2012年报道我国慢性肾脏病的发病率高达10.8%,已经成为我国严重的公共卫生问题[1-2]。肾间质纤维化是各种慢性肾脏病发展为终末期肾病的共同病理基础和共同途径[3-4],其纤维化的程度决定慢性肾脏病进展及恶化的速度[5-6]。肾间质纤维化的主要生物学事件是细胞外基质合成增加和大量积聚,其常由肾小管上皮细胞向间充质细胞转分化(EMT)所致[7]。新近发现的原癌基因metadherin(Mtdh),又名星形细胞上调基因- 1(AEG-1),最初在人胚胎初级星形胶质细胞中发现,其后进行体内噬菌体筛选试验发现它能介导乳腺癌细胞向肺部转移并命名为Mtdh[8]。HIV、肿瘤坏死因子α(TNF-α)、转移生长因子β(TGF-β1)等均可诱导Mtdh的表达[8-9]。Mtdh激活p38有丝分裂原激活蛋白激酶(p38 MAPK)通路参与TGF-β1诱导的肾小管上皮细胞EMT[9],且能放大TGF-β1致EMT的效应[10]。我们猜测Mtdh通过促进EMT参与肾脏纤维化,目前相关研究甚少。ARB类药物可改善肾脏纤维化,然而机制尚未完全阐明[11-14]。ARB类药物能否影响Mtdh表达改善肾脏纤维化尚无报道。我们假设替米沙坦通过抑制Mtdh改善肾脏纤维化并对该假设进行探讨。
1 材料和方法 1.1 主要材料 1.1.1 试剂抗小鼠GAPDH抗体(EarthOx)、抗小鼠纤连蛋白(FN)抗体和抗小鼠Mtdh抗体(Abcam)、抗小鼠钙连蛋白抗体(BD)、抗小鼠α-平滑肌肌动蛋白(α-SMA)抗体(Sigma)、抗小鼠胶原蛋白Ⅰ抗体(Col Ⅰ)和免疫组化显示剂(博士德)。重组人TGF-β1细胞因子(BD);lipofectamineTM2000(Invitrogen);胎牛血清和细胞培养基DMEM/F12(Gibco),Mtdh SiRNA(si-Mtdh)序列见我们课题组已经发表的文章[15],由Genepharma公司合成:(正义链5'-GGAUGAAGUUGUUAGAAAUTT-3',反义链5'-AUUUCUAACAACUUCAUCCT T-3')。
1.1.2 动物实验建立单侧输尿管梗阻小鼠肾脏纤维化模型:SPF级C57小鼠18只(南方医科大学动物中心),适应性饲养两周后,将18只小鼠随机分为3组,即假手术组、单侧输尿管结扎组(模型组)和单侧输尿管结扎并替米沙坦干预组(10 mg·kd-1·d-1),每组6只小鼠。单侧输尿管结扎时,用1%戊巴比妥钠腹腔注射麻醉小鼠,腹部正中切口,常规消毒、暴露、游离、结扎并剪断左侧输尿管,逐层缝合。假手术组仅游离,不结扎不剪断。替米沙坦干预组:将替米沙坦溶于生理盐水,于手术当天予替米沙坦10 mg·kd-1·d-1开始灌胃直至实验结束。假手术组和模型组予同体积生理盐水每天灌胃至实验结束。术后14 d结束实验。
1.1.3 标本收集实验结束时处死小鼠,通过球后静脉丛静脉取血,并快速分离肾脏组织,部分组织采用4%多聚甲醛固定液固定,常规脱水、石蜡包埋,切成3 μm的石蜡切片,进行MASSON染色和免疫组织化学检测,剩余组织液氮保存进行Western blot检测。取血清送珠江医院检验科检测血尿素氮和血肌酐。
1.1.4 免疫组织化学法肾脏石蜡切片置于62 ℃烤箱烤片1 h,常规脱蜡,枸橼酸盐缓冲液pH 6.0修复20 min,3% H2O2封闭10 min,2%山羊血清封闭15 min,相应的抗体Mtdh(1:100)、FN(1:200)、E-cadherin(1:100)、α-SMA(1:200)4 ℃孵育过夜,次日分别滴加通用型生物素标记二抗IgG及辣根酶标记链酶卵白素三抗各15 min,中间各步均需PBS洗涤3次,每次5 min,DAB显色。苏木素复染核3 min,封片。Masson染色按照试剂盒进行。
