2. 无锡市惠山区人民医院,江苏 无锡 214100
2. Department of Cardiology, Huishan District People's Hospital, Wuxi 214100, China
糖尿病心肌病是糖尿病患者最为严重的并发症之一,同时也是糖尿病患者主要死亡原因。高血糖致心肌病变的主要特点是以心肌结构改变为主[1-2],从而导致心力衰竭,并有证据表明糖尿病是致射血分数保留性心衰的主要原因之一[3]。因此对高糖环境对心肌细胞损伤的机制研究具有重要意义。游离钙离子作为第二信号广泛参与细胞生理活动的调节,其参与的心脏信号传导是十分复杂的,影响着心脏内多种信号通路的传导[4]。高糖可以通过钙库操纵的的钙离子内流(SOCE)使细胞内钙离子浓度升高[5],当心肌细胞长期受高糖环境刺激时,高糖可通过G蛋白偶联受体途径和受体酪氨酸激酶途径使细胞内三磷酸肌醇(IP3)浓度增加[6],IP3首先作用于位于细胞内钙库(内质网)上基质相互作用分子(STIM)使钙库内钙离子释放进入胞浆,当钙库内钙离子清空后,STIM寡聚体化后转移到位于细胞膜ORAI和TRPC蛋白附近,激活ORAI和TRPC通道介导的钙离子内流[7]。胞浆内浓度升高的钙离子可过度活化钙调神经磷酸酶(CaN)。CaN是迄今发现唯一一种受Ca2+调节的丝氨酸/苏氨酸蛋白磷酸酶, 在心脏表达的CaN的活性只与CaNAβ相关[8],CaN使活化的T细胞核因子(NFAT)去磷酸化激活,活化的NFAT蛋白能在核内保持长时间的活化状态[9]。NFAT3作为CaN/NFAT3信号通路的下游分子最终决定了相关目的基因的表达[10-11],使用钙调神经磷酸酶抑制剂证明NFAT3的入核能被显著抑制,这一发现证实CaN调控着NFAT3的激活入核[12]。研究表明,Ca2+-CaN-NFAT3信号通路在心血管疾病发病过程中有着重要的作用,其在心肌肥大和心肌纤维化等过程都有参与[13-15],在高糖刺激下Ca2+- CaN-NFAT3作为心肌肥大信号被激活并参与相关炎症因子的调控[16]。
线粒体乙醛脱氢酶2(ALDH2)是乙醛脱氢酶家族成员之一,是一种位于线粒体内重要的醛类氧化酶,不仅是体内重要的氧化应激分子,对细胞凋亡也具有抑制作用。研究证实,ALDH2对心血管系统的保护作用不仅在单纯心血管疾病方面[17-21]。在糖尿病大鼠模型中,通过低浓度酒精激活ALDH2,后者可以在大鼠心肌损伤中起保护作用[22]。但是,激活ALDH2对抗高糖引起的心肌细胞损伤,是否通过下调Ca2+-CaN-NFAT3信号通路活性实现的目前尚未见报道,本研究探讨了高糖处理的乳鼠心肌细胞中ALDH2对Ca2+-CaN-NFAT3信号通路影响及其可能机制。
1 材料和方法 1.1 动物和试剂SD大鼠由蚌埠医学院实验动物中心提供且符合动物伦理;胰酶消化液(碧云天);胶原酶2(索莱宝);Hanks液(碧云天);胎牛血清(四季青);DMEM低糖、高糖培养基(HyClone);Alda-1(SML0462, Sigma-Aldrich);11R-VIVIT(TOCRIS);钙调神经磷酸酶酶联免疫吸附试剂盒(上海酶联);钙离子荧光探针Fluo-3 AM(碧云天);乙醛脱氢酶2抗体、钙调神经磷酸抗体购自abcam;活化T细胞核因子-3抗体(CST);β-actin购自Biosharp;DNA酶Ⅰ、5-Brdu(索莱宝);BCA试剂盒(碧云天)。
1.2 方法 1.2.1 心肌细胞原代培养出生3 d内乳鼠取心脏心尖部剪碎后经混合酶消化成单个细胞,用含10%胎牛血清的DMEM低糖培养基接种于培养皿中,在5% CO2,饱和湿度,37 ℃培养箱培养,在培养1.5 h后取出培养皿中培养液离心重悬后接种于新培养皿中并且再加入5-Brdu继续培养,此时培养的细胞则为较高纯度心肌细胞可进行实验。
