2. Department of Rheumatology, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, Guangzhou 510330, China
2. 南方医科大学中西医结合医院风湿科,广东 广州 510330
Behcet's disease (BD) is a systemic inflammatory disorder affecting multiple sites of the body. The most common symptoms of BD include painful mouth sores, genital sores, eye inflammation, and arthritis. BD also has vascular, gastrointestinal and neurological involvements that are associated with a high morbidity and mortality[1]. While the Turkish dermatologist Hulusi Behçet first described the symptoms of BD in 1937 (BD was then named after him) [2], the documentation of Huhuo disease, manifested by oral and genital ulceration with systemic signs, has long been available in Jingui Yaolue (Golden Chamber Synopsis), an ancient classic work of traditional Chinese medicine (TCM) by Zhang Zhongjing (AD. 150-219)[3]. Currently the treatment for BD aims at easing the symptoms, reducing inflammation, and regulating the immune system, but the quality of evidence regarding the treatment of Behçet's disease-related oral ulcers remains poor[4]; actually, few options of medications are available for BD treatment due to their adverse effects, poor efficacy, or drug resistance.
As one of the most widely used drugs for treatment of diabetes, metformin is recently reported to restore the balance between regulatory T (Treg) and Th17 cells in mouse models with liver cancer[5], rheumatoid arthritis[6], systemic lupus erythematosus [7], and inflammatory bowel disease[8]. We thus hypothesized that metformin might have a potential therapeutic value for autoimmune diseases including BD, since these diseases shared a common pathogenesis of Treg/Th17 imbalance [9, 10]. To test this hypothesis, we conducted this clinical trial and evaluated the clinical effectiveness of metformin for treatment of mucocutaneous type BD, and analyzed its effects on the Treg/Th17 axis and the adverse effects associated with the treatment.
PATIENTS AND METHODS Study design and ethical approvalThis perspective study was conducted between May, 2016 and Feburary, 2017 following a single-blinded, before-after design with approval by the Ethics Committee of Huadong Hospital Affiliated to Fudan University, in strict accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and all applicable laws and regulations in China. This study was registered in the Chinese Clinical Trial Registry (registration No.: ChiCTR-ONC-16009621). All the participants were enrolled on a voluntary basis and had provided written informed consent to participate in any study-related procedures.
PatientsThirty patients meeting the diagnostic criteria of BD according to the International Study Group for BD [11] and admitted in our hospital between May and October, 2016 were enrolled in the current study by two senior rheumatologists. All the patients underwent systemic examinations including cranial magnetic resonance imaging (MRI), chest computed tomography (CT), gastrointestinal endoscopy, abdominal ultrasonography, vascular ultrasonography for important vessels, and blood tests for liver and kidney functions, glucose, cancer markers, Tuberculosis, HBV, Treponema pallidum, HIV and autoantibodies to exclude organ involvements and other diseases including infections, diabetes mellitus, rheumatoid arthritis, cancer, Crohn's disease and ulcerative colitis. All the patients enrolled in this study only had skin manifestations, including oral ulceration, genital ulceration, and skin erythema nodosum. Keratitis, conjunctivitis and anterior uveitis could be present in their history, but posterior uveitis that might influence vision and thus required intensive treatment was excluded.
Fourteen male and 16 female patients were enrolled, whose age ranged from 19 to 51 years (mean 36 years). The course of disease ranged from 1 to 20 years with a mean of 8.2 years. The patients enrolled were naive to treatments or had withdrawn medications at least 4 weeks before the study.
TreatmentThe patients were treated with metformin (Glucophage) alone at a dose of 500 mg for 2 or 3 times a day based on their individual body weight and the severity of side effects. The treatment and the follow-up period both lasted 3 months. A statistician with medical background was invited to evaluate the changes in the clinical manifestations and record the blood test results of the patients before and after the treatment in a single-blinded manner.
Clinical manifestationsThe mucocutaneous activity index for BD (MAIBD) established by G Mumcu et al[12] was used to assess the disease activity before and after the treatment. The relapse frequency before and after the treatment, as well as the side effects during the treatment were recorded.
Laboratory assessmentsBefore and at 1 and 3 months during the treatment, the patients were examined for C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR, Westergren method) as the clinical inflammatory indexes. Red blood cell (RBC) count, hemoglobin (HGB), alanine amino transferase (ALT), aspartate transaminase (AST), total protein (TP), albumin (ALB), alkaline phosphatase (ALP), serum creatinine (Cr), serum urea (Urea), glomerular filtration rate (GFR) and blood glucose (Glu) were all recorded before and after treatment to investigate potential side effects of metformin.
