索拉菲尼是目前用于晚期肝癌治疗相对有效的多靶点药物[1],但临床研究结果显示,患者通常在使用索拉菲尼治疗6月左右会发生耐药,导致索拉菲尼对肝癌的疗效不佳[2]。因此,深入研究肝癌对索拉菲尼耐药的机制对其临床治疗具有重要的现实意义。有文献报道,自噬在肿瘤的进展和耐药中扮演重要的角色[2-3],有研究发现自噬可能是导致索拉菲尼治疗肝癌发生耐药和疗效减弱的重要机制[4-7]。但目前对索拉菲尼如何诱导肝癌细胞发生自噬仍不清楚。巨噬细胞是肿瘤免疫微环境中的重要成员[8],M2型肿瘤相关巨噬细胞(TAMs)在肝癌中的表达显著增加,与肝癌恶性程度、预后、治疗耐药有关[9-11]。本研究通过体外诱导培养M2-TAMs,建立M2- TAMs与肝癌细胞共培养体系,探究M2-TAMs在诱导肝癌细胞自噬促进索拉菲尼耐药中的影响,以期为临床治疗索拉菲尼耐药提供更多的实验依据和治疗思路。
1 材料和方法 1.1 材料THP-1细胞、SMMC-7721肝癌细胞(中科院上海细胞库);DMEM高糖培养基(HyClone);胰蛋白酶、胎牛血清、CCK8细胞增殖检测试剂盒(上海碧云天公司);索拉菲尼(Selleck),PMA(Sigma),IL-4、IL-13(美国PeproTech),凋亡周期试剂盒(上海吉凯公司);CD163,LC3B、p62、Bcl-2、Bax、β-actin单克隆抗体(美国CST)。
1.2 方法 1.2.1 细胞培养THP-1细胞培养于含有10%胎牛血清和0.05 mmol/L β巯基乙醇的RPMI 1640培养基中,置于37 ℃、5% CO2培养箱。当细胞密度达到约8×105 cells/mL左右时,直接将细胞吸出,离心之后传代,传代浓度不少于1×105 cells/mL。SMMC-7721细胞用含10%胎牛血清DMEM培养液培养,当细胞生长至融合度约70%~80%时,加0.25%的胰酶对细胞进行1: 2传代培养。
1.2.2 M2-TAM细胞诱导及M2细胞条件培养液(M2-CM)的收集[12]将THP-1细胞按照1×106 cells/mL的密度接种在六孔板内,加入100 ng/mL的PMA作用6 h,而后加入20 ng/mL IL-4和20 ng/mL IL-13再作用18 h,去除刺激剂后,换新鲜无血清培养液2 mL培养24 h收集上清、冻存于-80 ℃备用。采用镜下形态和免疫荧光检测CD163的表达进行鉴定。
1.2.3 CCK-8法检测细胞增殖抑制用含5%胎牛血清的培养液制备细胞悬液(5×104 cell/mL),取90 μL加至96孔培养板,索拉菲尼组浓度分别有5个终浓度:1.25、2.5、5、10、20 μmol/L,10 μL,每组设6个复孔在37 ℃,5% CO2培养箱中培养24、48、72 h,终止培养前,每孔加入CCK-8 10 μL,继续培养2~4 h后测吸光度A490 nm。肿瘤细胞存活率(%)=(处理组A490 nm-空白对照组A490 nm)/(阴性对照组A490 nm-空白对照组A490 nm)×100%,通过软件计算IC50值,实验重复3次。