1.2.2 心肌细胞鉴定用免疫荧光对培养心肌细胞进行鉴定。将培养皿中心肌细胞用胰酶消化后取少量细胞接种于6孔板中,剩余细胞重新接种于培养皿中,待6孔板中细胞完全贴壁后进行实验。先用PBS清洗1 min/3次,4%多聚甲醛室温固定细胞15 min,PBS清洗5 min/3次;然后用0.1% TritonX-100对细胞进行打孔并放置培养箱中30 min,PBS清洗5 min/3次;用5% BSA封闭30 min并且PBS清洗5 min/3次后用1% BSA稀释的抗α-横纹肌肌动蛋白一抗(α-Sarcomeric Actin, α-SA, 1: 200,武汉博士德)孵育心肌细胞4 ℃过夜;次日PBS清洗5 min/3次后用含羊抗小鼠荧光标记二抗(1: 200,武汉博士德)孵育细胞1 h;PBS清洗后用DAPI(ZSGB,北京)进行核染;避光条件下用荧光显微镜观察。
1.2.3 实验分组实验共有以下分组:5.5 mmol/L糖对照组(M)、30 mmol/L高糖组(MH)、30 mmol/L高糖加乙醛脱氢酶2激动剂(Alda-1)组(MHA)、30 mmol/L高糖加乙醛脱氢酶2抑制剂(Daidzin)组(MHD)、30 mmol/L高糖加乙醛脱氢酶2激动剂(Alda-1)和NFAT3抑制剂(11R-VIVIT)组(MHAV)。
1.2.4 细胞内钙离子浓度测定Fluo-3 AM用无血清培养基配成2 μmol/L;弃去原细胞培养基,用PBS清洗细胞后给予2 μmol/L的Fluo-3于37 ℃,5% CO2孵育30 min;弃去Fluo-3,PBS洗涤细胞2次后加培养基适量后荧光显微镜检测。
1.2.5 Western blot检测CaN、ALDH2、NFAT3蛋白表达情况处理后细胞PBS洗涤1 min/3次后加适量胰酶消化细胞1 min,用含10%血清培养基终止消化,将混合液离心后PBS清洗再次离心,加入适量全细胞裂解液及PMSF裂解1 h后12 000 r/min 15 min离心,取上清。按照BCA试剂盒说明测蛋白浓度,制备10% SDS-PAGE分离胶和5%浓缩胶,每孔上样60 μg。用60 V恒压浓缩胶电泳30 min,恒压90 V分离胶电泳90 min。将凝胶中已分离的蛋白质恒流200 mA 150 min电转到PVDF膜上;5%脱脂奶粉室温封闭条带2 h;用TBST稀释的一抗孵育条带4 ℃过夜,TBST洗涤条带4次,5 min/次;将洗涤后条带放入TBST稀释的二抗中37 ℃水浴1 h,TBST清洗条带4次,10 min/次。ECL发光法显影。
1.2.6 细胞内CaN浓度测定从室温平衡20 min后的铝箔袋中取出板条,分别设置空白、标准、样品孔,除空白孔外各孔分别加入50 μL不同浓度标准品和样品。标准孔和样品孔每孔加入辣根过氧化物酶(HRP)标记的检测抗体,用封板膜封住反应孔,37 ℃恒温箱60 min。弃去液体,吸水纸吸干,每孔加满洗涤液,静置1 min,甩去洗涤液,吸水纸吸干,重复5次。每孔加入底物A、B个50 μL,37 ℃避光孵育15 min。每孔加入终止液50 μL,15 min内,测定各孔A450 nm波长。
1.3 统计学处理数据采用均数±标准差表示,采用GraphPad,prism 6进行统计学分析,多组均数间比较采用单因素方差分析,各组均数比较采用HolmSidak(recommended)校正的t检验,以P < 0.05为差异有统计学意义。
2 结果 2.1 原代心肌细胞培养免疫荧光鉴定结果通过差速贴壁结合5-Brdu得到纯度较高的心肌细胞,并且通过对特异性表达于心肌细胞和骨骼肌细胞中α-横纹肌肌动蛋白进行免疫荧光染色后,心肌α-SA抗原免疫荧光呈阳性反应,为绿色,位于细胞浆内,DAPI细胞核染色为蓝色,Merged为α-SA和DAPI合成结果(图 1)。

|
图 1 心肌细胞免疫荧光鉴定 Figure 1 Immunefluorescence identification of cardiomyocytes (Original magnification: ×100). |