Enzyme-linked immunosorbent assay (ELISA)Forkhead box P3 (Foxp3), interleukin-35 (IL-35), transforming growth factor-β (TGF-β), IL-17, and tumor necrosis factor-α (TNF-α) of the patients were detected using ELISA before and at 1 and 3 months during the treatment using ELISA kits (USCN Company) according to the manufacturer's protocols.
Quantitative real-time polymerase chain reaction (qRT-PCR)The mRNA expression levels of Foxp3, IL-35, TGF-β, retinoic acid receptor-related orphan receptor-γt (Ror-γt), IL-17, and TNF-α were quantified in the blood samples from patients before and at 1 and 3 months after the treatment. Briefly, the total RNAs were extracted from whole blood samples of the patients using RNAzol® BD (catalog number RB192, Molecular Research Center, Inc) following the manufacturer's protocol. The extracted total RNAs were reverse transcribed into cDNA using PrimeScriptTM RT Master Mix (Takara). qRT-PCR analysis was performed using the Applied Biosystems ViiATM 7 Real-Time PCR System (Wcgene Biotechnology, Shanghai) with the reaction mixture consisting of 5 μL of 2 × TB Green Premix Ex Taq Ⅱ (Takara), 3 μL of nuclease-free water, 1 μL of cDNA, 0.4 μL of each gene-specific primer and 0.2 μL of ROX reference dye. The target mRNAs were amplified individually in duplicate. The expression levels of the target mRNAs relative to GAPDH mRNA expression were calculated using the delta CT (ΔCT) method[13]. The primers for amplifying the target mRNAs are shown in Tab. 1.
| Tab.1 Sequences of qRT-PCR primers used in this study |
All the data of the continuous variables are presented as Mean±SD. One-way analysis of variance (ANOVA) was performed to test the changes in the variables after the treatment using GraphPad Prism 7 software (GraphPad Software, Inc., USA), and a P value less than 0.05 was deemed to indicate a statistically significant difference.
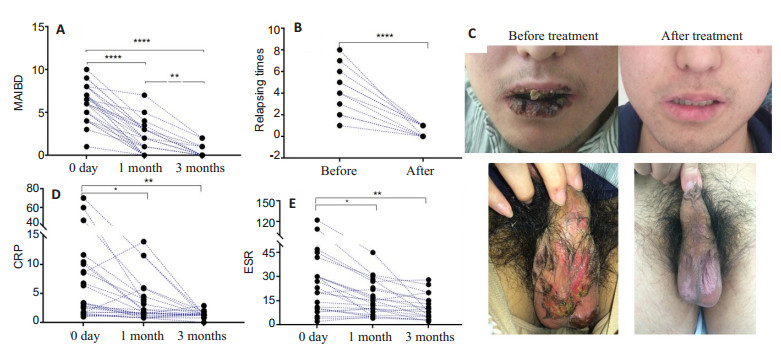
RESULTS Changes in clinical manifestationsFour of the 30 patients had withdrawn from the study in the first week of metformin treatment due to severe nausea and vomiting. In the remaining 26 patients, the overall favorable response rate was 88.46% (23/26); partial remission was achieved in 11.54% (3/26) of the patients, who were given other medications after the trial. At 1 month during the treatment, the MAIBD decreased significantly from 6.15±2.07 before treatment to 1.90±1.81 (P < 0.0001), and further to 0.30±0.63 at 3 months (Fig. 1A). The recurrent manifestations including oral and genital ulcerations and skin lesions were obviously relieved at 3 months (4.27±2.19 vs 0.45±0.52, P < 0.0001; Fig. 1B).
|
Fig.1 Comparison of ESR and CRP levels before and after treatment. A, B: MAIBD and relapse frequency of patients were decreased after metformin treatment; C: Oral and genital ulcerations were relieved after 1 week of metformin treatment. D, E: Clinical inflammatory indexes of CRP and ESR were significant decreased after metformin treatment. *P < 0.05, **P < 0.01, ****P < 0.0001 (n=30 before treatment, n=26 at 1 and 3 months) |
In a typical case, a 53 year-old male patient complained of oral and genital ulceration in the past 20 years with 2 or 3 attacks in a year. Ulcerations took more than 1 month to recover after the application of glucocorticoid. The patient was also diagnosed to have impaired glucose tolerance, for which no specific medications had been administered. Upon admission in our hospital, the patient presented with extensive mouth and genital sores, and after 1 week of metformin treatment at the dose of 500 mg twice a day, the lesions were obviously relieved (Fig. 1C). The patient required further metformin treatment for 2 years after the trial, and so far experienced no recurrence.
