肝细胞癌(HCC)是世界第六大常见恶性肿瘤[1]。流行病学数据显示, 全球每年新发HCC病例超过78万, 其中我国约占50%[2]。HCC已成为肿瘤相关死亡的第二大原因[3-4]。近年来, 尽管HCC的诊治已取得巨大进步, 但其远期生存率仍欠佳[5-6]。原因在于大多数患者在确诊时已属晚期。索拉菲尼是晚期HCC的标准治疗, 能够延长患者的生存期, 但总体效果仍欠佳[7-9]。HCC的分子靶向治疗是目前研究的热点[9-13]。极光激酶(AURK)在调控细胞周期方面起重要作用[14-15]。极光激酶A和B (AURKA、AURKB)在肝癌组织中存在过度表达, 与肝癌的侵袭性、术后复发、化疗耐药及预后欠佳等相关[16-20]。因此, AURKA及AURKB可作为潜在的抗肝癌靶点[21-23]。Danusertib (Danu)是近年来新合成的小分子化合物, 对AURK具有良好的抑制作用[24-26]。但Danu对HCC的作用和机制尚不明确。本研究旨在探讨Danu对HCC细胞株HepG2的增殖、细胞周期、凋亡和自噬的影响及其机制, 为体内实验奠定基础。
1 材料和方法 1.1 主要材料和试剂极光激酶抑制剂Danu (Selleck), DMEM培养基(Corning Cellgro)。胎牛血清、碘化丙啶(PI)、核糖核酸酶A、MTT、DMSO (Sigma), 自噬检测试剂盒Cyto-ID® (ENZ-51031-K200)(Enzo Life Sciences)。Annexin V/ PE细胞凋亡检测试剂盒(BD Biosciences)。RIPA蛋白裂解液、磷酸酶抑制剂、蛋白酶抑制剂、BCA蛋白定量试剂盒、SDS-PAGE配制试剂、显影液(Thermo Scientific)。聚偏氟乙烯膜(PVDF)(Bio-Rad), 电泳仪、转膜仪及成像系统(ChemiDocTM XRS)(Bio-Rad)。流式细胞仪为Becton Dickinson Immunocytometry系统。细胞周期相关蛋白抗体如Cyclin B1、CDK1/CDC2、p21、p53, 细胞凋亡相关蛋白抗体如细胞色素C (cytochrome C)、Bax、Bcl-xl、Bcl-2、cleaved caspase-3、cleaved caspase-9、cleaved PARP、PUMA, 以及自噬相关蛋白抗体如Beclin 1、LC3-I和LC3-II、PI3K、p-PI3K (Tyr458)、Akt、p-Akt (Ser473)、mTOR、p-mTOR (Ser2448)、PTEN、AMPK、p-AMPK (Thr172)等(CST)。β-actin及二抗(Santa Cruz)。
