渗出性胸腔积液主要见于各类炎症、结核以及恶性肿瘤[1-4]。肺炎性胸腔积液一般经过抗生素干预后预后较好[5]。结核性胸腔积液起病较为隐匿[6], 病程缓慢并缺乏特异性, 如早诊早治, 预后较好[7], 反之会因胸膜黏连及肥厚产生不可逆的肺功能损伤[8]。恶性胸腔积液中以肺癌居多, 约20%为首发症状, 30%~40%出现于病程中, 也提示预后不佳和生存期较短[9-11]。在临床实际工作中, 良恶性的胸腔积液分别以结核性胸膜炎和肺癌为著[12], 二者致病机理、治疗及愈后大相径庭, 及早明确诊断尤为重要。然而, 肺癌患者的年轻化、结核耐药变异株的增加[13]、传统细胞学的低灵敏度[14]、影像学在二者疾病的诊断中出现"同病异征, 同征异病"的征象[15]以及金标准病理学出现的滞后性和有创性导致二者的鉴别诊断仍然是困扰临床医生的一大挑战[16]。近年来, 有关良恶性胸腔积液诊断效能方面的研究较多[17-20], 将肺癌性胸腔积液从恶性胸腔积液中独立出来与结核性胸腔积液进行鉴别诊断的文献也有报道[21], 但将肺癌性胸腔积液分为鳞癌、腺癌、小细胞癌后利用癌胚抗原(CEA)与结核性胸腔积液进行鉴别的研究却未见报道。CEA由Gold[22]首次发现, 由多种恶性肿瘤分泌产生, 肿瘤侵及胸膜时, 大量分泌CEA释放入胸腔积液, 因其化学结构复杂, 相对分子质量较大, 极难由胸腔积液入血至肝脏代谢降解, 致使恶性胸腔积液中的CEA远高于血清[23]。但因恶性胸腔积液产生复杂, 起因于多种肿瘤(以肺癌为著)及多种病理类型, 导致国内外胸腔积液CEA尚无统一参考值范围[24], 本研究基于结核性与肺癌性胸腔积液的鉴别诊断, 探讨胸腔积液癌胚抗原(PCEA)和血清癌胚抗原(SCEA)和P/S在二者疾病诊断中的最适诊断临界值, 初步阐明三项指标单项和联合诊断的的鉴别价值, 提高诊断的准确率, 减少漏诊, 为临床及早确诊提供参考。
1 资料和方法 1.1 临床资料选择空军军医大学唐都医院呼吸科和胸腔外科2016年4月~2018年3月收治的202例临床资料比较完备的结核性与肺癌性胸腔积液初诊患者临床资料, 对其进行回顾性分析。其中, 结核性胸腔积液组82例, 男55例, 女27例, 年龄40~90岁, 61.94±13.25岁; 肺癌性胸腔积液组120例, 男80例, 女40例, 年龄41~85岁, 61.75± 10.85岁, 性别和年龄无统计学差异(P > 0.05)。两组均行PCEA和SCEA检测并随之计算P/S。结核性胸腔积液入选标准[25]:抗酸染色阳性或结核杆菌培养阳性; 病理查见结核病灶; 抗结核治疗胸腔积液明显吸收; 以上标准需满足至少1条。肺癌性胸腔积液入选标准[26]:影像学检查有肺部肿块影且抗结核治疗无效或病情加重; 胸腔积液为渗出性, 抗酸染色阴性或结核杆菌培养阴性; 组织学或细胞学检查证实肺部肿瘤; 以上标准需全部满足。
1.2 胸腔积液和血清CEA检测CEA检测采用电化学发光法, 试剂和仪器均为德国罗氏公司生产, 严格按照试剂和仪器的标准规程进行操作。
1.3 统计学分析PCEA和SCEA检测结果采用对数值进行分析, 数据服从正态分布, 用SPSS18.0软件进行统计分析, 计数资料用χ2检验, 计量资料用t检验, 多组之间比较用方差分析, ROC曲线确定最佳临界值(cut-off)及曲线下面积(AUC), 之后进行串并联试验, P < 0.05哦差异具有统计学意义。
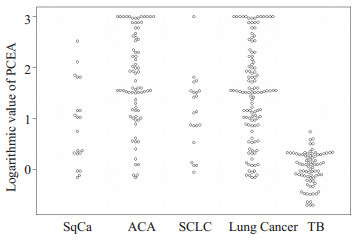
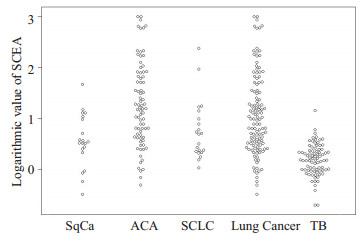
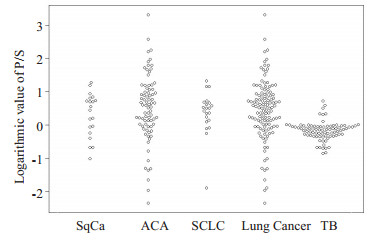
2 结果 2.1 PCEA、SCEA和P/S在两组中的检测结果比较结核性胸腔积液组和肺癌性胸腔积液组PCEA对数值分别为0.02±0.33 ng/mL、1.52±0.94 ng/mL; SCEA对数值分别为0.19±0.31 ng/mL、1.07±0.79 ng/mL; P/S对数值分别为-0.17±0.28、0.45±0.89, 后者均高于前者(P < 0.01);鳞癌组、腺癌组、小细胞癌组的3项指标水平也都高于结核性胸腔积液组(P < 0.01);鳞癌组、腺癌组和小细胞癌之间的P/S对数值比较无显著性差异(P> 0.05);腺癌组的PCEA对数值和SCEA对数值水平均高于鳞癌组和小细胞癌组(P < 0.01);鳞癌组和小细胞癌组的3项指标水平无显著性差异(P > 0.05, 表 1、图 1~3)。
