2. 蚌埠医学院生理学教研室,安徽 蚌埠 233000
2. Department of Physiology, Bengbu Medical College, Bengbu 233000, China
心血管疾病是严重威胁人类健康的常见病,糖尿病是心血管疾病的独立危险因素[1],高血糖可以直接损伤心肌细胞,也可以介导成纤维细胞和内皮细胞诱发心肌损伤。糖尿病心肌损伤与炎症反应、氧化应激及胰岛素抵抗等密切相关[2-3]。有研究报道35%乙醇长期过量摄入(>90 g/d)可降低胰岛素的敏感性,使糖尿病的危险性增加,而20%酒精摄入可以增加胰岛素敏感性,降低氧化应激和炎症反应,改善糖尿病患者心肌梗死后的心脏功能[4-5]。Miyamae等[6]通过对大鼠加入2.5%和5%的乙醇日常饮用发现,5%乙醇在暴露3周后即可对大鼠产生部分心脏保护作用,6周后这种保护作用更加明显。人脐静脉内皮细胞体外实验进一步说明,抑制乙醛脱氢酶2(ALDH2)活性可导致炎性分子升高,激活ALDH2后可通过调节MAPK及NF-κB信号通路影响斑块的发展和炎症的易感性[7],我们前期研究也表明采用低剂量乙醇摄入激动ALDH2能减轻糖尿病大鼠心肌缺血/再灌注所致的损伤[8]。而Matsuse等[9]报道10%的乙醇摄入后可诱导气道肥大细胞释放组胺,导致易感人群哮喘的加重,而在哮喘小鼠模型中,其代谢产物乙醛可导致GM-CSF和NF-κB的激活,增加螨虫过敏性炎症的发生。然而,乙醇干预对心肌组织NF-κB信号通路有何影响报道鲜少。
NF-κB是一类具有多向转录调节作用的核蛋白因子,广泛存在于多种细胞组织中,激活参与许多基因的转录调控,在免疫、炎症、氧化应激、细胞增殖和凋亡等生理病理过程中发挥作用[10-11]。IKK的诱导激活是NF- κB途径诱导活化的启动步骤[12]。糖尿病与NF-κB信号通路也存在着密切的关系[13-14]。炎症因子通过IKK/NF- κB信号通路,干扰胰岛素信号转导,导致胰岛素抵抗;糖尿病患者体内高血糖与蛋白质产生非酶糖化反应使NF-κB表达增加,产生大量的ROS,导致心血管内皮细胞损伤和平滑肌细胞增殖,最终引起糖尿病大鼠线粒体功能障碍及心功能不全[15-16]。Xu等[17]研究发现:乙醛脱氢酶2(ALDH2)活化后,可通过调节NF-κB信号通路,减少4-HNE在肺动脉平滑肌细胞的异常增值和迁移,进而改善肺动脉高压所引起的血管重构,ALDH2的心肌保护作用的发挥是否与NF-κB信号通路有关,引起了我们的兴趣。
本实验中,我们成功复制了糖尿大鼠心肌损伤模型,通过ALDH2的非特异性激动剂——低剂量乙醇干预,观察糖尿病心肌损伤后NF-κB信号通路存在何种变化,并探讨可能的机制,以便为糖尿病患者的心肌损伤寻求更多更有针对性的治疗措施。
1 材料和方法 1.1 实验动物和材料选取18只体质量在180~200 g的清洁级雄性SD大鼠,蚌埠医学院动物实验中心提供。乙醇(安特);链脲佐菌素(STZ,sigma);ECL发光试剂盒(Millipore);羊抗小鼠二抗(博士德);羊抗NF-κB、IKK、β-actin单克隆抗体(santa cruz)。
1.2 实验方法 1.2.1 制备糖尿病心肌损伤模型及实验分组雄性SD大鼠适应性喂养1周后随机分为3组,每组6只,分为正常对照组、糖尿病组及糖尿病+低剂量乙醇组。根据我们既往实验及Miyamae等实验方法[6, 8],糖尿病大鼠模型的制备采用腹腔注射STZ 55 mg/kg,3 d后测定大鼠空腹血糖,模型复制成功指标:血糖≥16.7 mmol/L持续1周。糖尿病组大鼠正常饮食、饮水(标准啮齿类动物饲料约345 g/d,饮水量约220 mL/d),糖尿病+低剂量乙醇组先以2.5%的乙醇适应性喂养1周(啮齿类动物饲料约300 g/d,饮水量约200 mL/d,乙醇含量20 mg/kg/d),之后以5%的乙醇持续喂养至8周(啮齿类动物饲料约300 g/d,饮水量约200 mL/d,乙醇含量39.45 mg/kg/d);正常对照组大鼠常规喂养持续至8周(啮齿类动物饲料约257 g/d,饮水量约125 mL/d)[2]。