1.2 细胞、细胞培养及药物浓度人肝癌细胞株HepG2(ATCC), 用含10%胎牛血清、1×105 U/L青霉素和100 mg/L的DMEM培养基, 置于37℃、5% CO2培养箱中常规培养。取对数生长期细胞用于实验。当细胞生长密度在75%左右时加药处理。Danu母液终浓度为50 mmol, 用新鲜DMEM培养基配制不同浓度的溶液。浓度效应实验药物浓度设为0.01、0.1、0.5 μmol, 作用时间24 h。时间效应实验设4、8、12、24、48、72 h组, Danu浓度0.5 μmol。
1.3 MTT法检测Danu对HepG2细胞的增殖抑制作用实验方法主要参照已发表文献[27]。实验组药物浓度为0.01、0.1、0.5、1、5、25和50 μmol。每组设6个复孔, 药物作用时间为24 h或48 h。
1.4 流式细胞仪检测Danu对HepG2细胞周期、凋亡和自噬的影响实验方法主要参照已发表文献[27]。药物作用足够时间后收集细胞, 随后用流式细胞仪进行检测。细胞周期实验用PBS-PI液染色。凋亡实验用PE和7AAD进行染色(按Annexin V/PE凋亡试剂盒说明书进行)。自噬实验用Cyto-ID®进行孵育(按Cyto-ID®自噬检测试剂盒说明书进行)。
1.5 Western blot检测Danu对HepG2细胞周期、凋亡及自噬相关蛋白表达的影响实验方法主要参照已发表文献[27]。在ChemiDocTM XRS成像系统上加入化学发光底物, 曝光显影。用Image Lab 3.0和Image J软件对条带进行分析。
1.6 MTT法检测抑制自噬后HepG2细胞的活力细胞接种同前。实验组设单纯Danu (0.5 µmol)组、单纯氯喹组(CQ组, 20 µmol)及联合组(0.5 µmol Danu+20 µmol CQ), 对照组不加药物。药物作用时间24 h。联合组药物同时孵育。余步骤同前。
1.7 统计学方法采用GraphPad Prism 7软件进行绘图和统计处理。实验结果以均数±标准差表示。多组间数据的比较采用完全随机设计方差分析(ANOVA, Tukey's), P < 0.05认为差异具有统计学意义。
2 结果 2.1 Danu对细胞增殖的影响Danu作用24 h和48 h后, HepG2细胞的增殖受到显著抑制。24 h和48 h的IC50分别为39.4 μmol和14.4 μmol (图 1)。
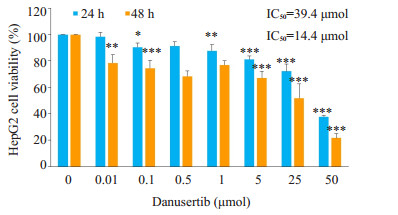
|
图 1 MTT法检测不同浓度Danu对HepG2细胞增殖活力的影响 Fig.1 Effect of danusertib at different concentrations on the viability of HepG2 cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs control (0 μmol) group. |
