2. 南京中医药大学附属中西医结合医院 神经外科,江苏 南京 210028
2. Department of Neurosurgery, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing 210028, China
感染性休克是感染引起的全身炎症反应失控导致的循环衰竭,其本质是组织灌注不足引起的组织缺氧及细胞代谢障碍,并最终导致多器官功能障碍[1],是危重症患者主要入院及死亡原因[2-4]。近年来以中心静脉血氧饱和度(ScvO2)>70%等为复苏目标的早期目标指导治疗(EGDT)有效减少了患者死亡率[5],但新近的3个临床试验[6-8]表明以ScvO2为目标并没有改善患者预后,且许多患者入院时及复苏前ScvO2已经是正常甚至更高,所以寻找新的组织灌注指标成为临床危重病研究重点[9-10]。良好的指标应具备实时、简便的优点。近年来研究表明中心静脉血与动脉血二氧化碳分压差值(Pcv-aCO2)能够反应感染性休克患者组织灌注情况且与预后相关[11-12]。Pcv-aCO2升高表示外周循环没有足够血流冲洗组织所产生的CO2,是组织低灌注的良好指标[13]。而休克患者由于交感神经兴奋循环血流分布不均一,外周组织灌注不足最早发生但最晚恢复,研究表明外周组织灌注能够反应内脏组织灌注[14]。既往动物实验显示逐渐减少实验犬的四肢血流会导致患肢外周动静脉CO2分压差值增加[15]。因此,我们推测外周静脉-动脉血二氧化碳分压差(Ppv-aCO2)可以提示组织灌注不足,可以用于对感染性休克患者的预后评估,尚未见文献报道。本研究通过观察本院重症医学科感染性休克患者Ppv-aCO2结果差异的变化,分析其与预后的相关性,以期寻找能够反映患者预后的指标。
1 资料和方法 1.1 临床资料采用前瞻性研究方法,选择2017年5月~2018年5月本院重症医学科收治的符合感染性休克诊断的患者。入选标准:年龄18岁~80岁;符合2016年感染性休克诊断国际标准[1]:存在感染或可疑感染,且序贯器官衰竭评分(SOFA)≥2分,经充分的容量复苏仍持续低血压,需缩血管药才能维持平均动脉血压(MAP)≥65 mmHg,以及血乳酸(Lactate, Lac)>2 mmol/L。排除标准:慢性肝肾功能不全、长期使用免疫抑制剂、恶性肿瘤晚期、获得性免疫缺陷、妊娠期、糖尿病长期服用二甲双胍、慢性阻塞性肺病、四肢动静脉血栓、外周血管病、放弃治疗患者。最终62例患者入选,年龄29岁~80岁,平均73岁,其中男性42例,女性20例。感染部位:肺部感染28例(45.2%),腹腔感染18例(29.0%),血流感染8例(12.9%),泌尿系感染8例(12.9%)。按患者28 d生存情况分为存活组35例,死亡组27例。本研究方案符合医学伦理学标准,经医院伦理委员会批准(审批号:2015LWKY003),所有入选患者家属均签署知情同意书。
1.2 研究方法 1.2.1 感染性休克治疗方法所有患者按照2016年国际指南给予标准6 h治疗[16]:入院后颈内静脉或锁骨下静脉深静脉置管,监测中心静脉压(CVP),入院后立即监测动脉乳酸,抗生素使用前留取血培养标本,并给与广谱抗感染治疗,3 h内给予30 mL/kg晶体液充分液体复苏;6 h内患者初始复苏后仍存在MAP < 65 mmHg,使用去甲肾上腺素(NE)维持MAP≥65 mmHg。
1.2.2 观察指标患者最初复苏6 h后使用血气生化仪(型号:Stat profile pHOX Ultra Analyzer,美国Nova)检测外周动脉血二氧化碳分压(PaCO2)、外周静脉血PCO2(PpvCO2)以及中心静脉血PCO2(PcvCO2),并按以下公式计算相应动静脉指标差值:Ppv-aCO2=PpvCO2-PaCO2;Pcv-aCO2=PcvCO2-PaCO2。动脉选择桡动脉或肱动脉,外周静脉选择同侧的手背或肘正中静脉。
1.3 统计方法呈正态分布计量资料以均数±标准差表示,组间比较采用独立样本t检验。非正态分布的计量资料以中位数(M)[下四分位数(QL),上四分位数(QU)]表示,组间比较采用Mann-whitney U检验。计数资料以率表示采用χ2检验。采用Pearson相关性分析Ppv-aCO2与PcvaCO2相关性,多因素Logistic回归分析法对预后进行多因素回归分析,绘制受试者工作特征曲线(ROC),以ROC曲线下面积(AUC)分析各指标的最佳诊断临界值及敏感度和特异度。以上数据的统计学分析均由SPSS19.0软件计算完成,采用双侧检验,P < 0.05为差异有统计学意义。