1.2.2 小动物超声测定大鼠心功能分别在实验的不同时期测定大鼠心功能,以大鼠心电图的QRS波群和T波作为收缩期和舒张期标志,结合图像上二尖瓣的开闭进行各房室内径的测量。在左心长轴切面上测量并记录大鼠心脏的左室内径(LVIDd),左房内径(LVIDs);通过M型超声获得射血分数(EF)和左室内径缩短率(FS),所有数据均测量3次取平均值,为减少大鼠个体差异所造成的误差,采用体表面积进行校正,体表面积(m2)= 0.09× [体质量(kg)] 2/3。
1.2.3 血浆IL-1及IL-4水平测定动物麻醉后,腹主动脉取血2 mL,离心分离血浆,试剂盒测定IL-1及IL-4的水平。
1.2.4 糖尿病大鼠心肌组织4-HNE测定取0.1 g心肌组织,在冰冻PBS缓冲液中匀浆。4-HNE根据试剂盒说明书操作,画出标准曲线,计算心肌组织样本的表达水平。
1.2.5 蛋白印迹测定心肌NF-κB、IKK表达剪取0.1 g心肌组织,组织匀浆后裂解蛋白,离心机离心后取上清液,制胶后进行样品处理,取蛋白60 µg加样,转膜封闭后,加入NF-κB、IKK及内参β-actin抗体,4 ℃冰箱过夜,次日,TBST洗膜5 min/次×4,加入2抗37 ℃孵育1 h,洗膜10 min/次×3,ECL系统进行检测;NF-κB、IKK蛋白相对表达量=NF-κB、IKK/β-actin条带灰度比值。
1.2.6 透视电镜观察心肌组织变化各组心脏标本取左心室同部位心肌组织切块,1 mm×1 mm×1 mm大小,立刻浸入30 g/L戊二醛前固定,10 g/L鋨酸后固定,梯度酒精脱水,之后浸透、包埋、切片,常规醋酸铀和柠檬酸铅染色,由电镜观察心肌超微结构变化。
1.2.7 免疫组化检测心肌组织NF-κB的蛋白胞内定位石蜡切片脱蜡水化,修复抗原,滴加封闭液后加一抗,冲洗,加二抗,孵育后DAB显色,苏木青复染,封片。免疫组织结果判断标准:阳性强度无色或少量棕黄色:(-);浅棕色:弱阳性(+);中等棕黄色:中等阳性(++),深棕黄色:强阳性(++++)。采用计算机图像分析技术进行定量分析:HPLAS-2000高清晰度彩色病理图文报告系统对蛋白进行分析,每张切片随机选取10个视野,阳性面积率=阳性反应总面积/单位面积中细胞总面积×100% [5]。
1.3 统计学分析实验数据以均数±标准差表示,应用SPSS17.0统计软件进行分析,组间采用One-way ANOVA进行统计学分析。P < 0.05表示差异有统计学意义。
2 结果 2.1 各组大鼠心功能比较与正常对照组比较,糖尿病组LVIDd、LVIDs增大,EF、FS降低(P < 0.01),与糖尿病组相比,糖尿病+低剂量乙醇组LVIDd、LVIDs减小,而EF、FS升高(P < 0.01,表 1)。
| 表 1 各组大鼠左室心功能变化 Table 1 Changes of left ventricular functions in different groups (Mean±SD, n=6) |
与正常对照组相比,糖尿病组心肌肌丝断裂、肌原纤维结构不完整、线粒体损伤;而糖尿病+低剂量乙醇组肌原纤维和线粒体损伤减轻(图 1)。
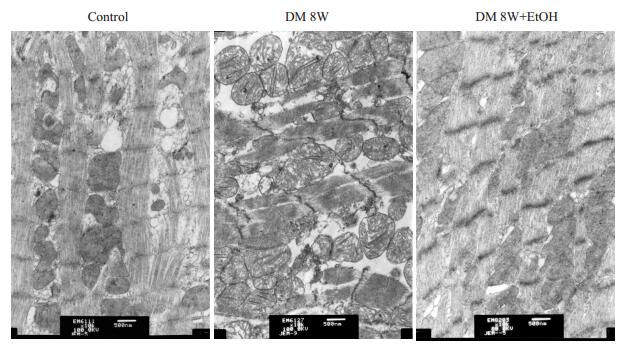
|