1.2.4 流式细胞仪检测细胞凋亡将细胞接种于6孔板,隔夜后待细胞完全贴壁后,加入索拉菲尼(2.5 μmol/L)和/或M2-CM继续培养24 h后,用冷PBS冲洗2遍,收集冲洗液至相应离心管中做好标记,每孔加入胰酶消化,收集消化后的细胞,1000 r/min,4 ℃离心5 min收集处理后的各组细胞,用PBS洗涤细胞2次1000 r/min,4 ℃离心5 min,加入100 μL的Binding Buffer重悬细胞;每管加入5 μL Annexin V-FITC、5 μL Propidium Iodide,轻轻混匀;避光、室温反应10 min;加入400 μL的Binding Buffer在1 h内,用流式细胞仪检测。
1.2.5 Western blot法检测LC3B、p62、Bcl-2、Bax等蛋白的表达收集各组处理后细胞,用4 ℃预冷的PBS洗涤3次,加入80 μL细胞裂解液(含相应蛋白酶、磷酸酶抑制剂),冰上裂解30 min,提取细胞总蛋白,BCA法测蛋白浓度。15%SDS-PAGE聚丙烯酰胺凝胶电泳分离蛋白,置于冰上220 mA恒流转移60 min转至PVDF上,用5%脱脂牛奶的封闭液4 ℃封闭过夜。洗膜后,加入相应一抗抗体(1: 1000稀释)过夜,辣根过氧化物酶标记的二抗(1: 2000稀释)反应2 h。按照ECL显影试剂盒说明,将化学发光增强液A和B两种试剂1:1混匀涂抹于PVDF膜上。用Bio-Rad凝胶成像系统检测和成像,实验重复3次。
1.2.6 统计学处理结果用均数±标准差表示,通过SPSS 16.0软件进行统计分析,组间比较采用单因素方差分析,以P < 0.05为差异有统计学意义。
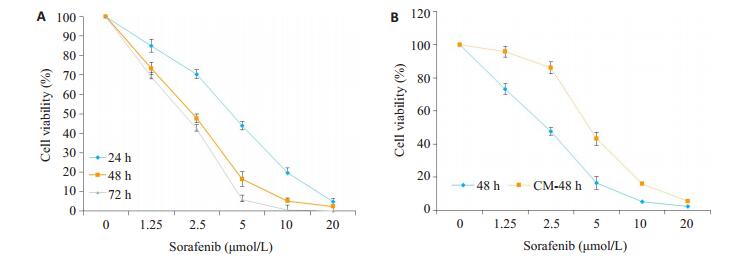
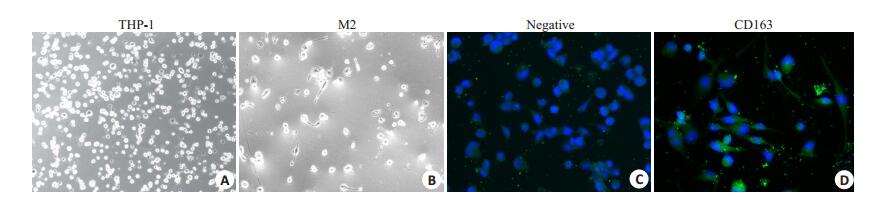
2 结果 2.1 M2-CM对索拉菲尼抑制SMMC-7721增殖的影响索拉菲尼(1.25、2.5、5、10、20 μmol/L)对SMMC- 7721的24、48、72 h增殖抑制作用呈现时间和剂量依赖效应(图 1A),为观察肿瘤微环境中M2-TAM在索拉菲尼治疗耐药中的作用,将THP1诱导为M2型巨噬细胞,分别计数5个镜下视野中CD163阳性细胞数,统计3次诱导实验的结果,THP1细胞诱导为M2型巨噬细胞的诱导效率为(91±3)%(图 2),收集M2-CM与SMMC- 7721共培养48 h,结果发现,M2-CM明显降低了索拉菲尼对SMMC-7721的增殖抑制作用(图 1B),索拉菲尼对肝癌细胞SMMC-7721作用48 h的IC50为2.25 μmol/L,与M2-CM共培养后索拉菲尼IC50为4.72 μmol/L。
|