1.1.5 Western bolt在细胞内或研碎的组织内加入适量RIPA裂解液,充分裂解后洗出液体至EP管,离心后吸取上清液。采用BCA法检测蛋白浓度。取适量蛋白100 ℃水浴锅变性10 min,加入制备好的10% SDSPAGE胶孔中,电泳分离结束后将蛋白转至PVDF膜上,5%脱脂奶37 ℃封闭1 h,分别加入相应的一抗抗体,按照说明书浓度,4 ℃孵育过夜,次日TBST洗3次,每次10 min,加入对应HRP抗兔或小鼠二抗(1:10 000),室温孵育1 h,TBST洗3次,每次10 min,ECL发光液避光显色,化学发光成像仪上发光并摄像。并用Quantity one Bio-Rad公司行半定量分析,将检测值与相应的内参灰度值相比表示检测值的表达水平。
1.1.6 细胞培养及分组小鼠来源肾小管上皮细胞(mTEC)于含5%胎牛血清的DMEM/F12培养基,37 ℃、5% CO2细胞培养箱培养。mTEC细胞转染Mtdh-siRNA,按LipofectamineTM 2000转染试剂盒说明书转染48 h。转染后收集提取总蛋白并行Western blot检测Mtdh蛋白表达。实验细胞分为4组:Negative Control SiRNA(si-NC)、si-NC+TGF-β1、Mtdh SiRNA(si-Mtdh)和si-Mtdh+TGF-β1组。转染前1 h,按上述分组,加或不加入重组人TGF-β1细胞因子(5 ng/mL)共刺激。转染方法按照LipofectamineTM 2000转染试剂盒说明转染48 h。实验结束时收集提取细胞总蛋白,通过Western blot检测FN、钙连蛋白、α-SMA和Col Ⅰ。
1.1.7 统计学方法采用SPSS 17.0进行统计分析,实验数据根据方差齐性检验结果采用均数±标准差或中位数四分位间距表示,多个样本均数比较采用One-way ANOVA分析,根据方差齐性检验结果,采用LSD或Dunnett进行组间比较,P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠血尿素氮及肌酐水平的变化3组小鼠血尿素氮与血肌酐值见表 1。与假手术组相比,模型组血尿素氮和血肌酐水平显著升高(P < 0.05);与模型组相比,替米沙坦干预后血尿素氮仍有明显升高,血肌酐水平显著下降(P < 0.05)。
| 表 1 3组小鼠血生化结果 Tab.1 Renal function in the 3 groups |
Masson染色观察肾脏的病理改变:假手术组肾脏基本无病变,模型组肾脏病变主要位于肾小管和肾间质,可见肾小管明显的扩张、肾间质增宽及间质纤维化形成,替米沙坦干预后可减轻上述病变(图 1)。
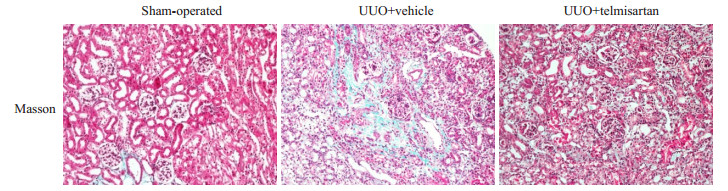
|
图 1 3组小鼠肾脏Masson染色变化 Fig.1 Masson's trichrome staining of the kidneys in the 3 group (Original magnification: ×200) |
模型组Fibronectin、α-SMA染色阳性面积明显高于假手术组,而E-cadherin则呈现相反趋势。替米沙坦干预组Fibronectin、α-SMA染色阳性面积明显低于模型组(图 2)。进一步通过Western blot法检测肾脏组织蛋白,发现模型组Fibronectin、α-SMA和Collagen I的蛋白水平高于假手术组组(P < 0.05),E-cadherin则显著低于假手术组(P < 0.01);替米沙坦干预组Fibronectin、α-SMA和Collagen I较模型组表达显著下降,E-cadherin较模型组表达显著上升(图 3)。与免疫组织化学染色结果一致。