图 1 各组大鼠心肌超微结构变化 Figure 1 Ultrastructure of the myocardium of the rat in different groups (Original magnification: ×10 000). |
与正常对照组比较,糖尿病组IL-1的水平增加,IL-4的水平降低(P < 0.01),与糖尿病组相比,糖尿病+低剂量乙醇组IL-1的水平降低,而IL-4的水平增加(P < 0.01,表 2)。
| 表 2 各组大鼠血浆IL-1和IL-4水平变化 Table 2 Changes of plasma IL-1 and IL-4 levels in different groups (Mean±SD, n=6) |
与正常对照组比较,糖尿病组NF-κB、IKK的蛋白表达增加(P < 0.01),而糖尿病+低剂量乙醇组NF-κB、IKK的蛋白表达无明显差异(P>0.05),与糖尿病组相比,糖尿病+低剂量乙醇组NF-κB、IKK的蛋白表达降低(P < 0.01,图 2)。
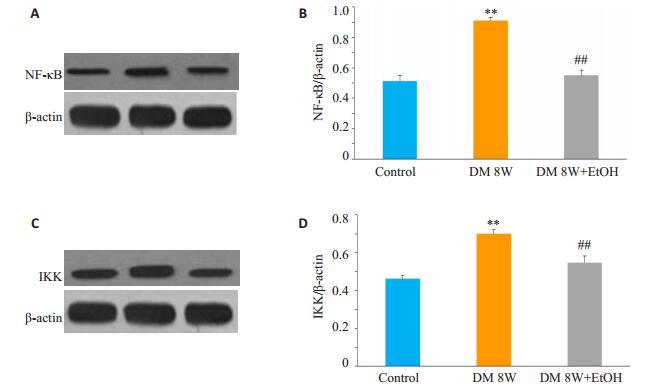
|
图 2 各组大鼠NF-ƘB、IKK蛋白表达和定量分析 Figure 2 Myocardial expressions of NF-κB and IKK protein detected by Western blotting (A, C) and quantitative analysis of their protein expressions (B, D) in diabetic rats (Mean±SD, n=6). **P < 0.01 vs Control group; ##P < 0.01 vs DM 8W. |
正常对照组心肌细胞胞浆内可见少量分布的棕黄色颗粒,阳性表达(+);糖尿病组心肌NF-κB蛋白分布增加,表达面积率明显高于正常对照组(P < 0.01);糖尿病+低剂量乙醇组心肌NF-κB蛋白分布减少,表达面积明显低于糖尿病组(P < 0.01),与正常对照组无明显差异(P> 0.05,图 3、4)。
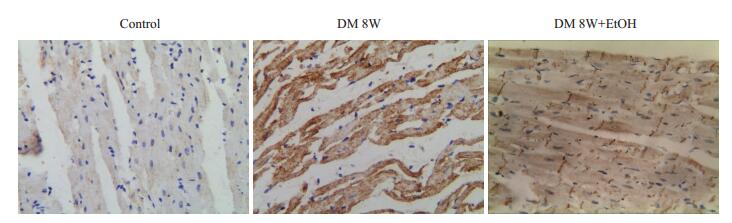
|
图 3 免疫组化检测各组大鼠NF-ƘB的蛋白定位分布 Figure 3 Immuohistochemistry of NF-κB protein in the myocardium of the rats (SP method, ×400). |
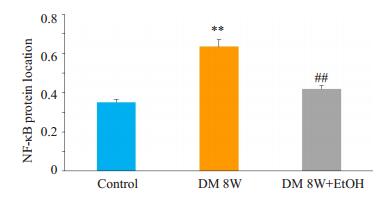
|
图 4 各组大鼠NF-κB的蛋白定位分布 Figure 4 Changes of NF-KB protein in different groups (Mean±SD, n=6). **P < 0.01 vs Control group; ##P < 0.01 vs DM 8W. |
与正常对照组比较,糖尿病组4-HNE的表达增加(P < 0.01),与糖尿病组相比,糖尿病+低剂量乙醇组4-HNE的表达降低(P < 0.01,表 3)。
| 表 3 各组大鼠心肌组织4-HNE变化 Table 3 Levels of myocardial 4-HNE in different groups (Mean± SD, n=6) |
糖尿病作为心血管疾病的等危症,通过炎症损伤、氧化应激、线粒体功能障碍以及心肌细胞凋亡等参与到慢性心血管病变中[18]。本实验中,与正常对照组比较,糖尿病组大鼠LVIDd、LVIDs增大,EF、FS降低,同时血浆IL-1水平升高、IL-4水平降低,心肌组织4-HNE的水平增加,心肌超微结构显示肌原纤维和线粒体损伤,提示糖尿病可引起心肌炎症损伤,导致左心功能障碍。