| 表 1 PCEA、SCEA和P/S在两组中的结果比较 Tab.1 PCEA, SCEA and P/S patients with tuberculosis and lung cancer (Mean±SD) |
|
图 1 PCEA在各组中的对数值比较 Fig.1 Logarithmic values of PCEA in each group. |
|
图 2 SCEA在各组中的对数值比较 Fig.2 Logarithmic values of SCEA in each group. |
|
图 3 P/S在各组中的对数值比较 Fig.3 Logarithmic values of P/S in each group. |
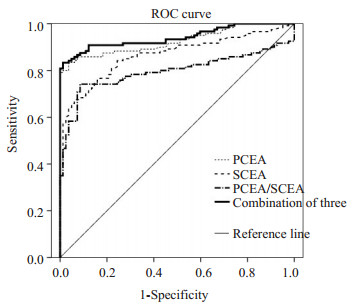
根据ROC曲线分析, PCEA、SCEA、P/S和三者联合的曲线下面积(AUC)分别为0.925(95%CI, 0.889~ 0.962)、0.866(95% CI, 0.816~0.915)、0.796(95% CI, 0.732~0.860)、0.941 (95%CI, 0.909~0.973), AUC愈大, 诊断效能愈高, 因此三者联合强于任一单独指标(表 2、图 4)。
| 表 2 PCEA、SCEA和P/S单项和三者联合诊断肺癌性胸腔积液的AUC Tab.2 AUC of the 3 indexes alone or in different combinations for diagnosing lung cancer-related pleural effusion |
|
图 4 PCEA、SCEA、P/S单项和三者联合诊断肺癌性胸腔积液的ROC曲线 Fig.4 ROC curves of the 3 indexes alone or in different combinations for diagnosing lung cancer-related pleural effusion. |
从SPSS输出表中查出, 3项单独指标的最佳诊断值分别为3.26、4.03、1.05 ng/mL, 相应的灵敏度分别为83.33%、67.50%、74.17%, 特异度分别为96.34%、93.90%、91.46%。在3项指标单独诊断肺癌性胸腔积液方面, PCEA在灵敏度、特异度、阴阳性预测值、正确率、约登指数和阴阳性似然比上诊断性能均优于SCEA和P/S, 另外SCEA在灵敏度、阴性预测值、正确率、约登指数和阴性似然比方面, 诊断性能最低; 在串联试验诊断肺癌性胸腔积液方面, 3者串联组合在诊断性能上并不优于两两串联组合, 反而大大降低了灵敏度; PCEA+P/ S在四种串联组合中显示出最好的诊断性能; 在并联试验诊断肺癌性胸腔积液方面, 并联组合明显提高了灵敏度、阴性预测值和阴性似然比, 以SCEA+P/S组合和3者组合为著, 特异度和阳性预测值仅有略微下降。在SCEA+P/S组合和3者组合中, 各组合之间的诊断性能并无明显差别, 3者联合相比较SCEA+P/S组合未显示出明显优势(表 3)。
| 表 3 PCEA、SCEA和P/S单项和三者联合对诊断肺癌性胸腔积液的价值 Tab.3 Diagnostic values of PCEA, SCEA and P/S alone or in different combinations for diagnosing lung cancer-related pleural effusion |
在本次研究中, 无论肺癌性胸腔积液组还是其3个亚组(鳞癌组、腺癌组、小细胞癌组)的PCEA、SCEA和P/S水平均显著高于结核性胸腔积液组, 差异均有统计学意义(P < 0.01), 显示出3项指标可作为两种疾病的鉴别诊断指标。另外, 鳞癌组、腺癌组和小细胞癌之间的P/S水平比较无显著性差异(P > 0.05), 但腺癌组的PCEA和SCEA水平均高于鳞癌组和小细胞癌组, 而鳞癌组和小细胞癌组之间却无显著性差异, 本次研究纳入的79例腺癌患者中, 利用ROC曲线确定PCEA和SCEA的诊断临界值(分别为PCEA>3.26 ng/mL和SCEA>4.03 ng/mL)作为诊断标准, 有72例PCEA阳性, 63例SCEA阳性, 阳性率分别为91.14%和79.75%, 高于文献报道[27], 因此笔者认为PCEA和SCEA对于诊断肺腺癌合并胸腔积液诊断价值更大, 具有更好的诊断价值。