Danu能够导致HepG2细胞出现G2/M周期阻滞和异倍体, 呈浓度和时间依赖。Danu作用细胞24 h后, 0.1 μmol组及0.5 μmol组G2/M期细胞比例分别为58.2%和66.5%, 异倍体比例分别为51.9%和44.5%, 显著高于对照组(图 2)。0.5 μmol Danu作用时间超过4 h后, G2/M期细胞及异倍体比例逐渐升高, 和对照组相比, 均具有统计学意义(图 3)。
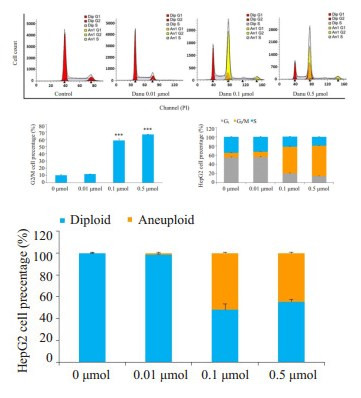
|
图 2 流式细胞仪检测不同浓度Danu作用24 h后HepG2细胞周期的变化 Fig.2 Effect of treatment with danusertib at different concentrations for 24 h on cell cycle distribution of HepG2 cells. ***P < 0.001 vs 0 μmol group. Dip: Diploid; An1: Aneuploid. |
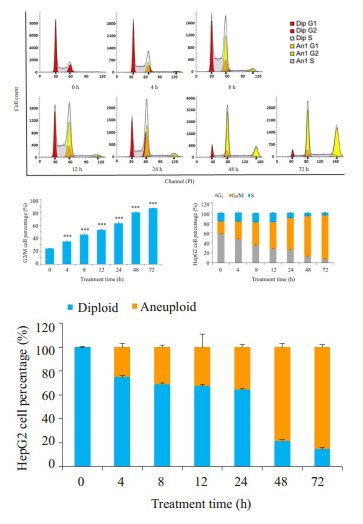
|
图 3 流式细胞仪检测0.5 μmol的Danu作用不同时间后HepG2细胞周期的变化 Fig.3 Effect of 0.5 μmol danusertib treatment for 0-72 h on cell cycle distribution of HepG2 cells. ***P < 0.001 vs 0 μmol group. |
0.5 μmol Danu能使肝癌细胞Cyclin B1及CDC2的表达下调41.3%和30.4%。0.1 μmol及0.5 μmol Danu使p53的表达上调96.4%和74.3%, p21的表达则被上调1.2倍和1.95倍(图 4)。
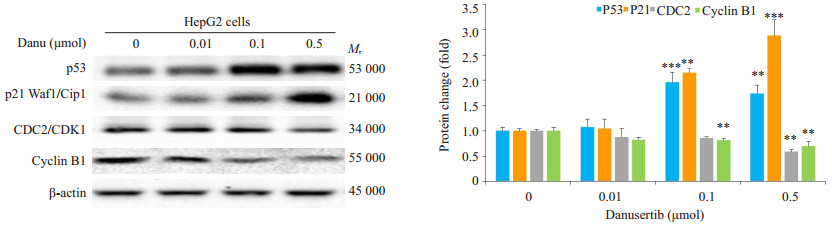
|
图 4 Western blot检测不同浓度Danu作用24 h后Cyclin B1、CDC2、p53及p21表达的变化 Fig.4 Effect of danusertib at different concentrations on expressions of cyclin B1, CDC2, p53 and p21 in HepG2 cells. **P < 0.01, ***P < 0.001 vs 0 μmol group. |
Danu能够诱导HepG2细胞凋亡, 呈浓度和时间依赖。Danu作用细胞24 h后, 0.1 μmol组及0.5 μmol组的总凋亡率分别为10.8%和12.0%, 显著高于对照组(4.9%)。0.5 μmol Danu作用24、48、72 h后, 实验组的凋亡率分别为3.9%、4.1%和11.4%, 和对照组(1.1%)相比, 具有统计学意义(图 5)。