2 结果 2.1 基线资料按患者28 d生存情况分为存活组35例,死亡组27例;两组年龄、性别及体质量指数(BMI)比较差异无统计学意义(P>0.05)。两组患者NE用量比较差异无统计学意义(P>0.05)。两组患者标准化6 h治疗后MAP、CVP和ScvO2比较差异无统计学意义(P>0.05)。与存活组比较,死亡组患者急性生理及慢性疾病Ⅱ评分(APACHE Ⅱ)及SOFA评分明显升高(P < 0.05,表 1)。
| 表 1 患者入院时及标准化6 h集束化治疗后基本信息 Table 1 Baseline characteristics of patients at admission and after 6-h bundle treatments |
两组患者PaCO2、PpvCO2、pH值、实际碳酸氢盐、标准碳酸氢盐及阴离子间隙比较差异无统计学意义(均P>0.05);与存活组比较,死亡组患者Pcv-aCO2、PpvaCO2及Lac明显升高(P < 0.05,表 2)。
| 表 2 两组患者复苏6 h后静脉及动脉血气分析比较 Table 2 Peripheral venous and arterial blood partial pressure of carbon dioxide in patients with septic shock in the survivor and non-survivor groups after 6-h bundle treatments (Mean±SD) |
Pearson相关性分析显示Ppv-aCO2与Pcv-aCO2明显相关,r=0.897,R2=0.805,P < 0.001,两者线性关系为Ppv-aCO2=1.75×Pcv-aCO2-1.5(图 1)。
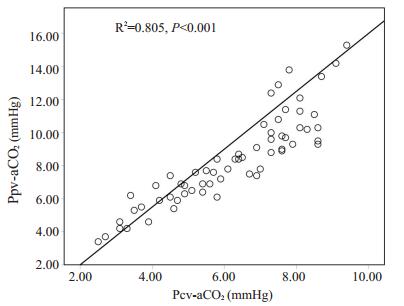
|
图 1 Ppv-aCO2与Pcv-aCO2相关性曲线 Figure 1 Correlation between peripheral venous-to-arterial carbon dioxide difference and central venous-to-arterial carbon dioxide difference. |
因为Ppv-aCO2与Pcv-aCO2显著相关,故将组间比较差异明显的PpvCO2、Ppv-aCO2、Lac、APACHEⅡ评分和SOFA评分纳入多因素Logistic回归分析,结果显示Ppv-aCO2及Lac为患者28 d生存率的独立预后因素([Ppv-aCO2:β =0.659,P=0.003,相对危险度(OR)= 1.934,95% CI:1.244~3.005;Lac:β =0.780,P=0.017,OR=2.182,95%CI:1.148-4.148,表 3)]。
| 表 3 多因素Logistic回归分析与患者28 d死亡的危险因素 Table 3 Logistic regression analysis of possible risk factors for 28-day mortality |
2.5 Ppv-aCO2、Pcv-aCO2与Lac对患者预后判断价值ROC曲线分析显示,Ppv-aCO2、Pcv-aCO2和Lac对患者预后均有预测价值,最佳临界值分别为9.05、7.05、3.45 mmol/L(图 2,表 4)。
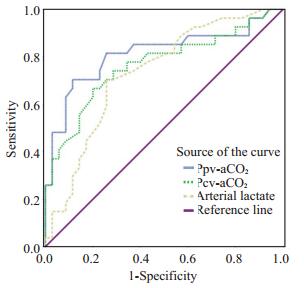
|
图 2 Ppv-aCO2、Pcv-aCO2与Lac判断28 d死亡的ROC曲线 Figure 2 Receiver-operating curves of peripheral venous-toarterial carbon dioxide difference, central venous-toarterial carbon dioxide difference and arterial lactate at 6 h for 28-day mortality. |