图 1 M2-CM对索拉菲尼抑制SMMC-7721增殖的影响 Fig.1 Inhibitory effect of sorafenib on SMMC-7721 cells culture aloned or in the presence of M2-CM (n=3).A: Effects of different concentrations of sorafenib on the cell viability of SMMC-7721 cells at 24, 48 and 72 h; B: Effects of M2-CM on cell viability of SMMC-7721 cells treated with sorafinib at different concentrations for 48 h. |
|
图 2 M2-TAM细胞的诱导及鉴定 Fig.2 Induction and identification of M2-TAM (Original magnification: ×200).A: THP-1 cells; B: THP-1 cells induced by 100 ng/mL PMA for 6 h and with 20 ng/mL IL-4 and 20 ng/mL IL-13 for 18 h; C: Negative control of FITC; D: CD163-FITC group. |
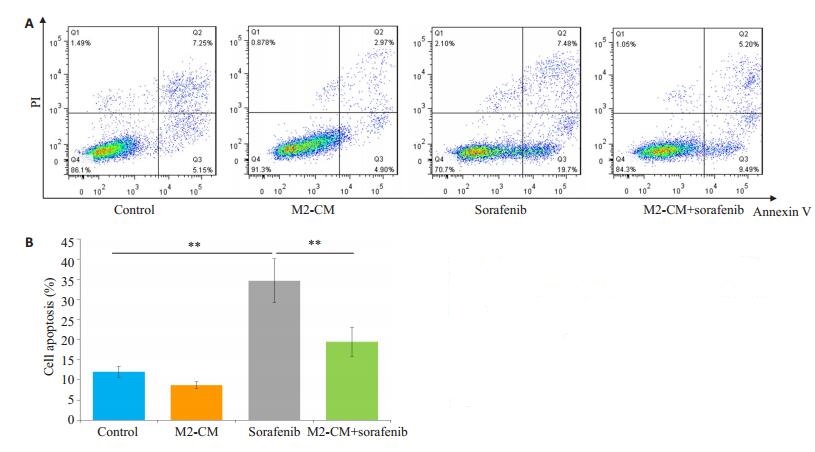
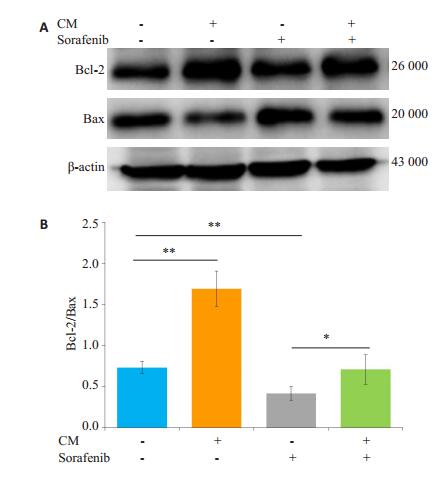
索拉菲尼作用24 h(单给药组)和与M2-CM共培养后索拉菲尼作用24 h(共培养给药组)SMMC-7721的平均凋亡率分别是34.6%和19.5%,共培养给药组与单给药组的差别具有统计学意义(P < 0.01,图 3)。Western blot检测抑凋亡蛋白Bcl-2和促凋亡蛋白Bax的表达,结果显示共培养给药组与单独给药组相比Bcl- 2表达明显增加,Bcl-2/Bax比值增加(P < 0.05,图 4)。
|
图 3 M2-CM对索拉菲尼促SMMC-7721细胞凋亡的影响 Fig.3 Effect of M2-CM on the pro-apoptotic effect of sorafenib (n=3).A: Effects of M2-CM on apoptosis of SMMC-7721 cells induced by sorafenib (2.5 μmol/L) for 24 h determined by flow cytometry; B: Quantitativel analysis of the cell apoptosis in each group. **P < 0.01. |
|
图 4 M2-CM对索拉菲尼作用SMMC-7721后凋亡相关蛋白表达的影响 Fig.4 Effect of sorafenib on the expression of apoptosisrelated proteins and the influence of M2-CM.A: Effects of M2-CM on the expression of Bcl-2 and Bax in SMMC-7721 cells induced by sorafenib (2.5 μmol/L) for 24 h; B: Standard quantitative analysis of Bcl-2/Bax with Image J grayscale scanning (n=3). *P < 0.05, **P < 0.01. |