使用Image-J软件进行图像分析,结果显示:各组细胞不同条件分别培养48 h后,与正常组相比,高糖组平均Ca2+荧光密度值明显增高(P < 0.01),与MH相比,MHA组荧光密度值显著降低(P < 0.01),MHD组荧光值进一步升高(P < 0.01,图 2)。
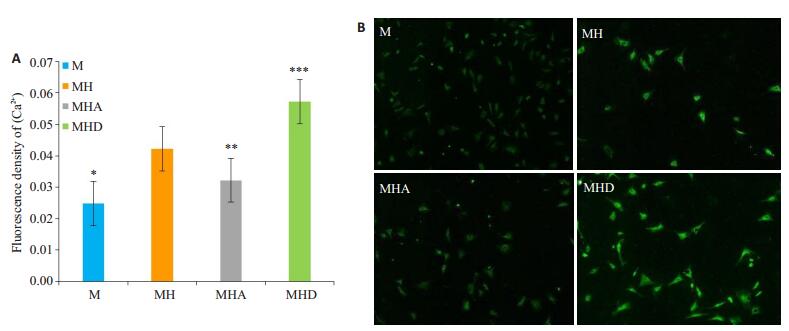
|
图 2 荧光探针检测各组[Ca2+]活性测定结果和各组[Ca2+]荧光染色观察 Figure 2 Determination of [Ca2+] level and fluorescence staining for detecting [Ca2+] in each group. A: Quantitative analysis of the mean fluorescence density of [Ca2+] in each group (Mean ± SD, n=5). *P < 0.01 vs MH, ***P < 0.01 vs MH; **P < 0.05 vs MH; B: Fluorescence density of [Ca2+] in primary cardiomyocytes with different treatments (×100). |
通过Image-J分析曝光后条带灰度值显示:使用Image-J软件进行图像分析,各组细胞不同条件分别培养48 h后,与MH组相比,MHA组ALDH2表达升高(P < 0.05),而CaN、NFAT3表达相应减弱(P < 0.05);与MHA相比,MHAV组ALDH2蛋白表达差异无统计学意义(P>0.05),NFAT3蛋白表达明显降低(P < 0.05,图 3)。
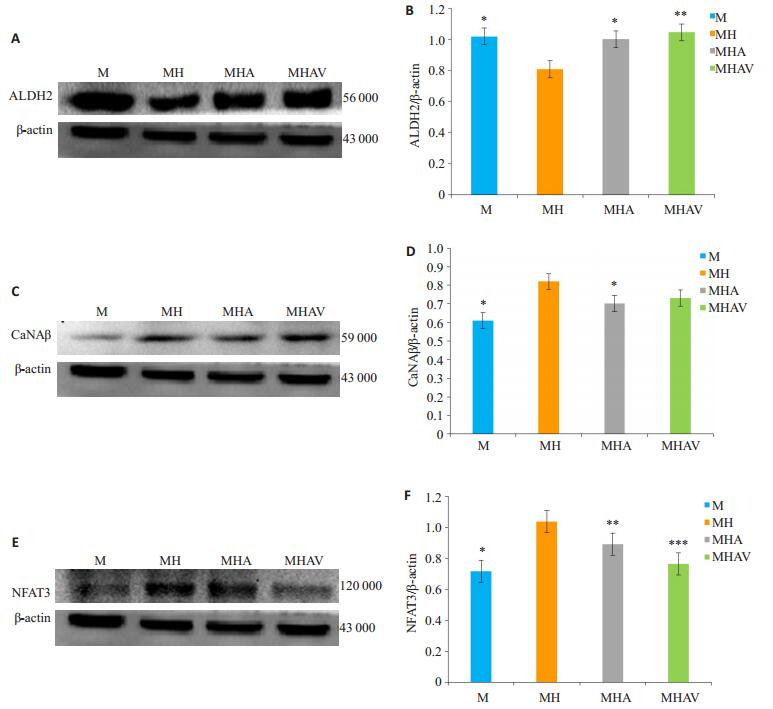
|
图 3 Western blotting检测各组ALDH2、CaNAβ、NFAT3蛋白表达 Figure 3 Western blotting for detecting ALDH2, CaNAβ, and NFAT3 protein levels in primary cultures of cardiomyocytes in each group (Mean±SD, n=3). A: Western blotting of ALDH2 and β-actin; B: ALDH2 protein levels normalized by β-actin (*P < 0.05 vs group MH; **P>0.05 vs MHA); C: Western blotting of CaNAβ and β-actin; D: CaNAβ protein levels normalized by β-actin (*P < 0.05 vs group MH); E: Western blotting of NFAT3 and β-actin; F: NFAT3 protein levels normalized by β-actin levels (*P < 0.01 vs MH; **P < 0.05 vs MH; ***P < 0.05 vs MHA). |
各组细胞分组如前,在不同条件下处理48 h结果显示:M组CaN浓度最低,在高糖诱导下细胞内CaN浓度显著升高(P < 0.01),给予Alda-1处理的MHA组细胞内CaN浓度有所降低(P < 0.01),而当单独给予Daidzin后CaN浓度较MH组升高显著(P < 0.05,图 4)。
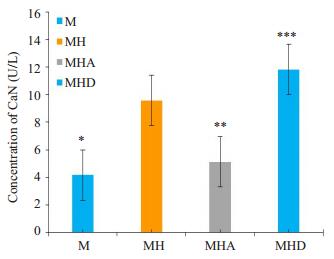
|