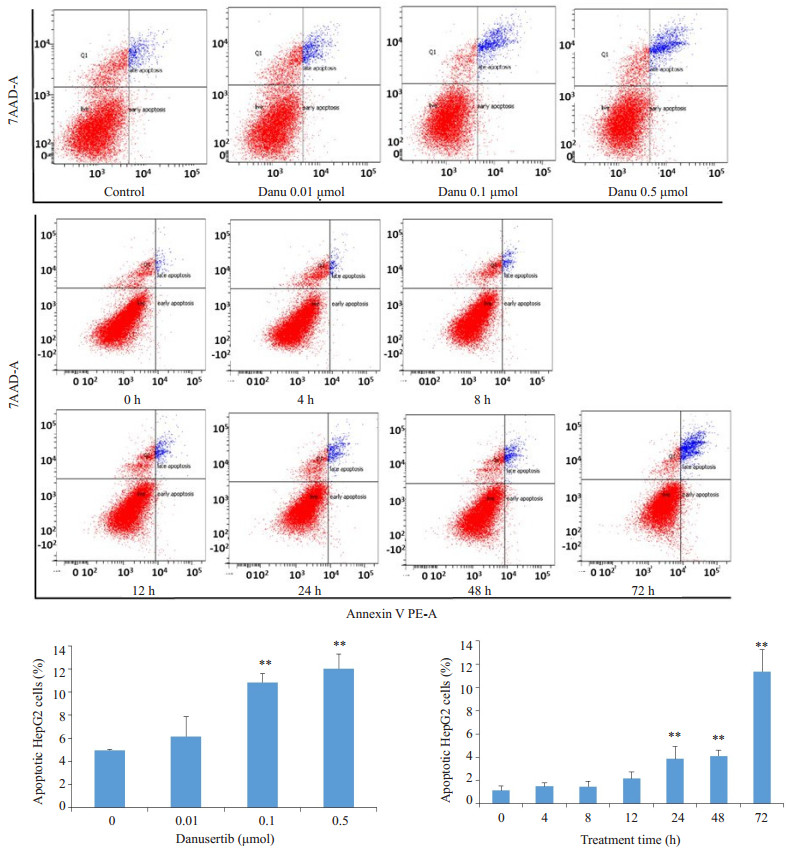
|
图 5 流式细胞仪检测不同浓度的Danu及不同作用时间对HepG2细胞凋亡的影响 Fig.5 Effect of danusertib on apoptosis of HepG2 cells. **P < 0.01 vs 0 μmol group. |
0.5 μmol Danu作用24 h能使cleaved caspase-3、cleaved caspase-9及cleaved PARP的表达分别上调72.4%、65.1%和42.3%, 促凋亡蛋白Bax和Puma的表达分别上调0.6和0.9倍, 凋亡抑制蛋白Bcl-xl和Bcl-2的表达则分别下调31.0%和45.5%。0.1 μmol及0.5 μmol组的胞浆细胞色素C表达升高0.9倍和1.5倍(图 6)。
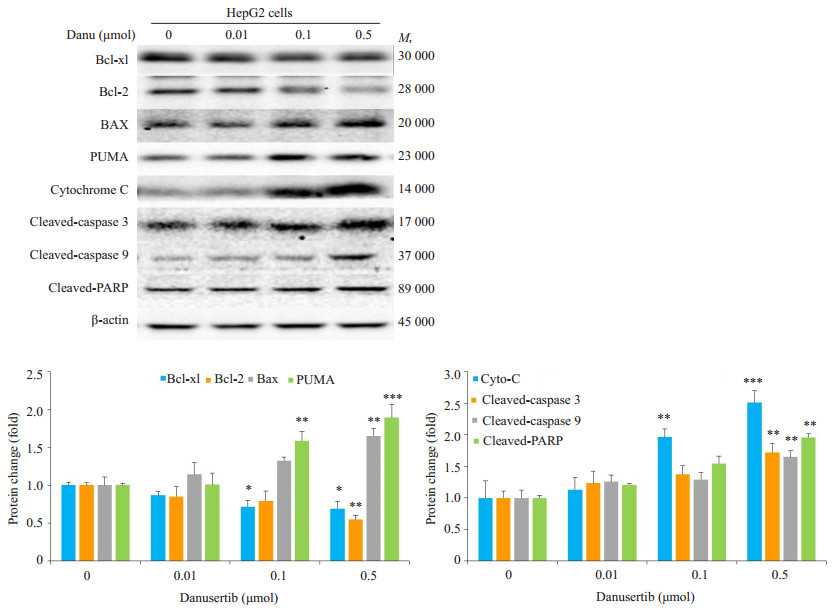
|
图 6 Western blot检测不同浓度Danu作用24 h后凋亡相关蛋白表达的变化 Fig.6 Effect of danusertib at different concentrations on expressions of apoptosis-related proteins. *P < 0.05, **P < 0.01, ***P < 0.001 vs 0 μmol group. |
Danu能够导致HepG2细胞发生自噬, 呈浓度和时间依赖。Danu作用细胞24 h后, 0.1 μmol组及0.5 μmol组的自噬细胞比例分别为21.5%和30.0%, 显著高于8.9%。0.5 μmol Danu作用12、24、48及72 h后, 实验组的凋亡率分别为3.2%、15.2%、23.8%和30.7%, 和对照组(8.6%)相比, 具有统计学意义(图 7)。
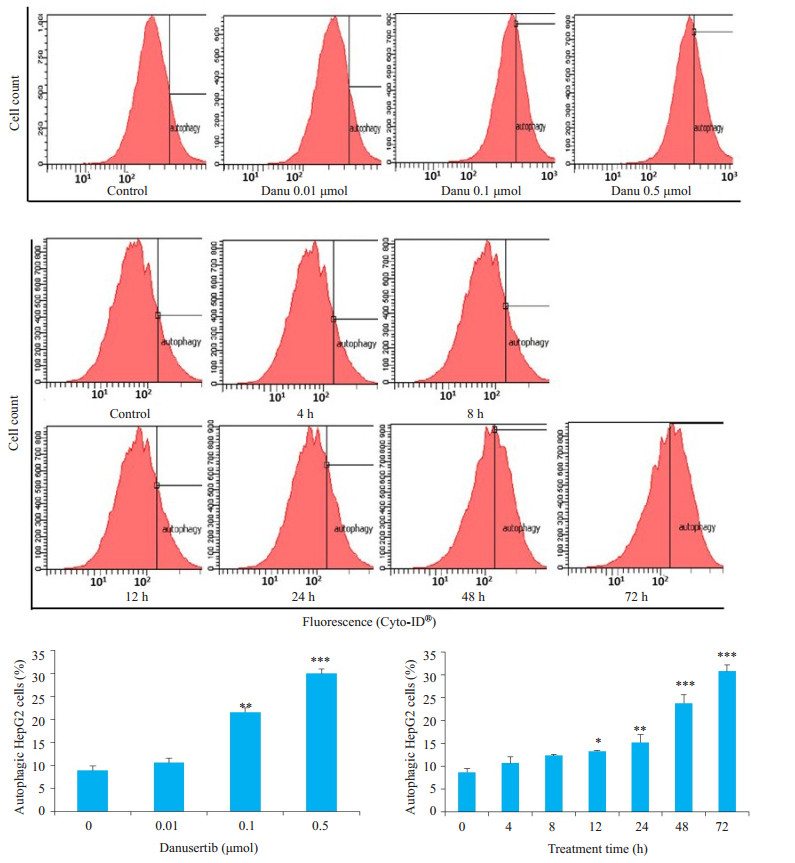
|
图 7 流式细胞仪检测不同浓度Danu及不同作用时间对HepG2细胞自噬的影响 Fig.7 Effect of danusertib at different concentrations and for different durations on autophagy of HepG2. *P < 0.05, **P < 0.01, ***P < 0.001 vs 0 μmol group. |
0.5 μM Danu使自噬标志蛋白Beclin 1的表达上调38.2%, 使LC3-II/LC3-I的比值上调40.7%。p-PI3K/ PI3K、p-AKT/AKT及p-mTOR/mTOR的比值分别被下调54.0%、28.2%和41.6%。PI3K/Akt/mTOR通路的负调节蛋白PTEN则被上调51.5%。p-AMPK/AMPK的比值亦被上调71.0%(图 8)。