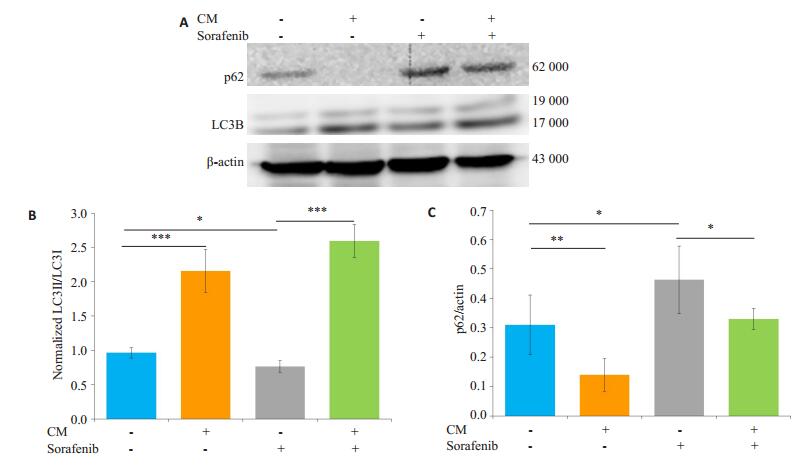
共培养给药组与单独给药组相比LC3B-II/I的比值升高,p62蛋白表达下调,共培养给药组SMMC-7721凋亡减少的同时自噬水平显著增强(图 5)。
|
图 5 M2-CM对索拉菲尼作用SMMC-7721后自噬相关蛋白表达的影响 Fig.5 Effect of sorafenib on the expression of autophagy-related proteins and the influence of M2-CM.A: Effects of M2-CM on the expression of autophagy associated proteins p62 and LC3B in SMMC-7721 cells induced by sorafenib (2.5 μmol/L) for 24 h; B: Standard quantitative analysis of LC3II/LC3I with Image J grayscale scanning; C: Standard quantitative analysis of p62/β-actin with Image J grayscale scanning (n=3). *P < 0.05, **P < 0.01, ***P < 0.001. |
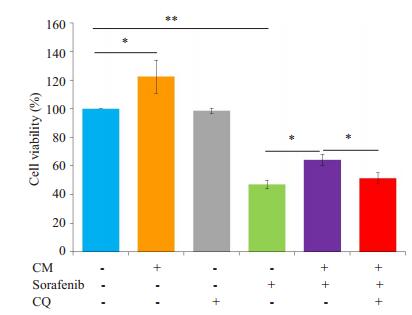
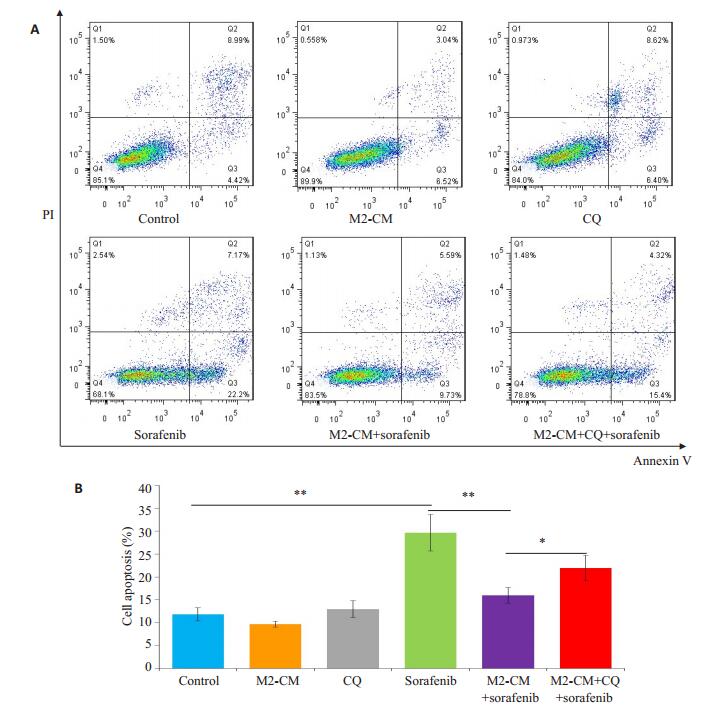
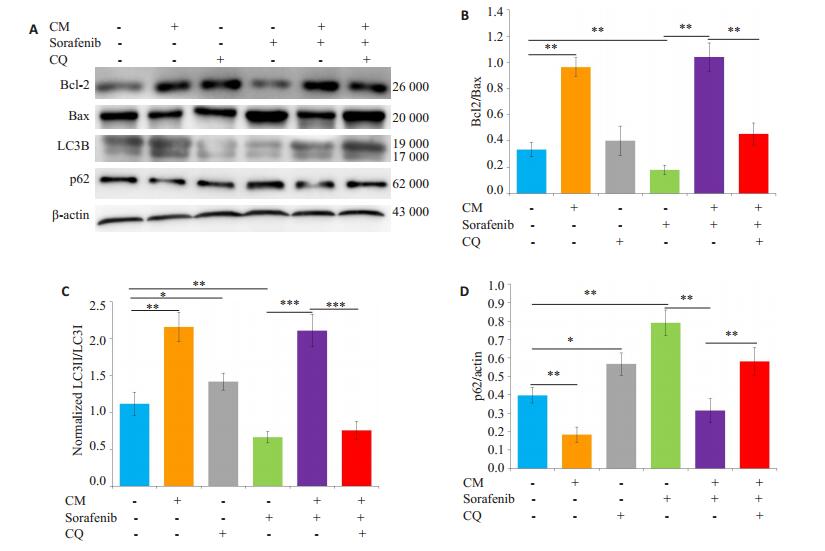
给予自噬抑制剂氯喹(10 μmol/L)预处理SMMC- 7721细胞后,观察M2-CM和索拉菲尼共培养(共培养给药组)24 h对细胞增殖、凋亡的影响及凋亡和自噬相关蛋白表达的变化。氯喹处理后的共培养给药组较未加氯喹处理的共培养给药组相比细胞增殖抑制更为明显(图 6),促细胞凋亡作用明显增强(图 7)。Western blot结果显示,氯喹可显著下调与M2-CM和索拉菲尼共培养的SMMC-7721细胞自噬相关蛋白LC3B-II/LC3-I的比值、上调负调控蛋白p62的表达;下调凋亡相关蛋白Bcl-2/Bax比值(图 8),从而促进细胞凋亡。
|
图 6 氯喹处理后对M2-CM与索拉菲尼共培养的SMMC- 7721细胞增殖水平影响 Fig.6 Effect of M2-CM and sorafenib treatment on the proliferation of SMMC-7721 cells after CQ treatment.*P < 0.05, **P < 0.01. |