图 4 ELISA检测不同处理组细胞内CaN浓度 Figure 4 Concentration of CaN in primary cardiomyocytes with different treatments detected by ELISA (Mean±SD, n=3). *P < 0.01 vs MH, **P < 0.01 vs MH; ***P < 0.05 vs MH. |
机体内长期高糖环境是导致心肌损伤重要因素,但具体机制尚不明确,因此本研究通过高糖诱导原代培养的SD大鼠乳鼠心肌细胞来探索高糖致心肌损伤可能机制。本实验发现高糖环境下心肌细胞内钙离子浓度、细胞内CaN浓度、CaNAβ和NFAT3蛋白水平都显著增加,这些结果进一步验证高糖可激活Ca2+-CaNAβ-NFAT3信号通路。
研究发现[5]原代培养的人脐静脉内皮细胞经高糖处理后,钙库操纵的钙离子内流(SOCE)相关蛋白活性升高,进而导致细胞内游离钙离子浓度升高,也有研究发现了类似的结果[23-24]。细胞内升高的钙离子浓度可使细胞内CaN浓度、CaN和NFAT3蛋白表达量均明显升高[22]。这些结果与我们的研究结果相符。以上说明CaN/NFAT3信号通路在介导高糖诱导的心肌损伤过程中发挥着重要作用,其机制可能是高糖环境引起心肌细胞内SOCE活性升高,引起细胞内游离钙离子浓度升高,导致依赖于细胞内Ca2+的CaN活化。使用钙调神经磷酸酶抑制剂证明NFAT3的入核能被显著抑制,这一发现证实CaN调控着NFAT3的激活入核[12]。活化的NFAT3去磷酸化入核调控多种参与心肌肥大和纤维化基因的表达[8, 23],以上研究提示了Ca2+-CaN-NFAT3信号通路介导了高糖诱导的心肌肥大及纤维化等病理过程。
既往研究发现ALDH2可对抗糖尿病诱导的心肌细胞损伤[19, 22, 26],ALDH2抗细胞损伤可能与其可清除细胞内产生的乙醛和氧自由基密切相关[27-28],Pan等[29]在培养的心肌细胞中发现了ALDH2的这种保护作用,我们研究发现ALDH2对于高糖诱导的心肌纤维化也具有一定的逆转作用[30],但ALDH2能否调控Ca2+-CaNNFAT3活性从而拮抗高糖引起的心肌细胞损伤目前未见报道。我们探讨了ALDH2与Ca2+-CaN-NFAT3通路的关系。首先我们使用高糖和乙醛脱氢酶2激动剂Alda-1共处理原代心肌细胞。结果显示ALDH2能够显著抑制高糖对钙离子浓度、CaNAβ浓度的上调作用。当进一步用高糖和ALDH2抑制剂Daidzin共处理原代心肌细胞,结果发现钙离子浓度、CaNAβ浓度较仅用高糖处理显著增高。此外,我们还应用了高糖、Alda-1和NFAT3抑制剂11R-VIVIT共处理心肌细胞,结果显示NFAT3蛋白表达水平较高糖与Alda-1共处理显著降低,而CaNAβ蛋白表达量却有所增高,但并无统计学意义。我们的研究结果表明高糖可激活心肌细胞内Ca2+-CaN-NFAT3信号通路,并推测该过程可被ALDH2有效拮抗。
因此,为了进一步验证上述猜测,我们通过检测ALDH2蛋白表达发现, 各组ALDH2蛋白表达与各组CaNAβ、NFAT3蛋白表达变化趋势相反,并且与各组细胞钙离子浓度和CaN浓度变化也呈相反趋势。在使用NFAT3特异性抑制剂11R-VIVIT后发现ALDH2蛋白表达量与无11R-VIVIT存在条件下并无明显差异,这些结果进一步证实了ALDH2可能作为Ca2+-CaN-NFAT3信号通路上游分子发挥调控作用。
综上所述,ALDH2可能通过下调Ca2+-CaN-NFAT3通路活性拮抗高糖引起乳鼠心肌细胞损伤。
| [1] |
Aydemir M, Ozturk N, Dogan S, et al. Sodium tungstate administration ameliorated diabetes-induced electrical and contractile remodeling of rat heart without normalization of hyperglycemia[J].