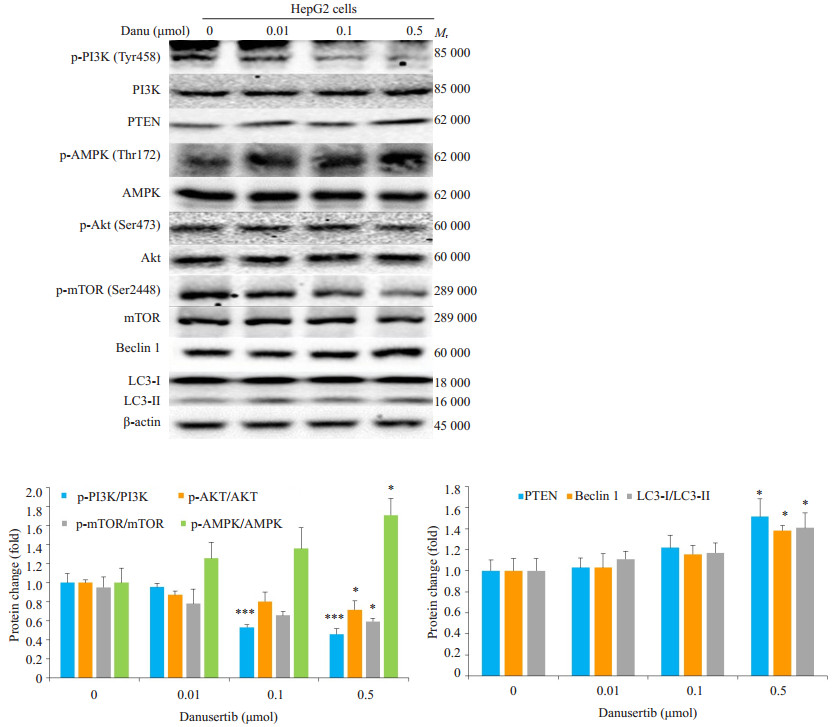
|
图 8 Western blot检测不同浓度Danu作用24 h后自噬相关蛋白表达的变化 Fig.8 Effect of danusertib treatment at different concentrations for 24 h on expressions of autophagy-related proteins. P < 0.05, ***P < 0.001 vs 0 μmol group. |
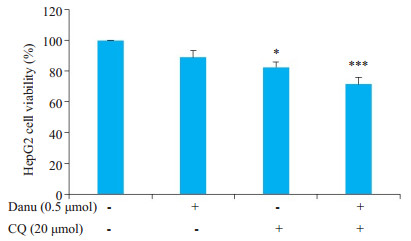
采用氯喹(20 μmol)抑制自噬后, 联合组细胞活力为72.0%, 显著低于Danu单药组(88.9%)以及氯喹单药组(82.4%)(图 9)。可见, 抑制自噬可增强Danu杀灭细胞的作用, 说明Danu所引起的自噬起到了保护细胞的作用。
|
图 9 MTT法检测采用氯喹抑制自噬后HepG2细胞的活力 Fig.9 Effect of 0.5 μmol danusertib on the viability of HegG2 cells after chloroquine-mediated autophagy inhibition. *P < 0.05, ***P < 0.001 vs 0 μmol group. |
中晚期HCC的生存期短仍旧是不争的事实[4, 28]。索拉菲尼在延长进展期HCC患者生存期方面具有一定优势, 但总体效果仍欠佳[5, 6, 29]。研发新药、寻求新的治疗方案是临床上亟待解决的难题[30]。研究表明, AURK在肝癌组织中的过度表达具有重要的临床意义[16-18], AURKA及AURKB可作为抗肝癌靶点。临床前研究和部分临床研究显示Danu对多种肿瘤具有较好的抗肿瘤效果[31-33], 本项Danu体外抗HCC实验表明, Danu能够有效抑制细胞增殖, 通过调控p53及p21的表达引起细胞出现G2/M细胞周期阻滞和异倍体, 通过活化线粒体依赖通路诱导细胞出现凋亡, 并通过抑制PI3K/Akt/mTOR及活化AMPK通路诱导细胞发生自噬。
AURK属于丝氨酸-苏氨酸激酶, 在调控细胞周期方面起重要作用[34]。本实验结果表明, Danu是通过抑制AURK和p53-p21信号轴来影响HepG2细胞周期分布的。AURK活性受到抑制后, 中心体成熟受限, 纺锤体非正确组装并延迟分裂退出, 最终导致HepG2细胞阻滞于G2/M期。此外, 胞质分裂被阻止, 导致异倍体的出现。Danu上调了p53和p21的表达, 进而抑制Cyclin B1-CDC2复合体的活性, 最终导致HepG2细胞阻滞于G2/M期。