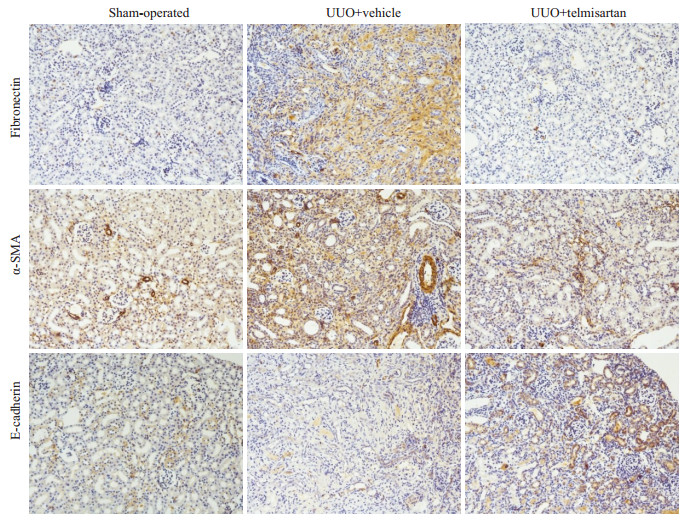
|
图 2 替米沙坦减轻了细胞外基质的沉积 Fig.2 Telmisartan attenuates ECM deposit in the mouse model of UUO (×200) |
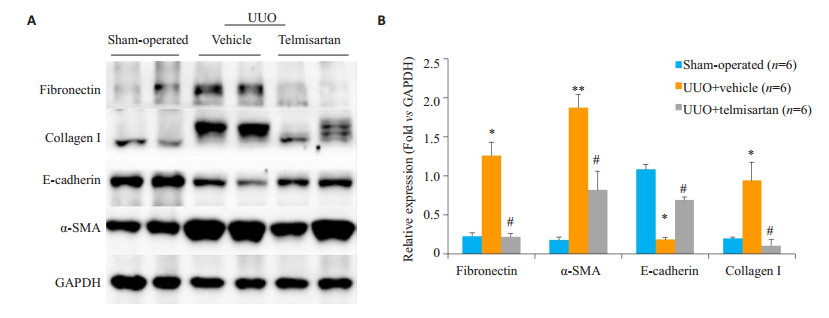
|
图 3 替米沙坦减轻细胞外基质及胶原沉积 Fig.3 Telmisartan alleviates renal fibrosis in the mouse model of UUO. A: Western blot analysis of fibronectin, collagen I, E-cadherin and α-SMA in the UUO kidney. B: Quantitative analysis of fibronectin, collagen I, E-cadherin and α-SMA in the UUO kidney. *P < 0.05, **P < 0.01 vs sham-operated mice; #P < 0.01 vs control treatment (UUO + vehicle) |
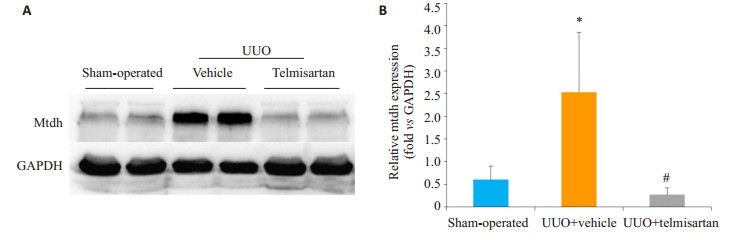
Mtdh主要表达于肾小管中,与假手术组相比,模型组Mtdh染色阳性面积显著增加;与模型组相比,替米沙坦干预组Mtdh染色阳性面积显著下降(图 4)。模型组Mtdh蛋白表达较假手术组显著增加(P < 0.05,图 5);替米沙坦干预组Mtdh蛋白表达较模型组显著下降(P < 0.05)。
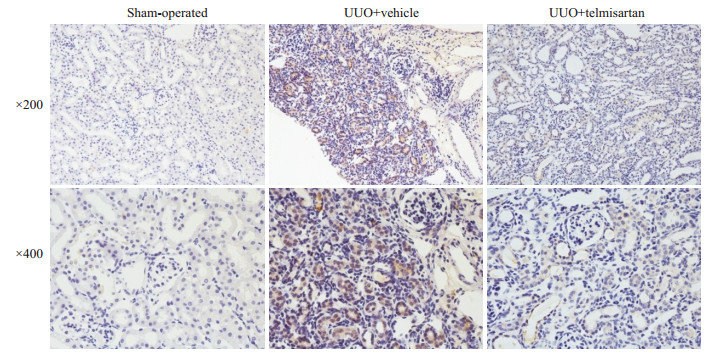
|
图 4 Mtdh在3组小鼠肾脏组织的表达(免疫组化) Fig.4 Renal Mtdh expression in the 3 groups of mice (Immunohistochemistry) |
|
图 5 替米沙坦减轻Mtdh的表达 Fig.5 Telmisartan alleviates Mtdh expression in the model of UUO. A: Western blot analysis of Mtdh in the UUO kidney. B: Quantitative analysis of Mtdh in the UUO kidney. *P < 0.05 vs sham-operated mice; #P < 0.05 vs UUO + vehicle |
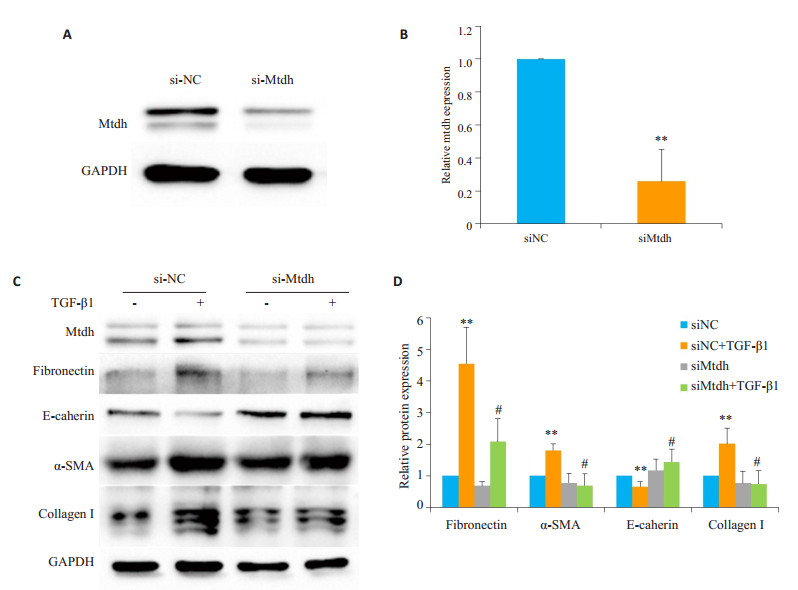
siMtdh能显著抑制Mtdh的表达(图 6A、B)。TGF- β1刺激组Fibronectin、α-SMA和Collagen I较对照组表达显著上升,E-cadherin表达显著下降,有统计学意义;siMtdh组Fibronectin、α-SMA和Collagen I较siNC组表达显著下降,E-cadherin表达显著上升,有统计学意义(图 6C、D)。
|