|
图 7 氯喹处理后对M2-CM与索拉菲尼共培养的SMMC-7721细胞凋亡的影响 Fig.7 Effect of M2-CM co-culture and sorafenib on apoptosis of SMMC-7721 cells after CQ treatment.A: Effects of M2-CM on apoptosis of SMMC-7721 cells pretreated with CQ (10 μmol/L) and then with sorafenib (2.5 μmol/L) for 24 h; B: Quantitatively analysis of cell apoptosis in each group (n=3). *P < 0.05, **P < 0.01. |
|
图 8 氯喹处理后对M2-CM与索拉菲尼共培养的SMMC-7721细胞凋亡和自噬相关蛋白表达的影响 Fig.8 Effect of sorafenib on the expression of apoptosis- and autophagy-related proteins and the influence of M2-CM after CQ treatment.A: Effects of M2-CM on the expressions of Bcl-2, Bax, p62, and LC3B in SMMC-7721 cells pretreated with CQ (10 μmol/L) and then treated with sorafenib (2.5 μmol/L) for 24 h; B: Standard quantitative analysis of Bcl-2/ Bax with Image J grayscale scanning; C: Standard quantitative analysis of LC3II/ LC3I with Image J grayscale scanning; D: Standard quantitative analysis of p62/β-actin with Image J grayscale scanning(n=3). *P < 0.05, **P < 0.01, ***P < 0.001. |
我国是原发性肝癌的高发区,集中了全世界约54%的新发病例,同时肝癌也是我国位居第2位的肿瘤死亡原因,严重威胁着人们的生命与健康[13]。索拉菲尼是目前美国FDA唯一批准用于晚期肝癌治疗的分子靶向药物,可延长患者平均生存期7.9~10.7月,虽然在一定程度上给患者带来了获益,但结局仍不理想,排除患者对药物的反应性外,发生耐药是限制其持续发挥治疗作用的关键问题[14]。