凋亡和自噬是癌症细胞程序性死亡的两种形式, 凋亡主要受外源性死亡受体通路和线粒体介导通路的调控[35]。本研究显示, Danu是通过活化线粒体介导通路诱导HepG2细胞凋亡的。促凋亡蛋白Bax和凋亡抑制蛋白Bcl-xl及Bcl-2在Danu的作用下表达失衡, 进而引起细胞色素C从线粒体释放至胞浆中, 激发caspase依赖性凋亡信号, 最终导致HepG2细胞出现凋亡。在多数情况下, 自噬是维持细胞稳态的(即维持细胞生存), 而抑制自噬则可以阻断细胞利用消耗自身能量物质维持生存的过程[36]。本研究发现Danu能够引起HepG2细胞发生自噬, 且自噬起到了细胞保护作用, 提示联合应用自噬抑制剂可以促进Danu的抗肿瘤效果。PI3K/Akt/mTOR通路抑制和AMPK通路活化能够引起细胞自噬[37]。本研究发现Danu能够下调p-PI3K/PI3K、pAKT/AKT及p-mTOR/mTOR的比值, 以及上调pAMPK/AMPK的比值, 可见Danu抑制了PI3K/Akt/ mTOR通路的磷酸化, 同时促进AMPK的磷酸化, 最终引起HepG2细胞发生自噬。
本研究结果表明, Danu具有较好的体外抗HCC效果, 为下一步进行体内实验奠定了基础。Danu引起的自噬起到了保护细胞的作用, 提示联合使用自噬抑制剂能够增强AURK抑制剂的抗HCC效果。此外, Danu联合索拉菲尼等现有的靶向药物从而增强抗HCC效果可能是日后的研究方向。
总之, Danu能够抑制HepG2细胞增殖, 引起G2/M细胞周期阻滞, 导致细胞凋亡和自噬, 具有良好的体外抗肿瘤效果。Danu能够引起细胞保护性自噬。为增强Danu的抗HCC效果, 联用用药可能是一种选择。
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].
CA Cancer J Clin, 2018, 68(6): 394-424.
DOI: 10.3322/caac.v68.6. |
| [2] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J].
CA Cancer J Clin, 2016, 66(2): 115-32.
DOI: 10.3322/caac.21338. |
| [3] |
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012[J].
Int J Cancer, 2015, 136(5): E359-86.
DOI: 10.1002/ijc.29210. |
| [4] |
Forner A, Reig M, Bruix J. Hepatocellular carcinoma[J].
Lancet, 2018, 391(10127): 1301-14.
