, c-rel核移位 Fig.3 Tristetraprolin blocks NF-кB nuclear translocation. A: H1975 cells were transiently transfected with tristetraprolin or control vector, and 40 h later, the transfected cells were treated with TNF-α (10 ng/mL) for 2 h, followed by immunostaining with anti-tristetraprolin Ab (red) and anti-NF-кB p65 Ab (green), or anti-NF-кB c-Rel Ab (green), or anti-NF-кB p50 (green) Ab. DAPI labeling was used to identify the cell nuclei (blue); B-D: Western blot analysis of the endogenous p65, c-rel, and p50 protein in cytoplasmic and nuclear extracts of tristetraprolin-overexpressing H1975 cells stimulated with TNF-α (10 ng/mL) for 2 h. β-actin was used as the cytoplasmic protein loading control. Lamin B was used as the nuclear protein loading control. EV: H1975 cells transfected with empty plasmid; tristetraprolin: H1975 cells transfected with tristetraprolin-overexpressing plasmid. *P < 0.05, **P < 0.01 vs EV. |
为进一步验证tristetraprolin通过NF-κB信号通路影响肺腺癌细胞自噬, 在H1975肺腺癌细胞中瞬时转染IκBα-mut质粒抑制NF-κB信号通路, 然后再转染tristetraprolin过表达质粒, 观察抑制NF-κB通路后, tristetraprolin+ IκBα-mut组和tristetraprolin组中自噬相关基因表达差异。结果显示, tristetraprolin+IκBα-mut组中, 自噬相关基因Beclin1在mRNA及蛋白水平较单过表达tristetraprolin组明显增加(图 4A、C、D, P < 0.05), LC3Ⅱ表达也增多, 差异具有统计学意义(图 4A、E, P < 0.05)。结果显示, 抑制NF-κB信号通路后, 肺腺癌细胞中tristetraprolin对自噬基因Beclin1及LC3Ⅱ抑制作用减弱。
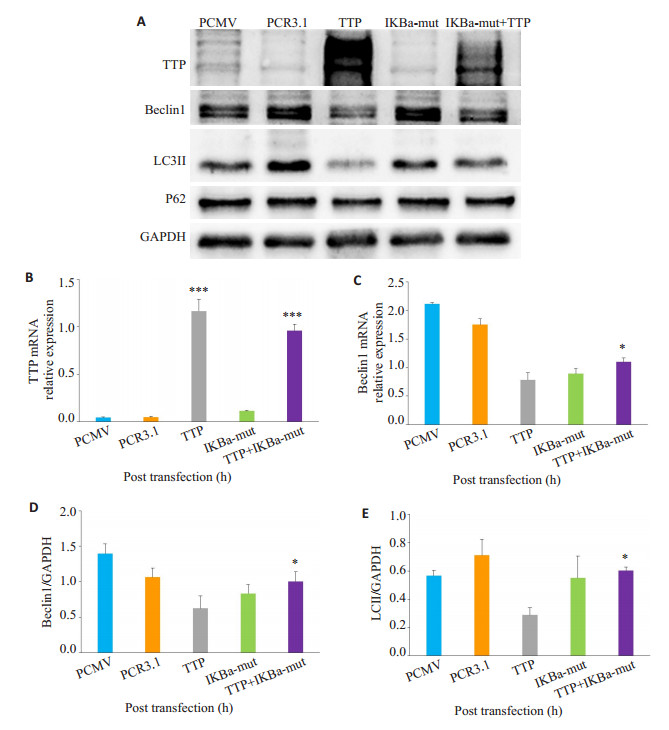
|
图 4 抑制NF-κB信号通路后, tristetraprolin对肺腺癌细胞自噬相关基因表达影响 Fig.4 Tristetraprolin overexpression inhibits autophagy in lung H1975 cancer cells through NF-κB signal pathway. H1975 cells were co-transfected with tristetraprolin and IκBα-mut plasmid for 48 h, and the mRNA and protein expressions of Beclin1 were measured by RT-qPCR and Western blotting. A, D, E: The expression of tristetraprolin and autophagy-related protein in lung H1975 cancer cells by Western blotting; B, C: The mRNA expression level of tristetraprolin and Beclin1 in lung H1975 cancer cells by RT-qPCR. PCMV: H1975 cells transfected with tristetraprolin-empty vector; PCR3.1: H1975 cells transfected with IκBα-mut-empty vector; tristetraprolin: H1975 cells transfected with tristetraprolin-overexpressing plasmid. IκBα-mut: H1975 cells transfected with IκBα-mut plasmid. *P < 0.05, **P < 0.01, ***P < 0.001 vs tristetraprolin. |