本实验结果显示M2-TAMs明显降低了索拉菲尼对SMMC-7721的增殖抑制作用。对与M2-TAMs共培养的SMMC-7721,索拉菲尼的IC50是单独用药的2.1倍。肿瘤微环境是肿瘤细胞赖以生存的复杂环境,主要由多种不同的细胞组成,包括肿瘤细胞、TAMs、血管内皮细胞、间充质来源细胞(成纤维细胞)及细胞外基质等[15-16]。巨噬细胞是体内微环境中的重要一员,其表型具有很大的异质性,随所在器官微环境和生理病理条件的不同而具有多样化功能[17-18]。炎症环境中巨噬细胞多呈M1型,肿瘤间质中的巨噬细胞生物学功能不同于炎症环境中的巨噬细胞多呈M2型,主要发挥免疫抑制作用,可促进新生血管形成和肿瘤进展和耐药[19-20]。已有多篇文献报道M2-TAMs在肝癌治疗耐药中发挥了十分重要的作用[21-26]。
在凋亡实验中我们发现,M2-TAMs与SMMC- 7721共培养可明显减弱索拉菲尼的促细胞凋亡作用,Bcl-2/Bax比值也较单独索拉菲尼组明显上调。这提示M2-TAMs降低索拉菲尼的增殖抑制作用可能与抑制其促凋亡作用有关。有研究发现M2-TAMs能产生IL-10,上调抗凋亡蛋白Bcl-2表达,激活信号转导与转录激活因子3(Stat3)信号通路,从而显著减低乳腺癌细胞对紫杉醇诱导的凋亡[27],因此,M2-TAMs可能通过分泌多种细胞因子如:IL-10、VEGF、TGF-β等促进自噬发生[27-29]。为此本研究进一步观察了M2-TAMs减弱索拉菲尼的促细胞凋亡作用是否与促进自噬的发生有关,结果显示,M2-TAMs上调索拉菲尼作用后Bcl-2/Bax比值的同时,显著增强了肝癌细胞的自噬水平。这些结果表明,肿瘤微环境中的巨噬细胞会在索拉菲尼杀灭肿瘤细胞的同时诱导其发生自噬,在这种情况下自噬的发生可能有助于肿瘤细胞消除药物的蓄积,保护细胞存活,从而使得肿瘤细胞对索拉菲尼产生耐药性,不利于肿瘤的治疗。
本研究进一步在实验中加入自噬抑制剂氯喹[30]进行处理,发现有氯喹的共培养给药组与无氯喹处理的共培养给药组相比细胞凋亡率明显增加,也就是说自噬抑制剂在一定程度上增加了与M2-TAMs共培养的肝癌细胞对索拉菲尼的敏感性,促进了肝癌细胞凋亡,这可能是由于细胞处于药物作用的应激状态下,抑制自噬使细胞失去保护机制,导致破坏线粒体等细胞器有害的物质,如ROS和大量超氧化物的累积,破坏线粒体,促进细胞的凋亡[31-32]。这提示肿瘤微环境中M2-TAMs诱导的细胞自噬增加是索拉菲尼治疗肝癌耐药的重要分子机制。接下来我们将通过临床标本和动物实验进一步对体外实验结果进行验证,并深入探讨M2-TAMs诱导肝癌细胞自噬的分子机制,为阐明索拉菲尼治疗肝癌耐药的分子机制和设计更为有效的治疗方法提供新的实验依据。
| [1] |
Abdel-Rahman O, Fouad M. Sorafenib-based combination as a first line treatment for advanced hepatocellular carcinoma: A systematic review of the literature[J].