Among the 26 patients who completed the study, the objective disease activity indexes of CRP and ESR decreased markedly at 1 month during the treatment compared with that before treatment (CRP: 11.57 ± 18.19 mg/L vs 3.73±4.23 mg/L, P < 0.05; ESR: 29.44± 29.88 mm/h vs 15.4±10.52 mm/h, P < 0.05). At 3 months, the mean CRP and ESR levels of the patients further decreased to 1.35 ± 0.55 mg/L and 10.75 ± 7.63 mm/h, respectively, significantly lower than those before treatment (P < 0.01; Fig. 1D, E).
Safety assessmentsOf the 30 patients enrolled, 8 (26.7%) reported nausea, vomiting or poor appetite after taking metformin, and 4 of them dropped out of the study because of intolerance of these side effects. In addition, 3 (10.0%) patients experienced fatigue, and 6 (20.0%) reported loose stool and mild diarrhea. All the 26 patients who completed the study well tolerated the side effects of metformin within 10-15 days. Follow-up laboratory tests did not reveal the occurrence of anemia or any damage of liver or kidney functions in these patients; metformin was not found to affect normal blood glucose levels (Tab. 2).
| Tab.2 Laboratory test results of the patients before and at 1 and 3 months during metformin treatment (Mean±SD) |
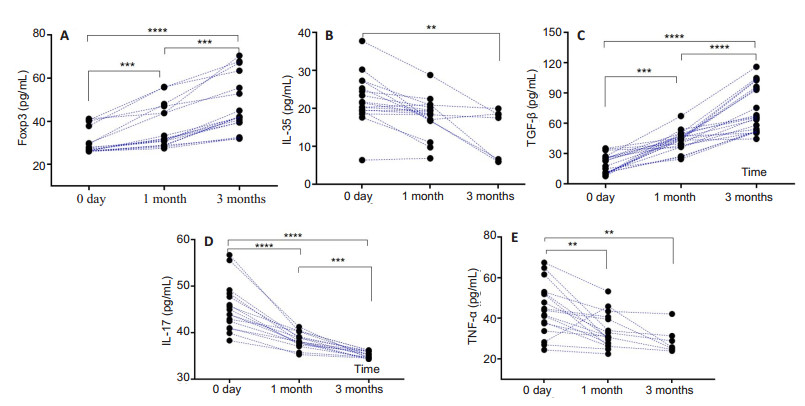
ELISA analysis showed that metformin treatment for 1 and 3 months significantly increased the protein levels of Foxp3 and TGF-β and lowered the expression levels of IL-35, IL-17 and TNF-α in the patients compared with the levels before treatment (P < 0.01; Fig. 2).
|
Fig.2 Changes of Treg/Th17-related cytokines, Foxp3 and TNF-ɑ after metformin treatment for 1 and 3 months in patients with BD (n=30 before treatment, n=26 at 1 and 3 months). **P < 0.01, ***P < 0.001, ****P < 0.0001 |
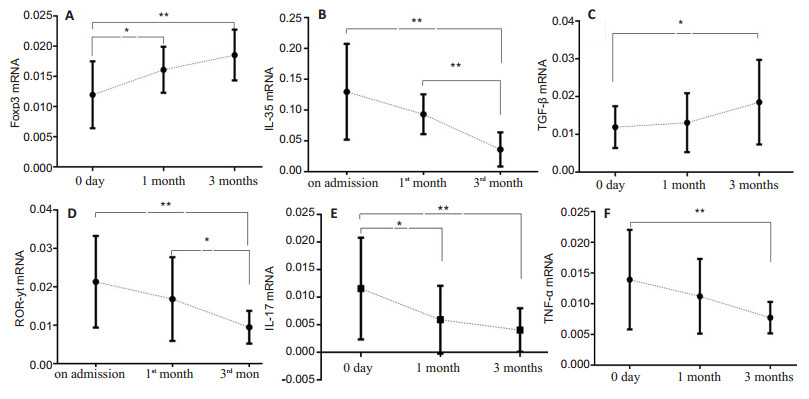
The results of qRT-PCR also demonstrated significantly increased expression levels of Foxp3 and TGF-β mRNA and decreased expression levels of IL-35, ROR-γt, IL-17 and TNF-α mRNA in the patients after metformin treatment for 1 and 3 months (P < 0.05; Fig. 3).