| 表 4 Ppv-aCO2、Pcv-aCO2与Lac判断28 d死亡的预测价值 Table 4 Prognostic value of peripheral venous-to-arterial carbon dioxide difference, central venous-to-arterial carbon dioxide difference and arterial lactate at 6 h for 28-day mortality |
感染性休克本质是微循环障碍导致的组织灌注不足及氧代谢障碍,当全身的宏观指标正常或恢复正常时,组织灌注不足仍可持续存在,且持续的组织灌注不足与患者器官功能障碍及死亡率增加等不良预后明显相关[17]。Rivers等[5]提出了以ScvO2>70%等为复苏目标的EGDT有效减少了患者死亡率。ScvO2反应的是感染性休克是氧输送(DO2)和氧消耗(VO2)之间的紊乱,间接反应休克时组织灌注不足。但正常的ScvO2并不能代表DO2的充足和组织不缺氧,因为休克时微循环障碍导致的毛细血管关闭以及线粒体功能障碍,氧消耗减少,可以导致ScvO2正常或升高,另一方面,中心静脉是混合了的全身血液,反应的是全身氧供是否充足,某些器官的缺氧可能会被其他正常氧供的组织来源的静脉血所掩盖,也导致ScvO2升高或正常。研究也证明ScvO2高于正常的感染性休克患者死亡率反而更高[18]。3个大规模临床试验表明以ScvO2为目标并没有改善患者预后,且许多患者入院时及复苏前ScvO2已经是正常甚至更高,说明组织灌注的异常有可能在ScvO2正常时仍旧存在[6-8]。本研究结果也显示虽然死亡组患者与存活组患者结局截然不同,但早期复苏后两组ScvO2并没有显著差异。因此,应该寻找更佳的实时、敏感反应组织灌注指标。
Pcv-aCO2代表细胞代谢所产生的CO2在中心静脉与动脉的分压差。近年来有研究表明其能够反应感染性休克患者组织灌注情况[11],持续的动静脉PCO2差值升高提示患者预后不良[19],使用动静脉PCO2差值可以指导患者的液体复苏[20]。而休克患者由于交感神经兴奋循环血流分布不均一,外周非重要器官组织如肌肉皮肤等灌注不足最早发生但最晚恢复,研究表明外周组织灌注能够反应患者病情危重程度[14]。且外周灌注指标较宏观指标具有更好的预测价值[21-23]。近年来临床使用有效的外周灌注评估指标包括毛细血管再充盈时间,皮肤花斑评分系统、外周灌注指数等,一定程度上反应的也是外周肌肉皮肤等组织灌注情况。既往动物实验也显示逐渐减少实验犬的四肢血流会导致患肢外周动静脉CO2分压差值增加[15]。因此,我们推测Ppv-aCO2可以提示组织灌注不足。采用外周静脉血相较中心静脉血避免了不必要的中心静脉置管,临床更易获取,且理论上可以反应外周组织灌注情况,更符合休克的病理生理学特征。
本研究结果显示与存活组患者相比,死亡组患者Ppv-aCO2及Pcv-aCO2都明显升高,Ppv-aCO2与Pcva-CO2具有良好相关性。回归分析显示Ppv-aCO2是感染性休克患者28 d死亡独立危险因素。进一步ROC分析显示Ppv-aCO2对于患者预后具有良好判断价值,研究结果证实我们的推测。Ppv-aCO2升高原因考虑休克时循环血容量绝对或相对不足,血流缓慢,血液通过毛细血管时间延长,致单位体积血液中的CO2含量增加[24]。另一方面由于组织灌注减少,组织缺氧,细胞无氧代谢增加也增加静脉CO2含量[25],最终导致Ppv-aCO2升高,这种升高不依赖于DO2及VO2之间的平衡,表明外周循环没有足够血流冲洗组织所产生的CO2 [13],是组织灌注不足的指标,不代表组织缺氧。
另外本研究比较了死亡与存活患者动脉乳酸差异,研究结果显示死亡组患者具有更高的复苏后动脉乳酸,回归分析显示动脉乳酸是患者死亡独立危险因素,虽然关于乳酸的判断价值尚存争议[26],但有研究表明乳酸仍是良好的复苏目标[27],复苏6 h后的乳酸值较乳酸清除率具有更好的预后价值,这与本研究结果吻合。
本研究的局限性:为单中心研究,大部分患者由其他科室直接转入,很多感染性休克患者入科时已经进入病程晚期或者是恢复期,从而导致选择偏倚;样本量有限,还需扩大样本进一步验证研究结果,所提供的诊断截断点仅供参考;本研究患者都使用了去甲肾上腺素维持血压,而去甲肾上腺素的使用可能导致外周血管收缩,引起外周血流异常进而导致外周静脉与动脉二氧化碳分压异常[28],作为混杂因素可能导致结果不可信。