图 6 siMtdh抑制TGF-β1诱导的EMT Fig.6 siMtdh reverses TGF-β1-induced EMT in mTECs. A: Western blotting showing lowered Mtdh expression in mTECs transfected with si-Mtdh; B: Quantitative analysis of Mtdh protein level normalized to GAPDH; C: Western blotting showing that downregulation of Mtdh inhibits TGF-β1-induced up-regulation of collage I, EMT (reduced E-cadherin and gained α- SMA), and fibronectin; D: Quantitative analysis of relative protein levels of collage I, E-cadherin, α-SMA, and fibronectin in TGF-β1-treated cells (normalized to GAPDH). **P < 0.01 vs siNC group without TGF-β1 treatment group; #P < 0.05 vs siNC group with TGF-β1 treatment |
Mtdh是一个新近发现的多功能基因,与肿瘤发生发展密切相关[16-18]。AEG-1能够调控PI3K/Akt、Wnt/β- catenin、NF-КB、MAPK、SND1等多条信号通路参与正常细胞转分化、肿瘤细胞的存活、增殖、血管形成、迁移侵袭、肿瘤耐药及炎症反应等多种生物学过程[19-20]。本实验研究中,我们采用单侧输尿管结扎建立肾脏纤维化模型,发现Mtdh在模型组中表达显著上调,给予替米沙坦治疗后Mtdh表达下调,肾脏纤维化明显减轻,细胞实验提示下调Mtdh能抑制TGF-β1诱导的EMT,说明替米沙坦可能通过影响Mtdh的表达改善小鼠肾脏纤维化。
我们认为替米沙坦抑制Mtdh的表达改善肾脏纤维化可能通过以下机制:(1)抑制炎症:炎症因子TNF-α可通过NF-КB途径上调Mtdh的表达,而Mtdh高表达又可刺激炎症因子的生成[21-22]。单侧输尿管梗阻肾脏的炎症因子升高可能与Mtdh表达上调互为因果。ARB类药物可抑制血管紧张素Ⅱ的活性、炎症细胞的浸润及炎症因子的生成[23-24]。因此替米沙坦可能通过抑制炎症抑制Mtdh的表达。(2)抑制Mtdh/TGF-β通路:Wei等[9]研究显示Mtdh在TGF-β1诱导的肾小管上皮细胞转分化发挥重要作用,具有放大TGF-β致EMT的效应[10]。肾间质纤维化的核心是肾小管上皮细胞向间充质细胞转分化形成的肾间质成纤维化细胞的活化增殖和功能加强进而发生细胞外基质合成增加和大量积聚[25-27]。众所周知,TGF-β1是EMT最重要的诱导调控因子[28-29]。ARB类药物可显著抑制TGF-β1表达进而抑制脏器纤维化[30-31]。我们的动物实验显示替米沙坦干预后Mtdh显著下调,同时细胞实验结果显示下调Mtdh能抑制TGF-β1诱导的肾小管EMT。进一步说明,ARB类药物可能通过抑制Mtdh/TGF-β1通路进而抑制肾脏纤维化。
综上所述,本研究发现Mtdh主要表达肾小管上皮细胞,且在UUO小鼠肾组织中呈高表达。经替米沙坦干预治疗后,肾脏纤维化显著改善,且Mtdh表达显著下降。且下调Mtdh可抑制TGF-β1诱导的EMT,提示替米沙坦可能通过影响Mtdh的表达进而改善肾脏纤维化,具体机制有待于进一步研究。本研究结果揭示了ARB治疗肾脏纤维化中的新的分子机制。
| [1] |
Liu ZH. Nephrology in China[J].