|
Fig.3 Relative expression levels of Treg/Th17 axis-related mRNAs in BD patients before (n=30) and after metformin treatment (n=26 at 1 and 3 months). *P < 0.05, **P < 0.01 |
Metformin has become the most widely used first-line drug for the treatment of type 2 diabetes mellitus [14]. Accumulating evidence has suggested that metformin can potentially prevent cardiovascular diseases [15], cancer complications of diabetes[16] as well as a wide range of other conditions. Inflammation is believed to play important roles in various diseases ranging from diabetes[17], atherosclerosis[18], cancers[19], chronic obstructive pulmonary disease [20], and Alzheimer's disease [21] to such autoimmune diseases as BD. Metformin may attenuate inflammatory reactions in these diseases and thus provides potential benefits. In this prospective study, we observed favorable responses in 88.46% of the patients with BD after metformin treatment, and the remaining 11.54% patients showed partial remission. Metformin treatment obviously decreased the levels of CRP and ESR in these patients at 1 month, and treatment for another 2 months further decreased CRP level and ESR without causing anemia or adverse effects on liver and kidney functions.
Treg cells play important roles in maintaining immune tolerance and suppressing pathological immune responses against self and foreign antigens through the effector molecules such as IL-10, TGF-β and IL-35[22]. Th17 cells in a healthy immune system can recognize and eliminate the potential harmful non-self pathogens, which is critical for the clearance of extracellular pathogens. But under pathological conditions, Th17 cells and their effector molecules, including IL-17, IL-21, IL-22, GM-CSF, and CCL20, are involved in the pathogenesis of autoimmune and inflammatory diseases[23]. Decreased Treg cells and increased Th17 cells, along with the cytokines they produce, have been shown to contribute to the pathogenesis of BD[24, 25].
Known for its immune regulatory activity, metformin can restore the balance of Treg/Th17 axis in multiple inflammation-related disorders. Kang et al reported that metformin could attenuate arthritis scores and bone destruction in a mouse model of collagen antibody-induced arthritis (CAIA) [7]. They found that metformin could significantly lower the serum levels of inflammatory cytokines including IL-17, TNF-a and IL-6 and reduce the number of Th17 cells in axillary draining lymph nodes, and confirmed that stimulation of CD4+ T cells with metformin in vitro dose-dependently reduced Th17 differentiation and down-regulated the expressions of the transcriptional factors of Th17 (STAT3 and RORγt). These findings indicate that the anti-inflammatory effect of metformin is achieved by inhibiting the differentiation of Th17 through the AMPK-mTOR pathway[6]. Metformin is also shown to suppress tumor growth, decrease IL-22 level, inhibit IL-22-induced STAT3 phosphorylation and its downstream genes Bcl-2 and cyclin D1, and suppress the de novo generation of Th1 and Th17 cells from naive CD4+ cells[5].
The disturbed Treg/Th17 axis participates in the pathogenesis of BD and CD4+ CD25+ FOXP3+ Treg and CD4+ FOXP3+ Treg are negatively correlated with the disease activity. The patients with clinically active BD are found to have decreased peripheral blood regulatory T cells [26]. Shimizu et al reported that Th17 cells were increased in BD patients in response to various inflammatory cytokines, and the pro-inflammatory cytokines (IL-1β, TNF-α, and IL-23) may be associated with Th17 cell proliferation[27]. Our findings suggest that metformin can restore Treg/Th17 balance in BD patients. Both ELISA and qRT-PCR results indicated that metformin treatment increased the protein and mRNA expression levels of Foxp3 and TGF-β in these patients. Foxp3 is a specific marker of natural T regulatory cells (nTregs) and adaptive/induced T regulatory cells (a/iTregs). Typically, Tregs that express Foxp3 are critical in the transfer of immune tolerance, especially for the self-tolerance[28]. TGF-β, which is secreted by multiple cell types including Foxp3+ Treg cells, executes important immune regulatory function. TGF-β1 has suppressive actions on different immune cells, such as T cells, B cells and macrophages; an increased TGF-β1 production is correlated with the protection against and/or recovery from autoimmune diseases[29].