据Moore[28]报道, ROC曲线下面积在大于0.9时有较高的准确度, 本研究中PCEA、SCEA、P/S和3者联合的AUC分别为0.925、0.866、0.796和0.941, PCEA和3者联合的AUC均大于0.9, 对于区分结核性和肺癌性胸腔积液有较高的准确度, 且3者联合强于任一单独指标。3项单独指标的最佳诊断值分别为3.26、4.03、1.05 ng/mL, 相应的灵敏度分别为83.33%、67.50%、74.17%, 特异度分别为96.34%、93.90%、91.46%, 与陆国军等[29]报道相符, 与Hernandez等[25]报道相比, 灵敏度高于其报道, 特异度与其相一致。在3项指标单独诊断肺癌性胸腔积液方面, PCEA的各项诊断性能均优于SCEA和P/S, 并且SCEA在灵敏度、阴性预测值、正确率、约登指数和阴性似然比方面, 诊断性能最低。分析原因可能为PCEA为肺癌时在闭合胸膜中产生, 受其大相对分子质量的影响, 不易进入血液循环被代谢降解, 致PCEA远高于SCEA, 故此升高也更为明显[30]。有研究者[31]认为约登指数综合了检测方法灵敏度和特异度的信息, 能较好反映检测方法的真实性, 如果约登指数大于0.7, 则该检测方法具有良好的临床诊断价值。本研究中的PCEA的约登指数为0.8, 仅次于PCEA + SCEA的并联组合(0.81), 高于其它任何单项检测和串并联的联合检测。本次串联试验中, SCEA+P/S组合和3者联合组合将诊断特异度升至100%, 大大提高了诊断特异性, 减少了误诊率; 并联试验明显提高了灵敏度、阴性预测值和阴性似然比, 特异度和阳性预测值仅有略微下降, 并且均显示出较高的约登指数(分别为0.81、0.77、0.79、0.78), 具备了较高的灵敏度和特异度。SCEA+P/S组合和3者组合极大地降低了漏诊率, 但两组合之间的诊断性能并无明显差别, 后者相比较前者未显示出明显优势, 所以在提高阳性检出率方面, 进行并联组合时, 应以SCEA+P/ S组合为最。另外, CEA是一种非特异性肿瘤标志物, 不具备器官特异性, 可在多种肿瘤患者血清中表达, SCEA检测具有取材方便、无创伤的特点, 可与其它血清肿瘤标志物联合检测对肿瘤类别进行初筛, 尤其在怀疑肺癌性胸腔积液时, 可作为进行胸腔穿刺进行PCEA检测的指征。毕竟胸腔穿刺是有创检查, 有手术并发症的风险(气胸、血胸、休克、感染)。SCEA检测还可以用到一些存在胸腔穿刺禁忌症(有严重出血倾向、大咯血、肺气肿)的患者。PCEA在3项单独指标中, 虽诊断性能优于SCEA和P/S, 但3者联合却强于任一单独指标, SCEA和P/S联合在串联试验中将特异度提高至100%, 减少了漏诊率, 在并联试验中大大提高了灵敏度, 降低了漏诊率。因此, 在肯定PCEA诊断优势的同时, 也要考虑到SCEA和P/S的不可或缺性。
本研究结果表明, 在结核性和肺癌性胸腔积液的诊断中, 单项PCEA检测的诊断效能均优于单项SCEA和二者比值, 相比较其他检查, 具有高敏感性和高特异性的双重性质, 并且易于取材、简单方便、创伤小的特点[32], 尤其在不能有效开展纤维支气管镜和胸腔镜的基层医院, 是鉴别诊断的一个可靠指标, 应将其像SCEA一样作为一项常规检查方法[33]。在3项指标中, SCEA灵敏度最低(67.50%), 漏诊率最高(32.50), 虽有一定诊断意义, 但不宜做排除性诊断, 同时应看到SCEA具有取材方便、无创伤的特点和可对肿瘤类别进行初筛以及用于胸腔穿刺禁忌症患者方面的重要性。P/S作为PCEA和SCEA的比值, 诊断性能上优于SCEA, 低于PCEA, 将其纳入联合诊断后可大大提高诊断性能, 减少误诊和漏诊。综上所述, 随时将3项指标有机结合, 进行串并联试验, 对有效区分结核性和肺癌性胸腔积液具有重要的诊断意义。
| [1] |
Zhang F, Wang JJ, Zheng XY, et al. Clinical value of jointly detection pleural fluid Midkine, pleural fluid adenosine deaminase, and pleural fluid carbohydrate antigen 125 in the identification of nonsmall cell lung cancer-associated malignant pleural effusion[J].