Nat Rev Nephrol, 2013, 9(9): 523-8.
DOI: 10.1038/nrneph.2013.146. |
| [2] |
Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J].
Lancet, 2012, 379(9818): 815-22.
DOI: 10.1016/S0140-6736(12)60033-6. |
| [3] |
Meguid EA, Bello AK. Chronic kidney disease: the global challenge[J].
Lancet, 2005, 365(9456): 331-40.
DOI: 10.1016/S0140-6736(05)70199-9. |
| [4] |
Sugimoto H, Lebleu VS, Bosukonda D, et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis[J].
Nat Med, 2012, 18(3): 396-404.
DOI: 10.1038/nm.2629. |
| [5] |
Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention[J].
J Am Soc Nephrol, 2004, 15(1): 1-12.
|
| [6] |
Meyer TW. Tubular injury in glomerular disease[J].
Kidney Int, 2003, 63(2): 774-87.
DOI: 10.1046/j.1523-1755.2003.00795.x. |
| [7] |
Schuttert JB, Liu MH, Gliem N, et al. Human renal fibroblasts derived from normal and fibrotic kidneys show differences in increase of extracellular matrix synthesis and cell proliferation upon angiotensin Ⅱ exposure[J].
Pflugers Arch, 2003, 446(3): 387-93.
DOI: 10.1007/s00424-003-1026-y. |
| [8] |
Su ZZ, Kang DC, Chen Y, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH[J].
Oncogene, 2002, 21(22): 3592-602.
DOI: 10.1038/sj.onc.1205445. |
| [9] |
Wei JL, Li ZR, Chen WF, et al. AEG-1 participates in TGF-beta1- induced EMT through p38 MAPK activation[J].
Cell Biol Int, 2013, 37(9): 1016-21.
DOI: 10.1002/cbin.v37.9. |
| [10] |
Zou MJ, Zhu W, Wang L, et al. AEG-1/MTDH-activated autophagy enhances human malignant glioma susceptibility to TGF-beta 1- triggered epithelial-mesenchymal transition[J].
Oncotarget, 2016, 7(11): 13122-38.
|
| [11] |
Mikami D, Kimura H, Kamiyama K, et al. Telmisartan activates endogenous peroxisome proliferator-activated receptor-delta and May have anti-fibrotic effects in human mesangial cells[J].
Hypertens Res, 2014, 37(5): 422-31.
DOI: 10.1038/hr.2013.157. |
| [12] |
Wang J, Pang T, Hafko R, et al. Telmisartan ameliorates glutamateinduced neurotoxicity: Roles of AT(1) receptor blockade and PPAR gamma activation[J].
Neuropharmacology, 2014, 79: 249-61.
DOI: 10.1016/j.neuropharm.2013.11.022. |
| [13] |
Michel MC, Brunner HR, Foster C, et al. Angiotensin Ⅱ type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease[J].
Pharmacol Ther, 2016, 164: 1-81.
DOI: 10.1016/j.pharmthera.2016.03.019. |
| [14] |
Yao YF, Li Y, Zeng XF, et al. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (EMT) under highfat diet-induced hyperglycemia[J].