本研究在前期实验基础上进一步探究在肺腺癌细胞中tristetraprolin与自噬关系及具体分子机制, 结果显示, tristetraprolin在肺腺癌中低表达, tristetraprolin抑制肺腺癌自噬相关基因Beclin1及LCⅡ/LCⅠ的表达, 同时tristetraprolin抑制肺腺癌中NF-κB p65, c-rel核移位, 阻断NF-κB信号通路后, tristetraprolin对自噬基因Beclin1及LC3Ⅱ的抑制作用减弱, 提示在肺腺癌细胞中, tristetraprolin可能通过NF-κB信号通路抑制自噬。tristetraprolin在多种肿瘤组织中低表达, 与恶性肿瘤的发生、发展密切相关[18]。肺癌患者中, tristetraprolin低表达患者预后较差, 生存率更低, 同时与肿瘤的基因突变频率相关[19]。同样, 本研究发现与正常肺上皮细胞MRC-5比较, 肺腺癌H1975及H1299中tristetraprolin表达降低, 这表明可能仅仅tristetraprolin的低表达就是肺腺癌发生的关键驱动力, 对其作用的具体机制的研究具有重要意义。
自噬与肿瘤的关系受到广泛关注, 自噬相关分子表达及自噬相关通路与肺癌的预后治疗相关, 但自噬对肺癌患者的预后治疗否有益存在争议[20-22]。目前tristetraprolin与自噬的关系研究较少, Bourcier等[13]发现tristetraprolin过表达能抑制黑色素瘤细胞的自噬。相反, 有研究发现tristetraprolin过表达能诱导细胞自噬[14-15]。María[16]发现tristetraprolin抑制细胞发生自噬性死亡, 其具体机制不明。本研究发现肺腺癌细胞过表达tristetraprolin后, 细胞中Beclin1在mRNA及蛋白表达水平降低, LC3 Ⅱ/LC3 Ⅰ比值降低, 同时, 当tristetraprolin表达减少时, Beclin1的表达增加。这说明在肺腺癌中, tristetraprolin可能通过调控Beclin1影响细胞自噬水平。Beclin1是自噬启动的关键基因, 在胃癌、肺癌及宫颈癌等肿瘤中表达降低, 同时在肺癌中发现Beclin1升高导致的自噬活性增强对肿瘤发展及耐药相关, 说明在肺癌的不同时期, Beclin1介导的自噬活性调控对肿瘤的发生发展具有不同的作用[23-25], 本研究发现在肺腺癌中tristetraprolin可能通过抑制Beclin1表达从而发挥抑癌作用, 而tristetraprolin对自噬基因Beclin1表达的直接调控机制还需进一步的证实。
NF-kappaB是重要的转录因子, tristetraprolin能抑制NF-kapaB p65亚基核移位从而抑制炎症相关因子及c-jun表达[4-6, 26]。同样, 我们发现肺腺癌中过表达tristetraprolin组和空载组比较, 细胞核中NF-κB p65, c-rel在蛋白水平表达降低, 但p50在细胞核及细胞质中表达无明显差异。细胞免疫荧光显示在tristetraprolin过表达组, 细胞核内NF-κB p65, c-rel减少, p50无明显差异, 这表明在肺腺癌细胞中tristetraprolin能抑制NF-κB p65, c-rel核移位, 对p50无明显影响。NF-κB对自噬的调控因细胞背景不同而不同, 具有细胞特异性[27], 为研究tristetraprolin是否通过NF-κB信号通路调控肺腺癌细胞自噬, 我们在H1975肺腺癌细胞中转染IκBα-mut质粒抑制NF-κB信号通路, 结果显示, 抑制NF-κB信号通路后, tristetraprolin对Beclin1及LC3Ⅱ抑制作用消除。这说明tristetraprolin可能通过NF-κB信号通路调控自噬相关基因, 抑制肺腺癌细胞自噬, 从而调控肿瘤的发生发展。
p62也是自噬标志物, 自噬诱导时, p62表达降低, 当自噬抑制时, p62表达升高[28]。本研究发现, 过表达tristetraprolin抑制自噬后p62在蛋白表达水平不受影响。这可能是由于p62作为自噬底物降解的标记物时, 其表达变化具有滞后性, 同时p62也参与了其他多种细胞活动, 这些途径的变化也会导致p62水平发生变化[29]。同时也说明tristetraprolin可能不直接调控p62的表达, 需对其相关机制做进一步研究。
综上所述, 本研究揭示了tristetraprolin可能通过NF-κB信号通路抑制肺腺癌细胞自噬, 进一步抑制肺腺癌发生发展, 深化了tristetraprolin的抑癌机制研究, 同时为tristetraprolin可能作为肺腺癌治疗的靶点提供理论依据。
| [1] |
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics[J].