Biol Trace Elem Res, 2012, 148(2): 216-23.
DOI: 10.1007/s12011-012-9350-8. |
| [2] |
Thackeray JT, Radziuk J, Harper ME, et al. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunctionin a rat model of insulin resistance with hyperglycemia[J].
Cardiovasc Diabetol, 2011, 10(8): 75.
|
| [3] |
Pan G, Munukutla S, Kar A, et al. Type-2 diabetic aldehyde dehydrogenase 2 mutant mice (ALDH 2*2) exhibiting heart failure with preserved ejection fraction phenotype can be determined by exercise stress echocardiography[J].
PLoS One, 2018, 13(4): e0195796.
DOI: 10.1371/journal.pone.0195796. |
| [4] |
Dewenter M, von der Lieth A, Katus HA. Calcium signaling and transcriptional regulation in cardiomyocytes[J].
Circ Res, 2017, 121(8): 1000-20.
DOI: 10.1161/CIRCRESAHA.117.310355. |
| [5] |
Tamareille S, Mignen O, Capiod T, et al. Highglucose-induced apoptosis throughstore-operated calcium entry and calcineurin in human umbilical vein endothelial cells[J].
Cell Calcium, 2006, 39(1): 47-55.
DOI: 10.1016/j.ceca.2005.09.008. |
| [6] |
Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCRdependent Ca2+ signalling[J].
Cell Signal, 2003, 15(3): 243-53.
DOI: 10.1016/S0898-6568(02)00074-8. |
| [7] |
Daskoulidou N, Zeng B, Berglund LM, et al. High glucose enhances store-operated Calcium entry by upregulating ORAI/STIM via calcineurin-NFAT signalling[J].
J Mol Med (Berl), 2015, 93(5): 511-21.
DOI: 10.1007/s00109-014-1234-2. |
| [8] |
Bueno OF, Wilkins BJ, Tymitz KM, et al. Impaired cardiac hypertrophic response in calcineurin a beta-deficient mice[J].
Proc Natl Acad Sci USA, 2002, 99(7): 4586-91.
DOI: 10.1073/pnas.072647999. |
| [9] |
Bendickova K, Tidu F, Fric J. Calcineurin-NFAT signalling in myeloid leucocytes: new prospects and pitfalls in immunosuppressive therapy[J].
EMBO Mol Med, 2017, 9(8): 990-9.
DOI: 10.15252/emmm.201707698. |
| [10] |
Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes[J].
J Biol Chem, 2001, 276(5): 3524-30.
DOI: 10.1074/jbc.M004275200. |
| [11] |
Bai SM, Kerppola TK. Opposing roles of FoxP1 and Nfat3 in transcriptional control of cardiomyocyte hypertrophy[J].
Mol Cell Biol, 2011, 31(14): 3068-80.
DOI: 10.1128/MCB.00925-10. |
| [12] |
Liu CJ, Cheng YC, Lee KW, et al. Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells[J].
Mol Cell Biochem, 2008, 313(1/2): 167-78.
|
| [13] |
Asadi F, Razmi A, Dehpour AR, et al. Tropisetron inhibits high glucose-induced calcineurin/NFAT hypertrophic pathway in H9c2 myocardial cells[J].
J Pharm Pharmacol, 2016, 68(4): 485-93.
DOI: 10.1111/jphp.2016.68.issue-4. |
| [14] |
Dierck F, Kuhn C, Rohr C, et al. The novel cardiac z-disc protein CEFIP regulates cardiomyocyte hypertrophy by modulating calcineurin signaling[J].
J Biol Chem, 2017, 292(37): 15180-91.
DOI: 10.1074/jbc.M117.786764. |
| [15] |
Wang J, Wang Y, Zhang W, et al. Phenylephrine promotes cardiac fibroblast proliferation through calcineurin-NFAT pathway[J].