综上所述,本研究显示外周静脉与动脉二氧化碳分压差和中心静脉与动脉二氧化碳分压差具有一定相关性,外周静脉与动脉二氧化碳分压差可作为感染性休克患者死亡风险的独立预测因素,既往文献未见报道,且临床方便快捷,易于普及,对临床实践有一定指导意义。
| [1] |
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J].
JAMA, 2016, 315(8): 801-10.
DOI: 10.1001/jama.2016.0287. |
| [2] |
Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: propensity matched cohort study[J].
BMJ, 2016, 353(8): 353.
|
| [3] |
Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003[J].
Crit Care Med, 2007, 35(5): 1244-50.
DOI: 10.1097/01.CCM.0000261890.41311.E9. |
| [4] |
Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the united states[J].
Crit Care Med, 2013, 41(5): 1167-74.
DOI: 10.1097/CCM.0b013e31827c09f8. |
| [5] |
Rivers E, Nguyen B, Havstad S, et al. The early goal-directed therapy collaborative group: early goal-directed therapy in the treatment of severe sepsis and septic shock[J].
N Engl J Med, 2001, 345(7): 1368-77.
|
| [6] |
Mouncey PR, Osborn TM, Power G, et al. Trial of early, Goaldirected resuscitation for septic shock[J].
N Engl J Med, 2015, 372(14): 1301-11.
DOI: 10.1056/NEJMoa1500896. |
| [7] |
ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock[J].
N Engl J Med, 2014, 370(18): 1683-93.
DOI: 10.1056/NEJMoa1401602. |
| [8] |
ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. goal-directed resuscitation for patients with early septic shock[J].
N Engl J Med, 2014, 371(16): 1496-506.
DOI: 10.1056/NEJMoa1404380. |
| [9] |
Marini JJ, De Backer D, Ince C, et al. Seven unconfirmed ideas to improve future ICU practice[J].
Critical Care, 2017, 21(3): 315.
|
| [10] |
Duenser MW, Takala J, Brunauer A, et al. Re-thinking resuscitation: leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion- based approach[J].
Critical Care, 2013, 17(5): 326.