CA Cancer J Clin, 2012, 65(2): 87-108.
|
| [2] |
Guo J, Wang H, Jiang SY, et al. The cross-talk between tristetraprolin and cytokines in cancer[J].
Anticanc Agents Med Chem, 2017, 17(11): 1477-86.
|
| [3] |
Gu L, Ning H, Qian XE, et al. Suppression of IL-12 production by tristetraprolin through blocking NF-kappa B nuclear translocation[J].
J Immunol, 2013, 191(7): 3922-30.
DOI: 10.4049/jimmunol.1300126. |
| [4] |
Schichl YM, Resch U, Martin R. Tristetraprolin impairs NF-kappaB/p65 nuclear translocation[J].
J Biol Chem, 2009, 284(43): 29571-81.
DOI: 10.1074/jbc.M109.031237. |
| [5] |
Liang J, Lei TH, Song YT, et al. RNA-destabilizing factor tristetraprolin negatively regulates NF-kappa B signaling[J].
Biol Chem, 2009, 284(43): 29383-90.
DOI: 10.1074/jbc.M109.024745. |
| [6] |
Park JM, Lee TH, Kang TH. Roles of tristetraprolin in tumorigenesis[J].
Int J Mol Sci, 2018, 19(11): 3384-95.
DOI: 10.3390/ijms19113384. |
| [7] |
Al-Haj A, L, Blackshear PJ, et al. Regulation of p21/CIP1/WAF-1 mediated cell-cyclearrest by RNase L and tristetraprolin, and involvement of AU-rich elements[J].
Nucleic Acids Res, 2012, 40(16): 7739-52.
DOI: 10.1093/nar/gks545. |
| [8] |
Marderosian M, Sharma A, Funk AP, et al. Ttristetraprolin regulates cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling[J].
Oncogene, 2006, 25(47): 6277-90.
DOI: 10.1038/sj.onc.1209645. |
| [9] |
Lee HH, Lee SR, Leem SH. Tristetraprolin regulates prostate cancer cell growth throughsuppression of E2F1[J].
Microbiol Biotechnol, 2014, 24(2): 287-94.
|
| [10] |
Lee HH, Vo MT, Kim HJ, et al. Stability of the LATS2 tumor suppressor gene is regulated by tristetraprolin[J].
J Biol Chem, 2010, 285(23): 17329-37.
DOI: 10.1074/jbc.M109.094235. |
| [11] |
Wang H, Ding NN, Guo J, et al. Dysregulation of TTP and HuR plays an important role in cancers[J].
Tumour Biol, 2016, 37(11): 14451-61.
DOI: 10.1007/s13277-016-5397-z. |
| [12] |
Hai DD, Koch A, Allister A, et al. Treatment with MAPKAP2 (Mk2) inhibitor and DNA methylation inhibitor, 5-aza dC, synergistically triggers apoptosis in hepatocellular carcinoma (HCC) via tristetraprolin (TTP)[J].
Cell Signal, 2016, 28(12): 1872-80.
DOI: 10.1016/j.cellsig.2016.09.002. |
| [13] |
Bourcier C, Griseri P, Grépin R, et al. Constitutive ERK activity induces downregulation of tristetraprolin, a major protein controlling interleukin8/CXCL8 mRNA stability in melanoma cells[J].
Am J Physiol Cell Physiol, 2011, 301(3): C609-18.