糖尿病中存在的胰岛素抵抗是一种低度炎症状态,通过炎症反应和氧化应激引发心肌慢性损害,影响左心室功能[19-20]。各种炎症因子在糖尿病心肌损害的发展中起着重要角色,IL-1参与糖尿病心肌病的炎症反应并导致心肌结构改变,抗炎细胞因子IL-4则对心肌有着保护作用[21-22]。4—羟基壬烯醛(4-HNE)是氧化应激过程中产生的脂质过氧化醛基终产物,可引起组织的纤维化并导致细胞凋亡,并可导致炎性介质的释放,引起组织的炎症反应[23-24]。本实验中,我们观察到,与正常对照组相比,糖尿病组大鼠IL-1、4-HNE水平升高,IL-4水平降低,提示糖尿病可明显加重心肌细胞的氧化应激和炎性损伤。与糖尿病组相比,低剂量乙醇干预后,糖尿病大鼠IL-1、4-HNE水平降低,IL-4水平升高,提示低剂量乙醇可减轻氧化应激和炎性损伤。
线粒体乙醛脱氢酶2(ALDH2)是线粒体内重要的醛类氧化酶。乙醇在乙醇脱氢酶的作用下转化成乙醛,ALDH2可分解乙醛及其代谢产物4-HNE,ALDH2减轻其对细胞的损伤。近年的研究表明ALDH2参与氧化应激、细胞凋亡等病理和生理过程。Gomes等[25]发现激动ALDH2的表达可以通过改善线粒体功能,抑制心衰大鼠心脏细胞色素C的释放,从而改善心功能;孙爱军等[26]发现ALDH2对心肌细胞的保护作用与加快4- HNE的消除有关。而我们前期研究表明作为ALDH2的非特异性激动剂,低剂量乙醇干预能显著提高心肌ALDH2的表达,使ALDH2的活性增加,减轻DM组大鼠心肌缺血再灌注损伤,激活ALDH2可显著抑制高糖诱导的心肌细胞所致的细胞凋亡和心肌纤维化[8, 27]。在此基础上,我们进一步观察糖尿病状态下,低剂量乙醇是否通过NF-κB信号通路发挥心肌保护作用。
NF-κB是调节氧化还原和炎症反应的敏感核转录因子,被4-HNE通过线粒体ROS活化而表达增加,并使IL-1、IL-6等炎症因子过度表达[28]。κB激酶(IKK)由IKKα、IKKβ、IKKBγ、IKAP等4个亚基组成,其接受外界刺激被激活并使NF-κB磷酸化,调节下游基因的转录表达。研究发现NF-κB、IL-1在缺血再灌注受损脑组织及小鼠受损肝细胞中表达升高,同时,肠道免疫功能也受IL-1、IL-4及NF-κB的调节[29-31]。在糖尿病大鼠心肌细胞中,Hou等[32]也检测到高表达的IL-1和NF-κB,并使心肌细胞最终走向凋亡。本实验中,糖尿病大鼠炎症反应和氧化应激增加的同时,心肌细胞NF-κB及其激酶IKK表达增加,NF-κB胞内分布增加,提示NF-κB可能参与了糖尿病的心肌损伤。给予低剂量乙醇干预后,糖尿病大鼠炎症反应、氧化应激、心肌超微结构损伤降低,心脏舒缩功能改善,同时观察到NF-κB、IKK蛋白表达降低,NF-κB胞内分布减少,提示:低剂量乙醇可能通过抑制NF-κB信号通路减轻糖尿病心肌损伤。
综上所述,糖尿病大鼠炎症反应和凋亡增加时,心肌细胞NF-κB、IKK的蛋白表达增加,NF-κB蛋白的胞内分布增加,给予低剂量乙醇处理后,糖尿病大鼠IL-1、4-HNE表达减少,血浆IL-4表达增加,左心室功能和心肌超微结构均较普通糖尿病大鼠改善,而NF-κB、IKK的蛋白表达降低,NF-κB蛋白的胞内分布降低,我们推测:NF-κB通路参与了糖尿病对心肌的损伤过程,低剂量乙醇可能是通过抑制NF-κB信号通路来减轻糖尿病对心肌的损伤。
| [1] |
Tokoro F, Matsuoka R, Abe S, et al. Association of a genetic variant of the ZPR1 Zinc finger gene with type 2 diabetes mellitus[J].