DOI: 10.1186/cc12727. |
| [11] |
Ospina-Tascon GA, Umana M, Bermudez WF, et al. Can venous-toarterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock[J].
Intensive Care Med, 2016, 42(2): 211-21.
DOI: 10.1007/s00134-015-4133-2. |
| [12] |
刘光云, 黄惠斌, 秦含玉, 等. 中心静脉-动脉血二氧化碳分压差评估感染性休克患者容量反应性的前瞻性临床研究[J].
中华危重病急救医学, 2018, 30(5): 449-55.
DOI: 10.3760/cma.j.issn.2095-4352.2018.05.011. |
| [13] |
Antonelli M, Levy, et al. Hemodynamic monitoring in shock and implications for management. international consensus conference, Paris, France, 27-28 april 2006[J].
Intensive Care Med, 2007, 33(4): 575-90.
DOI: 10.1007/s00134-007-0531-4. |
| [14] |
Brunauer A, Kokoefer A, Bataar OA, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study[J].
J Crit Care, 2016, 35(8): 105-9.
|
| [15] |
Vallet B, Teboul JL, Cain S, et al. Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia[J].
J Appl Physiol, 2000, 89(4): 1317-21.
DOI: 10.1152/jappl.2000.89.4.1317. |
| [16] |
Dellinger RP, Schorr CA, Levy MM. A users' guide to the 2016 surviving sepsis guidelines[J].
Intensive Care Med, 2017, 45(3): 381-5.
|
| [17] |
Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock[J].
Crit Care Med, 2004, 32(9): 1825-31.
DOI: 10.1097/01.CCM.0000138558.16257.3F. |
| [18] |
Textoris J, Fouché L, Wiramus S, et al. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality[J].
Crit Care, 2011, 15(4): R176.
DOI: 10.1186/cc10325. |
| [19] |
Ospina-Tascon GA, Bautista-Rincon DF, Umana M, et al. Persistently high venous- to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock[J].
Crit Care, 2013, 17(6): R294.
DOI: 10.1186/cc13160. |
| [20] |
Mallat J, Lemyze M, Tronchon L, et al. Use of venous- to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock[J].
World J Crit Care Med, 2016, 5(1): 47-56.
DOI: 10.5492/wjccm.v5.i1.47. |
| [21] |
Lima A, Jansen TC, van Bommel J, et al. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients[J].
Crit Care Med, 2009, 37(3): 934-8.
DOI: 10.1097/CCM.0b013e31819869db. |
| [22] |
Lima A, Takala J. Clinical significance of monitoring perfusion in non-vital organs[J].
Intensive Care Med, 2014, 40(7): 1052-4.
DOI: 10.1007/s00134-014-3345-1. |
| [23] |
van Genderen ME, Engels N, van der Valk RJ, et al. Early peripheral perfusion-guided fluid therapy in patients with septic shock[J].
Am J Respir Crit Care Med, 2015, 191(4): 477-80.
DOI: 10.1164/rccm.201408-1575LE. |
| [24] |
Lamia B, Monnet X, Teboul JL. Meaning of arterio-venous PCO2 difference in circulatory shock[J].
Minerva Anestesiol, 2006, 72(6): 597-604.
|
| [25] |
Jr RH, Cohen JJ. Anaerobic CO2 production by dog kidney in vitro[J].
Am J Physiol, 1966, 211(2): 493-505.
|
| [26] |
Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial[J].
JAMA, 2010, 303(8): 739-46.
DOI: 10.1001/jama.2010.158. |
| [27] |
Ryoo SM, Lee JB, Lee YS, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3[J].
Crit Care Med, 2018, 46(6): E489-95.
DOI: 10.1097/CCM.0000000000003030. |
| [28] |
Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis[J].
Crit Care Med, 2005, 33(10): 2194-201.
DOI: 10.1097/01.CCM.0000182798.39709.84. |
 2018, Vol. 38
2018, Vol. 38