Crit Rev Oncol Hematol, 2014, 91(1): 1-8.
|
| [2] |
Tian L, Zhao ZF, Xie L, et al. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/ Akt/mTOR pathway[J].
Oncotarget, 2018, 9(4): 4886-96.
|
| [3] |
Wang L, Shang Z, Zhou Y, et al. Autophagy mediates glucose starvation-induced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle and survival[J].
Cell Death Dis, 2018, 51(2): 213-8.
|
| [4] |
Prieto-Domínguez N, Ordóñez R, Fernández A, et al. Modulation of autophagy by sorafenib: effects on treatment response[J].
Front Pharmacol, 2016, 7(2): 151-63.
|
| [5] |
Rybstein MD, Bravo-San JM, Kroemer G. The autophagic network and cancer[J].
Nat Cell Biol, 2018, 20(3): 243-51.
DOI: 10.1038/s41556-018-0042-2. |
| [6] |
Li J, Wu PW, Zhou Y, et al. Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy[J].
Cell Death Dis, 2018, 9(2): 225-36.
DOI: 10.1038/s41419-018-0329-z. |
| [7] |
Ling SB, Song L, Fan N, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway[J].
Int J Oncol, 2017, 50(1): 297-309.
DOI: 10.3892/ijo.2016.3799. |
| [8] |
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity[J].
Curr Opin Immunol, 2010, 22(2): 231-7.
DOI: 10.1016/j.coi.2010.01.009. |
| [9] |
Dong P, Ma L, Liu L, et al. CD86 +/CD206 +, diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis[J].
Int J Mol Sci, 2016, 17(3): 320-9.
DOI: 10.3390/ijms17030320. |
| [10] |
Jia XH, Feng GW, Wang ZL, et al. Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects[J].
Oncotarget, 2016, 7(15): 20934-44.
|
| [11] |
Kazankov K, Rode A, Simonsen K, et al. Macrophage activation marker soluble CD163 May predict disease progression in hepatocellular carcinoma[J].
Scand J Clin Lab Invest, 2016, 76(1): 64-73.
DOI: 10.3109/00365513.2015.1099722. |
| [12] |
Smith MP, Young H, Hurlstone A, et al. Differentiation of THP1 cells into macrophages for transwell co-culture assay with melanoma cells[J].
Bio Protoc, 2015, 5(21): 3357-69.
|
| [13] |
Zuo TT, Zheng RS, Zhang SW, et al. Incidence and mortality of liver cancer in China in 2011[J].
Chin J Cancer, 2015, 34(3): 56-67.
DOI: 10.1186/s40880-015-0056-0. |
| [14] |
Guan YS, He Q. Sorafenib: activity and clinical application in patients with hepatocellular carcinoma[J].
Expert Opin Pharmacother, 2011, 12(2): 303-13.