Front Biosci (Landmark Ed), 2016, 21(3): 502-13.
DOI: 10.2741/4405. |
| [16] |
Williams CR, Gooch JL. Calcineurin a beta regulates NADPH oxidase (Nox) expression and activity via nuclear factor of activated T cells (NFAT) in response to high glucose[J].
J Biol Chem, 2014, 289(8): 4896-905.
DOI: 10.1074/jbc.M113.514869. |
| [17] |
Dassanayaka S, Zheng YT, Gibb AA, et al. Cardiac-specific overexpression of aldehyde dehydrogenase 2 exacerbates cardiac remodeling in response to pressure overload[J].
Redox Biol, 2018, 17(2): 440-9.
|
| [18] |
Shen C, Wang C, Fan F, et al. Acetaldehyde dehydrogenase 2 (ALDH2) deficiency exacerbates pressure overload-induced cardiac dysfunction by inhibiting Beclin-1 dependent autophagy pathway[J].
Biochim Biophys Acta, 2015, 1852(2, SI): 310-8.
DOI: 10.1016/j.bbadis.2014.07.014. |
| [19] |
Guo YL, Yu WJ, Sun DD, et al. A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetesinduced cardiac dysfunction: Role of AMPK-regulated autophagy[J].
Biochim Biophys Acta, 2015, 1852(2, SI): 319-31.
DOI: 10.1016/j.bbadis.2014.05.017. |
| [20] |
Kang PF, Wu WJ, Tang Y, et al. Activation of ALDH2 with low concentration of ethanol attenuates myocardial ischemia/reperfusion injury in diabetes rat model[J].
Oxid Med Cell Longev, 2016, 18(10): 6190504.
|
| [21] |
Estrada IA, Donthamsetty R, Debski P, et al. STIM1 restores coronary endothelial function in type 1 diabetic mice[J].
Circ Res, 2012, 111(9): 1166-75.
DOI: 10.1161/CIRCRESAHA.112.275743. |
| [22] |
徐小红, 阮骆阳, 田小华, 等. 高糖激活Ca~(2+)-CaN-NFAT3信号通路致H9c2细胞肥大[J].
中国病理生理杂志, 2015, 31(11): 2016-20.
DOI: 10.3969/j.issn.1000-4718.2015.11.015. |
| [23] |
Li M, He HP, Gong HQ, et al. NFATc4 and myocardin synergistically up-regulate the expression of LTCC alpha 1C in ET-1-induced cardiomyocyte hypertrophy[J].
Life Sci, 2016, 155(6): 11-20.
|
| [24] |
Fang T, Cao R, Wang W, et al. Alterations in necroptosis during ALDH2mediated protection against high glucoseinduced H9c2 cardiac cell injury[J].
Mol Med Rep, 2018, 18(3): 2807-15.
|
| [25] |
Ueta CB, Campos JC, Prestes e Albuquerque R, et al. Cardioprotection induced by a brief exposure to acetaldehyde: role of aldehyde dehydrogenase 2[J].
Cardiovasc Res, 2018, 114(7): 1006-15.
DOI: 10.1093/cvr/cvy070. |
| [26] |
Zhang T, Zhao Q, Ye F, et al. Alda-1, an ALDH2 activator, protects against hepatic ischemia/reperfusion injury in rats via inhibition of oxidative stress[J].
Free Radic Res, 2018, 52(6): 629-38.
DOI: 10.1080/10715762.2018.1459042. |
| [27] |
Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction[J].
Curr Heart Fail Rep, 2014, 11(4): 354-65.
DOI: 10.1007/s11897-014-0223-7. |
| [28] |
Hua Y, Chen HM, Zhao XY, et al. Alda1, an aldehyde dehydrogenase2 agonist, improves longterm survival in rats with chronic heart failure following myocardial infarction[J].
Mol Med Rep, 2018, 18(3): 3159-66.
|
| [29] |
Pan GD, Deshpande M, Thandavarayan RA. ALDH2 inhibition potentiates high glucose stress-induced injury in cultured cardiomyocytes[J].
J Diabetes Res, 2016, 12(2): 1390861.
|
| [30] |
Gu X, Fang T, Kang P, et al. Effect of ALDH2 on high glucoseinduced cardiac fibroblast oxidative stress, apoptosis, and fibrosis[J].
Oxid Med Cell Longev, 2017, 10(9): 9257967.
|
 2018, Vol. 38
2018, Vol. 38