Front Pharmacol, 2018, 9: 1213.
DOI: 10.3389/fphar.2018.01213. |
| [15] |
Liu WT, Peng FF, Li HY, et al. Metadherin facilitates podocyte apoptosis in diabetic nephropathy[J].
Cell Death Dis, 2016, 7(11): e2477.
DOI: 10.1038/cddis.2016.335. |
| [16] |
Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis[J].
Cancer Cell, 2004, 5(4): 365-74.
DOI: 10.1016/S1535-6108(04)00079-0. |
| [17] |
Britt DE, Yang DF, Yang DQ, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells[J].
Exp Cell Res, 2004, 300(1): 134-48.
DOI: 10.1016/j.yexcr.2004.06.026. |
| [18] |
Yoo BK, Emdad L, Lee SG, et al. Astrocyte elevated gene-1 (AEG- 1): A multifunctional regulator of normal and abnormal physiology[J].
Pharmacol Ther, 2011, 130(1): 1-8.
DOI: 10.1016/j.pharmthera.2011.01.008. |
| [19] |
Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: clinical significance[J].
Adv Cancer Res, 2013, 120: 39-74.
DOI: 10.1016/B978-0-12-401676-7.00002-4. |
| [20] |
Vartak-Sharma N, Nooka S, Ghorpade A. Astrocyte elevated gene-1 (AEG-1) and the A(E)Ging HIV/AIDS-HAND[J].
Prog Neurobiol, 2017, 157(SI): 133-57.
|
| [21] |
Li G, Wang Z, Ye J, et al. Uncontrolled inflammation induced by AEG-1 promotes gastric cancer and poor prognosis[J].
Cancer Res, 2014, 74(19): 5541-52.
DOI: 10.1158/0008-5472.CAN-14-0968. |
| [22] |
Srivastava J, Robertson CL, Ebeid KA, et al. A novel role of astrocyte elevated gene-1 (AEG-1) in regulating nonalcoholic steatohepatitis (NASH)[J].
Hepatology, 2017, 66(2): 466-80.
DOI: 10.1002/hep.v66.2. |
| [23] |
Dandona P, Kumar V, Aljada A, et al. Angiotensin Ⅱ receptor blocker valsartan suppresses reactive Oxygen species Generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: Evidence of an antiinflammatory action[J].
J Clin Endocrinol Metab, 2003, 88(9): 4496-501.
DOI: 10.1210/jc.2002-021836. |
| [24] |
Zhou GY, Cheung AK, Liu X, et al. Valsartan slows the progression of diabetic nephropathy in db/db mice via a reductiorr in podocyte injury, and renal oxidative stress and inflammation[J].
Clin Sci, 2014, 126(9/10): 707-20.
|
| [25] |
Lovisa S, Zeisberg M, Kalluri R. Partial Epithelial-to-Mesenchymal transition and other new mechanisms of kidney fibrosis[J].
Trends Endocrinol Metab, 2016, 27(10): 681-95.
DOI: 10.1016/j.tem.2016.06.004. |
| [26] |
Grande MT, Sánchez-Laorden B, López-Blau C, et al. Snail1- induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease[J].
Nat Med, 2015, 21(9): 989-97.
DOI: 10.1038/nm.3901. |
| [27] |
Lovisa S, Lebleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis[J].
Nat Med, 2015, 21(9): 998.
DOI: 10.1038/nm.3902. |
| [28] |
Nlandu-Khodo S, Neelisetty S, Phillips MA, et al. Blocking TGFbeta and beta-catenin epithelial crosstalk exacerbates CKD[J].
J Am Soc Nephrol, 2017, 28(12): 3490-503.
DOI: 10.1681/ASN.2016121351. |
| [29] |
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition[J].
Nat Rev Mol Cell Biol, 2014, 15(3): 178-96.
DOI: 10.1038/nrm3758. |
| [30] |
Molteni A, Wolfe LF, Ward WF, et al. Effect of an angiotensin Ⅱ receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alphaactomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis[J].
Curr Pharm Des, 2007, 13(13): 1307-16.
DOI: 10.2174/138161207780618777. |
| [31] |
El-Agroudy AE, Hassan NA, Foda MA, et al. Effect of angiotensin Ⅱ receptor blocker on plasma levels of TGF-beta 1 and interstitial fibrosis in hypertensive kidney transplant patients[J].
Am J Nephrol, 2003, 23(5): 300-6.
DOI: 10.1159/000072820. |
 2019, Vol. 39
2019, Vol. 39