Biomed Rep, 2015, 3(1): 88-92.
DOI: 10.3892/br.2014.379. |
| [2] |
Liu P, Su JF, Song XX, et al. Activation of nuclear beta-catenin/cMyc axis promotes oxidative stress injury in streptozotocin-induced diabetic cardiomyopathy[J].
Biochem Biophys Res Commun, 2017, 493(4): 1573-80.
DOI: 10.1016/j.bbrc.2017.10.027. |
| [3] |
Williams LJ, Nye BG, Wende AR. Diabetes-Related cardiac dysfunction[J].
Endocrinol Metab (Seoul), 2017, 32(2): 171-9.
DOI: 10.3803/EnM.2017.32.2.171. |
| [4] |
Peng HC, Chen YL, Yang SY, et al. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats[J].
Hepatobiliary Surg Nutr, 2013, 2(3): 132-41.
|
| [5] |
Hong J, Smith RR, Harvey AE, et al. Alcohol consumption promotes insulin sensitivity without affecting body fat levels[J].
Int J Obes (Lond), 2009, 33(2): 197-203.
DOI: 10.1038/ijo.2008.266. |
| [6] |
Miyamae M, Diamond I, Weiner MW, et al. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury[J].
Proc Natl Acad Sci USA, 1997, 94(7): 3235-9.
DOI: 10.1073/pnas.94.7.3235. |
| [7] |
Pan C, Xing JH, Zhang C, et al. Aldehyde dehydrogenase 2 inhibits inflammatory response and regulates atherosclerotic plaque[J].
Oncotarget, 2016, 7(24): 35562-76.
|
| [8] |
Kang PF, Wu WJ, Tang Y, et al. Activation of ALDH2 with low concentration of ethanol attenuates myocardial ischemia/ reperfusion injury in diabetes rat model[J].
Oxid Med Cell Longev, 2016, 2016: 6190504.
|
| [9] |
Matsuse H, Fukushima C, Shimoda T, et al. Effects of acetaldehyde on human airway constriction and inflammation[J].
Novartis Found Symp, 2007, 285: 97-106; discussion 106-9, 198-9.
|
| [10] |
Jiang C, Tong YL, Zhang D, et al. Sinomenine prevents the development of cardiomyopathy in diabetic rats by inhibiting inflammatory responses and blocking activation of NF-κB[J].
Gen Physiol Biophys, 2017, 36(1): 65-74.
DOI: 10.4149/gpb_2016033. |
| [11] |
Ma Z, Chalkley RJ, Vosseller K. Hyper-O-GlcNAcylation activates nuclear factor k-light-chain-enhancer of activated B cells(NF-kB) signaling through interplay with phosohorylation and acetylation[J].
J Biol Chem, 2017, 292(22): 9150-63.
DOI: 10.1074/jbc.M116.766568. |
| [12] |
Freitas RHCN, Fraga CAM. NF-κB-IKKβ pathway as a target for drug development: realities, challenges and perspectives[J].
Curr Drug Targets, 2018, 2018: 2174.
|
| [13] |
Hu B, Xu G, Zheng Y, et al. Chelerythrine attenuates renal ischemia/ reperfusion-induced myocardial injury by activating CSE/H2S via PKC/NF-κB pathway in diabetic rats[J].
Kidney Blood Press Res, 2017, 42(2): 379-88.
DOI: 10.1159/000477948. |
| [14] |
Jayachandran M, Vinayagam R, Ambati RR, et al. Guava leaf extract diminishes hyperglycemia and oxidative stress, prevents β- cell death, inhibits inflammation, and regulates NF-kB signaling pathway in STZ induced diabetic rats[J].
Biomed Res Int, 2018, 2018: 4601649.
|
| [15] |
Mariappan N, Elks CM, Sriramula S, et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type Ⅱ diabetes[J].
Cardiovasc Res, 2010, 85(3): 473-83.
DOI: 10.1093/cvr/cvp305. |
| [16] |
Annibaldi A, Wicky John S, Vanden Berghe T, et al. UbiquitinMediated regulation of RIPK1 kinase activity Independent of IKK and Mk2[J].
Mol Cell, 2018, 69(4): 566-580.