DOI: 10.1517/14656566.2011.546346. |
| [15] |
Galdiero MR, Bonavita E, Barajon IA, et al. Tumor associated macrophages and neutrophils in cancer[J].
Immunobiology, 2013, 218(11, SI): 1402-10.
DOI: 10.1016/j.imbio.2013.06.003. |
| [16] |
Goswami KK, Ghosh T, Ghosh S, et al. Tumor promoting role of antitumor macrophages in tumor microenvironment[J].
Cell Immunol, 2017, 316(1): 1-10.
|
| [17] |
Fujita N, Nishie A, Aishima S, et al. Role of tumor-associated macrophages in the angiogenesis of well-differentiated hepatocellular carcinoma: Pathological-radiological correlation[J].
Oncol Rep, 2014, 31(6): 2499-505.
DOI: 10.3892/or.2014.3138. |
| [18] |
Li Y, Cao F, Li M, et al. Hydroxychloroquine induced lung cancer suppression by enhancing chemo-sensitization and promoting the transition of M2-TAMs to M1-like macrophages[J].
J Exp Clin Cancer Res, 2018, 37(1): 259-68.
DOI: 10.1186/s13046-018-0938-5. |
| [19] |
Shirabe K, Mano Y, Muto J, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma[J].
Surg Today, 2012, 42(1): 1-7.
DOI: 10.1007/s00595-011-0058-8. |
| [20] |
Tan BH, Shi XJ, Zhang J, et al. Inhibition of Rspo-Lgr4 facilitates checkpoint blockade therapy by switching macrophage polarization[J].
Cancer Res, 2018, 78(17): 4929-42.
DOI: 10.1158/0008-5472.CAN-18-0152. |
| [21] |
Yao WB, Ba Q, Li XG, et al. A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer[J].
Ebiomedicine, 2017, 22(1): 58-67.
|
| [22] |
Zhang C, Gao L, Cai Y, et al. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model[J].
Biomaterials, 2016, 84(10): 1-12.
|
| [23] |
Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects[J].
Clin Cancer Res, 2010, 16(13): 3420-30.
DOI: 10.1158/1078-0432.CCR-09-2904. |
| [24] |
Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice[J].
Hepatology, 2015, 61(5): 1591-602.
DOI: 10.1002/hep.27665. |
| [25] |
Tan HY, Wang N, Man K, et al. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin[J].
Cell Death Dis, 2015, 38(6): e1942-56.
|
| [26] |
Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma[J].
World J Gastroenterol, 2018, 24(41): 4643-51.
DOI: 10.3748/wjg.v24.i41.4643. |
| [27] |
Yang C, He L, He P, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway[J].
Med Oncol, 2015, 32(2): 352-66.
|
| [28] |
Zhao QQ, Wang ZP, Wang ZX, et al. Aspirin may inhibit angiogenesis and induce autophagy by inhibiting mTOR signaling pathway in murine hepatocarcinoma and sarcoma models[J].
Oncol Lett, 2016, 12(4): 2804-10.
DOI: 10.3892/ol.2016.5017. |
| [29] |
Zhang C, Zhang X, Xu R, et al. TGF-β2 initiates autophagy via Smad and non-Smad pathway to promote glioma cells'invasion[J].
J Exp Clin Cancer Res, 2017, 36(1): 162-76.
DOI: 10.1186/s13046-017-0628-8. |
| [30] |
Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research[J].
Cell, 2010, 140(3): 313-26.
DOI: 10.1016/j.cell.2010.01.028. |
| [31] |
Datta S, Choudhury D, Das A, et al. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway[J].
Apoptosis, 2019, 95(19): 15-26.
|
| [32] |
Liu D, Fan YF, Li J, et al. Inhibition of cFLIP overcomes acquired resistance to sorafenib via reducing ER stress-related autophagy in hepatocellular carcinoma[J].
Oncol Rep, 2018, 40(4): 2206-14.
|
 2019, Vol. 39
2019, Vol. 39