IL-35 is secreted by regulatory Treg cells, which can suppress the inflammatory responses of immune cells. However, IL-35 is not constitutively expressed in tissues, and the gene encoding IL-35 is transcribed by vascular endothelial cells, smooth muscle cells and monocytes after activation upon pro-inflammatory stimuli[30]. Studies in mice showed that the absence of IL-35 chain from regulatory Tregs reduced the capacity for cells to suppress inflammation [31]. IL-35 can also induce the proliferation of Treg cell populations while reducing the activity of Th17 cell populations[32]. In this study, IL-35 level was decreased in the patients after disease remission, probably indicating that the remission of inflammatory reaction lowered IL-35 to a non-stimulated level. Nevertheless, the changes in IL-35 was not parallel with those of the Treg cell marker Foxp3 and the cytokine TGF-β, and the exact mechanism of IL-35 in human body remains unclear. We also detected lowered mRNA expression levels of RORγt (the marker of Th17 cells) and reduced expressions of IL-17 and TNF-α at both the protein and mRNA levels in the patients.
We recorded adverse events associated with metformin in 8 patients such as nausea and vomiting. The majority of the patients well tolerated these side effects and benefited from metformin treatment with obviously improved symptoms. We did not design a placebo control group in this study; but as pain and difficulty in eating caused by oral ulcerations can not possibly be relieved by a placebo, we believe that the improvements in the symptoms of the patients are indeed the results of metformin treatment. Importantly, the decreased CRP, ESR and Th17 cell marker and increased Treg cell markers provide laboratory evidence to support the effectiveness of metformin for treatment of BD. Still, the inconsistency between the changes of IL-35 secreted by Treg cells and the variations of the Treg cell markers Foxp3 and TGF-β following metformin treatment awaits further investigation.
| [1] | Chen Y, Cui JS, Cai JF, et al. Surgical intervention for behcet's disease with aorta aneurysm and pseudoaneurysm: opposite outcomes in two cases[J]. Chin Med J, 2017, 130(20): 2503-5. DOI: 10.4103/0366-6999.216414. |
| [2] | Mendes D, Correia M, Barbedo M, et al. Behcet's disease--a contemporary review[J]. J Autoimmun, 2009, 32(3-4): 178-88. DOI: 10.1016/j.jaut.2009.02.011. |
| [3] | Chen Y, Shen Y, Ma HF, et al. Infliximab associated with life-threatening lung infection in a patient with Behcet disease with intestinal and hematopoietic system involvement: a case report[J]. Medicine, 2017, 96(50): e9202. DOI: 10.1097/MD.0000000000009202. |
| [4] | Taylor J, Glenny AM, Walsh T, et al. Interventions for the management of oral ulcers in Behçet's disease[J]. Cochrane Database Syst Rev, 2014, 25(9): CD011018. |
| [5] | Zhao D, Long XD, Lu TF, et al. Metformin decreases IL-22 secretion to suppress tumor growth in an orthotopic mouse model of hepatocellular carcinoma[J]. Int J Cancer, 2015, 136(11): 2556-65. DOI: 10.1002/ijc.v136.11. |
| [6] | Kang KY, Kim YK, Yi H, et al. Metformin downregulates TH17 differentiation and attenuates murine autoimmune arthritis[J]. Int Immunopharmacol, 2013, 16(1): 85-92. DOI: 10.1016/j.intimp.2013.03.020. |
| [7] | Lee SY, Moon SJ, Kim EK, et al. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/ STAT3[J]. J Immunol, 2017, 198(7): 2661-72. DOI: 10.4049/jimmunol.1403088. |
| [8] | Lee SY, Lee SH, Yang EJ, et al. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance[J]. Plos One, 2015, 10(9): e135858. |
| [9] | Kim JR, Chae JN, Kim SH, et al. Subpopulations of regulatory T cells in rheumatoid arthritis, systemic lupus erythematosus, and Behcet's disease[J]. J Korean Med Sci, 2012, 27(9): 1009-13. DOI: 10.3346/jkms.2012.27.9.1009. |