J Clin Lab Anal, 2018, 32(8): e22576.
DOI: 10.1002/jcla.2018.32.issue-8. |
| [2] |
王鹏, 张东, 郭学光, 等. 老年非小细胞肺癌合并慢性阻塞性肺病患者的临床特征[J].
南方医科大学学报, 2017, 37(7): 889-94.
|
| [3] |
Wei JY, Shi YH, Hou YJ, et al. Knockdown of thioredoxin-interacting protein ameliorates high glucose-induced epithelial to mesenchymal transition in renal tubular epithelial cells[J].
Cell Signal, 2013, 25(12): 2788-96.
DOI: 10.1016/j.cellsig.2013.09.009. |
| [4] |
Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin[J].
Arthritis Res Ther, 2005, 7(4): R784-95.
DOI: 10.1186/ar1733. |
| [5] |
Garske LA, Kunarajah K, Zimmerman PV, et al. In patients with unilateral pleural effusion, restricted lung inflation is the principal predictor of increased dyspnoea[J].
PLoS One, 2018, 13(10): e202621.
|
| [6] |
Sahn SA, Huggins JT, San Jose ME, et al. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone[J].
Int J Tuberc Lung Dis, 2013, 17(6): 787-93.
DOI: 10.5588/ijtld.12.0892. |
| [7] |
唐洁, 陈策, 查成, 等. 基于结核杆菌耐热抗原小分子多肽刺激人外周血T细胞产生TNF-α和IFN-γ鉴别肺结核和潜伏性结核感染[J].
南方医科大学学报, 2017(11): 1442-7.
|
| [8] |
Rahimkhani M, Einollahi N, Khavari Daneshvar H, et al. Survey of serum procalcitonin in cirrhotic patients[J].
Acta Med Iran, 2013, 51(3): 153-6.
|
| [9] |
Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions[J].
Respirology, 2015, 20(4): 654-9.
DOI: 10.1111/resp.12496. |
| [10] |
Wang XF, Wu YH, Wang MS, et al. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause[J].
Asian Pac J Cancer Prev, 2014, 15(1): 363-8.
|
| [11] |
Hawatmeh A, Thawabi M, Jmeian A, et al. Amiodarone-induced loculated pleural effusion without pulmonary parenchymal involvement:A case report and literature review[J].
J Nat Sci Biol Med, 2017, 8(1): 130-3.
DOI: 10.4103/0976-9668.198345. |
| [12] |
Ryu JS, Ryu HJ, Lee SN, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung Cancer[J].
J Clin Oncol, 2014, 32(9): 960-7.
DOI: 10.1200/JCO.2013.50.5453. |
| [13] |
Fan L, Chen Z, Hao XH, et al. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis:a systematic review and meta-analysis[J].
FEMS Immunol Med Microbiol, 2012, 65(3): 456-66.
DOI: 10.1111/j.1574-695X.2012.00972.x. |
| [14] |
Sriram KB, Relan V, Clarke BE, et al. Diagnostic molecular biomarkers for malignant pleural effusions[J].
Future Oncol, 2011, 7(6): 737-52.