DOI: 10.1016/S0140-6736(18)30010-2. |
| [5] |
Ikeda K.Recent advances in medical management of hepatocellular carcinoma[J].Hepatol Res, 2018, [Epub ahead of print].
|
| [6] |
Tovoli F, Negrini G, Benevento F, et al. Systemic treatments for hepatocellular carcinoma:challenges and future perspectives[J].
Hepat Oncol, 2018, 5(1): HEP01.
DOI: 10.2217/hep-2017-0020. |
| [7] |
Marisi G, Cucchetti A, Ulivi P, et al. Ten years of sorafenib in hepatocellular carcinoma:Are there any predictive and/or prognostic markers?[J].
World J Gastroenterol, 2018, 24(36): 4152-63.
DOI: 10.3748/wjg.v24.i36.4152. |
| [8] |
Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma[J].
Nat Rev Clin Oncol, 2018, 15(10): 599-616.
DOI: 10.1038/s41571-018-0073-4. |
| [9] |
Pinter M, Peck-Radosavljevic M. Review article:systemic treatment of hepatocellular carcinoma[J].
Aliment Pharmacol Ther, 2018, 48(6): 598-609.
DOI: 10.1111/apt.2018.48.issue-6. |
| [10] |
Yamamoto S, Kondo S. Oral chemotherapy for the treatment of hepatocellular carcinoma[J].
Expert Opin Pharmacother, 2018, 19(9): 993-1001.
DOI: 10.1080/14656566.2018.1479398. |
| [11] |
Raoul JL, Kudo M, Finn RS, et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma:Sorafenib and beyond[J].
Cancer Treat Rev, 2018, 68: 16-24.
DOI: 10.1016/j.ctrv.2018.05.006. |
| [12] |
Abdel-Rahman O, Cheung WY. The Expanding Role of Systemic Therapy in the Management of Hepatocellular Carcinoma[J].
Can J Gastroenterol Hepatol, 2018, 2018: 4763832.
|
| [13] |
Contratto M, Wu J. Targeted therapy or immunotherapy?Optimal treatment in hepatocellular carcinoma[J].
World J Gastrointest Oncol, 2018, 10(5): 108-14.
DOI: 10.4251/wjgo.v10.i5.108. |
| [14] |
Willems E, Dedobbeleer M, Digregorio M, et al. The functional diversity of Aurora kinases:a comprehensive review[J].
Cell Div, 2018, 13: 7.
DOI: 10.1186/s13008-018-0040-6. |
| [15] |
Afonso O, Figueiredo AC, Maiato H. Late mitotic functions of Aurora kinases[J].
Chromosoma, 2017, 126(1): 93-103.
|
| [16] |
Jeng YM, Peng SY, Lin CY, et al. Overexpression and amplification of Aurora-A in hepatocellular carcinoma[J].
Clin Cancer Res, 2004, 10(6): 2065-71.
DOI: 10.1158/1078-0432.CCR-1057-03. |
| [17] |
Lin ZZ, Jeng YM, Hu FC, et al. Significance of Aurora B overexpression in hepatocellular carcinoma.Aurora B Overexpression in HCC[J].
BMC Cancer, 2010, 10: 461.
DOI: 10.1186/1471-2407-10-461. |
| [18] |
Zhang K, Chen J, Chen D, et al. Aurora-A promotes chemoresistance in hepatocelluar carcinoma by targeting NFkappaB/microRNA-21/PTEN signaling pathway[J].
Oncotarget, 2014, 5(24): 12916-35.
|
| [19] |
Liu F, Wang G, Wang X, et al. Targeting high Aurora kinases expression as an innovative therapy for hepatocellular carcinoma[J].
Oncotarget, 2017, 8(17): 27953-65.
|
| [20] |
Lu L, Han H, Tian Y, et al. Aurora kinase A mediates c-Myc's oncogenic effects in hepatocellular carcinoma[J].
Mol Carcinog, 2015, 54(11): 1467-79.
DOI: 10.1002/mc.v54.11. |
| [21] |
Borisa AC, Bhatt HG. A comprehensive review on Aurora kinase: Small molecule inhibitors and clinical trial studies[J].
Eur J Med Chem, 2017, 140: 1-19.
DOI: 10.1016/j.ejmech.2017.08.045. |
| [22] |
Tang A, Gao K, Chu L, et al. Aurora kinases:novel therapy targetsin cancers[J].