| [10] | Touzot M, Cacoub P, Bodaghi B, et al. IFN-α induces IL-10 production and tilt the balance between Th1 and Th17 in Behçet disease[J]. Autoimmun Rev, 2015, 14(5): 370-81. DOI: 10.1016/j.autrev.2014.12.009. |
| [11] | Wechsler B, Davatchi F, Mizushima Y, et al. Criteria for diagnosis of Behçet's disease. International Study Group for Behcet's Disease[J]. Lancet, 1990, 335: 1078-80. |
| [12] | Mumcu G, Inanc N, Taze A, et al. A new mucocutaneous activity index for Behcet's disease[J]. Clin Expe Rheumatol, 2014, 32(4 Suppl 84): S80-6. |
| [13] | Luthra R, Singh R M, Li Y, et al. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers[J]. Oncogene, 2008, 27(52): 6667-72. DOI: 10.1038/onc.2008.256. |
| [14] | Sanchez Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes[J]. Diabetologia, 2017: 1-8. |
| [15] | Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study[J]. Ann Intern Med, 2012, 157(9): 601-10. DOI: 10.7326/0003-4819-157-9-201211060-00003. |
| [16] | Malek M, Aghili R, Emami Z, et al. Risk of Cancer in Diabetes: The effect of metformin[J]. Isrn Endocrinolo, 2013, 2013(2013): 636927-36. |
| [17] | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes[J]. J Clin Invest, 2005, 115(5): 1111-9. DOI: 10.1172/JCI25102. |
| [18] | Libby P. Inflammation in atherosclerosis[J]. Arch Cardio Mexico, 2002, 48(2): 265-6. |
| [19] | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-99. DOI: 10.1016/j.cell.2010.01.025. |
| [20] | Colarusso C, Terlizzi M, Molino A, et al. Role of the inflammasome in chronic obstructive pulmonary disease (COPD)[J]. Oncotarget, 2017, 8(47): 81813-24. |
| [21] | Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease[J]. Nat Rev Neurosci, 2015, 16(6): 358-72. DOI: 10.1038/nrn3880. |
| [22] | Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function[J]. Nature, 2007, 450(7169): 566-9. DOI: 10.1038/nature06306. |
| [23] | Maddur MS, Miossec P, Kaveri SV, et al. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies[J]. Am J Pathol, 2012, 181(1): 8-18. DOI: 10.1016/j.ajpath.2012.03.044. |
| [24] | Pekiner FN, Aytugar E, Demirel GY, et al. Interleukin-2, interleukin-6 and T regulatory cells in peripheral blood of patients with Behcet's disease and recurrent aphthous ulcerations[J]. J Oral Pathol Med, 2012, 41(1): 73-9. DOI: 10.1111/jop.2011.41.issue-1. |
| [25] | Na SY, Park MJ, Park S, et al. Up-regulation of Th17 and related cytokines in Behcet's disease corresponding to disease activity[J]. Clin Exp Rheumatol, 2013, 31(3 Suppl 77): 32-40. |
| [26] | Gündüz E, Teke HÜ, Bilge NY, et al. Regulatory T cells in Behçet's disease: Is there a correlation with disease activity? Does regulatory T cell type matter?[J]. Rheumatol Int, 2013, 33(12): 3049-54. DOI: 10.1007/s00296-013-2835-8. |
| [27] | Shimizu J, Takai K, Takada E, et al. Possible association of proinflammatory cytokines including IL1β and TNFα with enhanced Th17 cell differentiation in patients with Behcet's disease[J]. Clin Rheumatol, 2015, 35(7): 1857-63. |
| [28] | Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function[J]. Annu Rev Immunol, 2012, 30(1): 531-64. DOI: 10.1146/annurev.immunol.25.022106.141623. |
| [29] | Prud'Homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-β1) in autoimmune diseases[J]. J Autoimmun, 2000, 14(1): 23-42. DOI: 10.1006/jaut.1999.0339. |
| [30] | Li X, Mai J, Virtue A, et al. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines[J]. Plos One, 2012, 7(3): e33628. DOI: 10.1371/journal.pone.0033628. |
| [31] | Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function[J]. Nature, 2007, 450(7169): 566-9. DOI: 10.1038/nature06306. |
| [32] | Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells[J]. Eur J Immunol, 2007, 37(11): 3021-9. DOI: 10.1002/(ISSN)1521-4141. |
 2019, Vol. 39
2019, Vol. 39