DOI: 10.2217/fon.11.45. |
| [15] |
Wong C-, Al-faham Z, Jolepalem P, et al. Time sensitivity-corrected retention index:an enhanced metabolic index from F-18-FDG PET to differentiate between benign and malignant pulmonary nodules[J].
Nucl Med Commun, 2014, 35(12): 1220-3.
DOI: 10.1097/MNM.0000000000000190. |
| [16] |
李锐成, 刘昕阳, 沈建军, 等. T-SPOT.TB联合ADA和TB-DNA在诊断结核性胸膜炎中的应用价值[J].
东南国防医药, 2016(5): 483-5, 504.
DOI: 10.3969/j.issn.1672-271X.2016.05.010. |
| [17] |
Woo CG, Son SM, Han HS, et al. Diagnostic benefits of the combined use of liquid-based cytology, cell block, and carcinoembryonic antigen immunocytochemistry in malignant pleural effusion[J].
J Thorac Dis, 2018, 10(8): 4931-9.
DOI: 10.21037/jtd. |
| [18] |
Liu Q, Yu YX, Wang XJ, et al. Diagnostic accuracy of interleukin-27 between tuberculous pleural effusion and malignant pleural effusion:a Meta-Analysis[J].
Respiration, 2018, 95(6): 469-77.
DOI: 10.1159/000486963. |
| [19] |
Servonnet A, Frederic C, Fargeau FA. Pleural adenosine deaminase cut-off value for the diagnosis of tuberculous pleural effusion using the Diazyme assay[J].
Hong Kong Med J, 2018, 24(2): 207.
|
| [20] |
Zhang XR, Duan HF, Yu Y, et al. Differential diagnosis between benign and malignant pleural effusion with dual-energy spectral CT[J].
PLoS One, 2018, 13(4): e193714.
|
| [21] |
Samanta S, Sharma A, Das B, et al. Significance of total protein, albumin, globulin, serum effusion albumin gradient and LDH in the differential diagnosis of pleural effusion secondary to tuberculosis and cancer[J].
J Clin Diagn Res, 2016, 10(8): BC14-8.
|
| [22] |
Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques[J].
J Exp Med, 1965, 121(1): 439-62.
|
| [23] |
Hernandez L, Espasa A, Fernandez C, et al. CEA and CA 549 in serum and pleural fluid of patients with pleural effusion[J].
Lung Cancer, 2002, 36(1): 83-9.
|
| [24] |
Enz N, Fragoso F, Gamrekeli A, et al. Carcinoembryonic antigenpositive pleural effusion in early stage non-small cell lung cancer without pleural infiltration[J].
J Thorac Dis, 2018, 10(5): E340-3.
DOI: 10.21037/jtd. |
| [25] |
胡建林, 杨和平.
呼吸疾病鉴别诊断与治疗学[M]. 2版. 北京: 人民军医出版社, 2015: 379-80.
|
| [26] |
Radjenovic-Petkovic T, Pejcic T, Nastasijević-Borovac D, et al. Diagnostic value of CEA in pleural fluid for differential diagnosis of benign and malign pleural effusion[J].
Med Arh, 2009, 63(3): 141-2.
|
| [27] |
Moore HT, Andlauer O, Simon N, et al. Exploring medical diagnostic performance using interactive, multi-parameter sourced receiver operating characteristic scatter plots[J].
Comput Biol Med, 2014, 47(3): 120-9.
|
| [28] |
陆国军, 张宇, 解卫平, 等. ESM-1和CEA联合检测在鉴别良恶性胸腔积液中的价值[J].
南京医科大学学报:自然科学版, 2017(1): 77-80.
|
| [29] |
Wang WW, Zhou XL, Song YJ, et al. Combination of long noncoding RNA MALAT1 and carcinoembryonic antigen for the diagnosis of malignant pleural effusion caused by lung cancer[J].
Onco Targets Ther, 2018, 11(2): 2333-44.
|
| [30] |
Larson ML, Sauaia H. The feulgen reaction:importance of the wash after hydrolysis[J].
Acta Histochem, 1980, 67(1): 6-12.
DOI: 10.1016/S0065-1281(80)80047-X. |
| [31] |
Kim HR, Lee GO, Choi KH, et al. SRSF5:a novel marker for smallcell lung cancer and pleural metastatic cancer[J].
Lung Cancer, 2016, 99(5): 57-65.
|
| [32] |
Sharma SK, Bhat S, Chandel V, et al. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, and cytokeratin 19 fragments in patients with effusion from nonsmall cell lung cancer[J].
J Carcinog, 2015, 14(2): 7.
|
 2019, Vol. 39
2019, Vol. 39