Oncotarget, 2017, 8(14): 23937-54.
|
| [23] |
Falchook GS, Bastida CC, Kurzrock R. Aurora Kinase Inhibitors in Oncology Clinical Trials:Current State of the Progress[J].
Semin Oncol, 2015, 42(6): 832-48.
DOI: 10.1053/j.seminoncol.2015.09.022. |
| [24] |
Bavetsias V, Linardopoulos S. Aurora Kinase Inhibitors:Current Status and Outlook[J].
Front Oncol, 2015, 5: 278.
|
| [25] |
Rossari F, Minutolo F, Orciuolo E. Past, present, and future of BcrAbl inhibitors:from chemical development to clinical efficacy[J].
J Hematol Oncol, 2018, 11(1): 84.
|
| [26] |
Gavriilidis P, Poutahidis T, Giakoustidis A, et al. Targeting hepatocarcinogenesis model in C56BL6 mice with pan-aurora kinase inhibitor Danusertib[J].
J Cancer, 2018, 9(5): 914-22.
DOI: 10.7150/jca.22329. |
| [27] |
Zhu Q, Luo M, Zhou C, et al. A proteomics-based investigation on the anticancer activity of alisertib, an Aurora kinase A inhibitor, in hepatocellular carcinoma Hep3B cells[J].
Am J Transl Res, 2017, 9(8): 3558-72.
|
| [28] |
Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma:rationale for the design and evaluation of therapeutic trials[J].
Hepatology, 1999, 29(1): 62-7.
DOI: 10.1002/hep.510290145. |
| [29] |
Daher S, Massarwa M, Benson AA, et al. Current and future treatment of hepatocellular carcinoma:an updated comprehensive review[J].
J Clin Transl Hepatol, 2018, 6(1): 69-78.
|
| [30] |
Sun W, Cabrera R. Systemic treatment of patients with advanced, unresectable hepatocellular carcinoma:emergence of therapies[J].
J Gastrointest Cancer, 2018, 49(2): 107-15.
DOI: 10.1007/s12029-018-0065-8. |
| [31] |
He SJ, Shu LP, Zhou ZW, et al. Inhibition of Aurora kinases induces apoptosis and autophagy via AURKB/p70S6K/RPL15 axis in human leukemia cells[J].
Cancer Lett, 2016, 382(2): 215-30.
DOI: 10.1016/j.canlet.2016.08.016. |
| [32] |
Zi D, Zhou ZW, Yang YJ, et al. Danusertib induces apoptosis, cell cycle arrest, and autophagy but inhibits epithelial to mesenchymal transition involving PI3K/Akt/mTOR signaling pathway in human ovarian cancer cells[J].
Int J Mol Sci, 2015, 16(11): 27228-51.
DOI: 10.3390/ijms161126018. |
| [33] |
Schoffski P, Besse B, Gauler T, et al. Efficacy and safety of biweekly i.v.administrations of the Aurora kinase inhibitor danusertib hydrochloride in independent cohorts of patients with advanced or metastatic breast, ovarian, colorectal, pancreatic, smallcell and non-small-cell lung cancer:a multi-tumour, multiinstitutional phase Ⅱ study[J].
Ann Oncol, 2015, 26(3): 598-607.
DOI: 10.1093/annonc/mdu566. |
| [34] |
Lok W, Klein RQ, Saif MW. Aurora kinase inhibitors as anti-cancer therapy[J].
Anticancer Drugs, 2010, 21(4): 339-50.
DOI: 10.1097/CAD.0b013e3283350dd1. |
| [35] |
Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer:a review of apoptosis, autophagy and programmed necrosis[J].
Cell Prolif, 2012, 45(6): 487-98.
DOI: 10.1111/cpr.2012.45.issue-6. |
| [36] |
Denton D, Nicolson S, Kumar S. Cell death by autophagy:facts and apparent artefacts[J].
Cell Death Differ, 2012, 19(1): 87-95.
DOI: 10.1038/cdd.2011.146. |
| [37] |
Polivka J, J r., Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway[J].
Pharmacol Ther, 2014, 142(2): 164-75.
DOI: 10.1016/j.pharmthera.2013.12.004. |
 2018, Vol. 38
2018, Vol. 38