DOI: 10.1152/ajpcell.00506.2010. |
| [14] |
Suswam EA, Shacka JJ, Walker K, et al. Mutant tristetraprolin:a potent inhibitor of malignant glioma cell growth[J].
Neuro Oncol, 2013, 113(2): 195-205.
DOI: 10.1007/s11060-013-1112-8. |
| [15] |
Tilija PN, Park PH. Adiponectin inhibits inflammatory cytokines production by Beclin-1 phosphorylation and Bcl-2 mRNA destabilization:role for autophagy induction[J].
Br J Pharmaco, 2018, 175(7): 1066-84.
DOI: 10.1111/bph.v175.7. |
| [16] |
María VG, Albana G, Johanna M. Expression of the mRNA stability regulator Tristetraprolin is required for lactation maintenance in the mouse mammary gland[J].
Oncotarget, 2018, 9(9): 8278-89.
|
| [17] |
Dong F, Li C, Xu L. The RNA binding protein tristetraprolin downregulates autophagy in lung adenocarcinoma cells[J].
Exp Cell Res, 2018, 367(1): 89-96.
DOI: 10.1016/j.yexcr.2018.03.028. |
| [18] |
Hitti E, Bakheet T, Al-Souhibani N, et al. Systematic analysis of AURich element expression in cancer reveals common functional clusters regulated by key RNA-Binding proteins[J].
Cancer Res, 2016, 76(14): 4068-80.
DOI: 10.1158/0008-5472.CAN-15-3110. |
| [19] |
Mohammad F, Antonio LA, John LC. CREB targets define the gene expression signature of malignancies having reduced levels of the tumor suppressor tristetraprolin[J].
PLoS One, 2014, 9(12): e115517-26.
DOI: 10.1371/journal.pone.0115517. |
| [20] |
Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer[J].
Oncogene, 2016, 35(1): 1-11.
DOI: 10.1038/onc.2015.99. |
| [21] |
Liu GB, Pei F, Yang FQ, et al. Role of autophagy and apoptosis in non-small-cell lung cancer[J].
Int J Mol Sci, 2017, 18(2): E367-75.
DOI: 10.3390/ijms18020367. |
| [22] |
Karsli UG, Jy G, Price S, et al. Autophagy is required for glucose homeostasis and lung tumor maintenance[J].
Cancer Discov, 2014, 4(8): 914-27.
DOI: 10.1158/2159-8290.CD-14-0363. |
| [23] |
Lee SJ, Kim HP, Jin Y, et al. Beclin1 deficiency is associat-ed with increased hypoxia-induced angiogenesis[J].
Autophagy, 2011, 7(8): 829-39.
DOI: 10.4161/auto.7.8.15598. |
| [24] |
Wang XF, Du ZY, Li LY, et al. Beclin1 and p62 expression in nonsmall cell lung cancer:relation with malignant behaviors and clinical outcome[J].
Int J Clin Exp Pathol, 2015, 8(9): 10644-52.
|
| [25] |
Wu SF, Su J, Qian H, et al. SLC27A4 regulate ATG4B activity and control reactions to chemotherapeutics-induced autophagy in human lung cancer cells[J].
Tumour Biol, 2016, 37(5): 6943-52.
DOI: 10.1007/s13277-015-4587-4. |
| [26] |
Xu L, Ning H, Gu L, et al. Tristetraprolin induces cell cycle arrest in breast tumor cells through targeting AP-1/c-Jun and NF-kappa B pathway[J].
Oncotarget, 2015, 6(39): 41679-91.
|
| [27] |
Salminen A, Hyttinen JM, Kauppinen A, et al. Context-dependent regulation of autophagy by IKK-NF-κB signaling:impact on the aging process[J].
Int J Cell Biol, 2012, 20(18): 849541-53.
|
| [28] |
Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy[J].
J Biol Chem, 2007, 282(33): 24131-45.
DOI: 10.1074/jbc.M702824200. |
| [29] |
Seibenhener ML, Geetha T, Wooten MW. Sequestosome 1/p62-more than just a scaffold[J].
FEBS Lett, 2007, 581(2): 175-9.
DOI: 10.1016/j.febslet.2006.12.027. |
 2019, Vol. 39
2019, Vol. 39