DOI: 10.1016/j.molcel.2018.01.027. |
| [17] |
Xu T, Liu S, Ma T, et al. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension[J].
Redox Biol, 2017, 11: 286-96.
DOI: 10.1016/j.redox.2016.12.019. |
| [18] |
Dasu MR, Devaraj S, Zhao L, et al. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation[J].
Diabetes, 2008, 57(11): 3090-8.
DOI: 10.2337/db08-0564. |
| [19] |
Suthahar N, Meijers WC, Brouwers FP, et al. Heart failure and inflammation-related biomarkers as predictors of new-onset diabetes in the general population[J].
Int J Cardiol, 2018, 250: 188-94.
DOI: 10.1016/j.ijcard.2017.10.035. |
| [20] |
Srilatha K, Bobby Z, Subrahmanyam DK, et al. Insulin resistance and elevated C-reactive protein among first-degree relatives of ischemic stroke patients[J].
Diabetes Metab Syndr, 2017, 11(Suppl 2): S873-S8738.
|
| [21] |
Korkmaz-Icöz S, Lehner A, Li S, et al. Left ventricular pressurevolume measurements and myocardial gene expression profile in type 2 diabetic Goto-Kakizaki rats[J].
Am J Physiol Heart Circ Physiol, 2016, 311(4): H958-71.
DOI: 10.1152/ajpheart.00956.2015. |
| [22] |
Feng B, Chen S, Gordon AD, et al. miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes[J].
J Mol Cell Cardiol, 2017, 105: 70-6.
DOI: 10.1016/j.yjmcc.2017.03.002. |
| [23] |
Chandramoorthy HC, Bin-Jaliah I, Karari H, et al. MSCs ameliorates DPN induced cellular pathology via[Ca2 + ]i homeostasis and scavenging the pro-inflammatory cytokines[J].
J Cell Physiol, 2018, 233(2): 1330-41.
DOI: 10.1002/jcp.26009. |
| [24] |
Shoeb M, Ansari NH, Srivastava SK, et al. 4-Hydroxynonenal in the pathogenesis and progression of human diseases[J].
Curr Med Chem, 2014, 21(2): 230-7.
|
| [25] |
Gomes KM, Campos JC, Bechara LR, et al. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling[J].
Cardiovasc Res, 2014, 103(4): 498-508.
DOI: 10.1093/cvr/cvu125. |
| [26] |
孙爱军, 徐丹令, 邹云增, 等. 乙醛脱氢酶2通过缺氧诱导因子/热休克蛋白因子及P53途径抑制心肌细胞凋亡[J].
上海医学, 2007, 30(S1): 174.
|
| [27] |
Gu X, Fang T, Kang P, et al. Effect of ALDH2 on high glucoseinduced cardiac fibroblast oxidative stress, apoptosis, and fibrosis[J].
Oxid Med Cell Longev, 2017, 2017: 9257967.
|
| [28] |
Müller I, Beissert S, Kulms D. Anti-apoptotic NF-κB and "gain of function" mutp53 in concert act pro-apoptotic in response to UVB+ IL-1 via enhanced TNF production[J].
J Invest Dermatol, 2015, 135(3): 851-60.
DOI: 10.1038/jid.2014.481. |
| [29] |
Liu XE, Zhang XY, Wang FL, et al. Improvement in cerebral ischemia-reperfusion injury through the TLR4/NF kappa B pathway after Kudiezi injection in rats[J].
Life Sci, 2017, 191: 132-40.
DOI: 10.1016/j.lfs.2017.10.035. |
| [30] |
Zheng X, Feng L, Jiang WD, et al. Dietary pyridoxine deficiency reduced growth performance and impaired intestinal immune function associated with TOR and NF-kappa B signalling of young grass carp (Ctenopharyngodon idella)[J].
Fish Shellfish Immunol, 2017, 70: 682-700.
DOI: 10.1016/j.fsi.2017.09.055. |
| [31] |
Liu ZW, Zhao N, Zhu HL, et al. Circulating interleukin-1 beta promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2[J].
Cardiovasc Diabetol, 2015, 14: 125.
DOI: 10.1186/s12933-015-0288-y. |
| [32] |
Hou J, Zheng DZ, Fung G, et al. Mangiferin suppressed advanced glycation end products (AGEs) through NF-kappa B deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats[J].
Can J Physiol Pharmacol, 2016, 94(3): 332-40.
DOI: 10.1139/cjpp-2015-0073. |
 2018, Vol. 38
2018, Vol. 38

