2. 广东省妇幼保健院药学部,广东 广州 511400;
3. 广东省心血管病研 究所,广东省人民医院//广东省医学科学院医学研究中心,广东 广州 510080
2. Department of Pharmacy, Guangdong Women and Children's Hospital, Guangzhou 511400, China;
3. Research Center of Medical Sciences, Guangdong Cardiovascular Institute, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
心肌纤维化是多种心血管系统疾病终末期的共同病理表现,它可能导致局部胶原失衡和代谢紊乱[1-2],常伴有多种心血管疾病,如心律失常、心力衰竭、动脉粥样硬化等[3-4]。当心肌因缺血、炎症、衰老等原因受损时,局部心肌细胞凋亡,取而代之的是心肌成纤维细胞增殖和以胶原纤维为主的细胞外基质增多,最终导致心肌纤维化发生[5]。纤维化的心肌僵硬度增加、顺应性减少、收缩力下降、同步性降低,使得心脏功能逐步减退[6-7]。由于持续性心肌纤维化可导致慢性缺血性心脏病,因此研究心肌纤维化的调控机制具有重要意义。
微小RNA(miRNA)通过作用于靶基因3'非翻译区(3'UTRs)促进mRNA降解或抑制mRNA的翻译来调控基因表达[8-9]。目前,许多研究报道了miRNAs在多种病理生理过程中的关键作用,其中包括心肌纤维化[10-11]。我们前期发现,miR-199a-3p在血管紧张素Ⅱ(Ang-Ⅱ)诱导纤维化的小鼠心肌中表达显著增强,但其对心肌纤维化的作用机制尚不明确。
Smad1属于转化生长因子-β(TGF-β)超家族,TGF-β是一类在进化上高度保守的大家族多效行的细胞因子。Smad1蛋白属于TGF-β家族中AMH和BMPs的通路受体底物[12],被证实具有抑制肿瘤发生的功能[13-14]。然而,Smad1在小鼠心肌纤维中的作用机制尚未完全阐明,也未见miR-199a-3p是否调控Smad1表达的报道。本文将探究miR-199a-3p对小鼠心肌成纤维细胞中纤维化相关基因表达的调控作用及其作用的靶基因。
1 材料和方法 1.1 主要试剂DMEM/F12细胞培养基(Hyclone);特级澳洲胎牛血清(Gibco);0.25% EDTA-胰蛋白酶(Gibco);限制性内切酶Xho I、EcoR I、转染试剂Lipofectamine 2000、TRIzol、4×SDS loading buffer(Invitrogen)和逆转录试剂盒;2 × SYBR Green Mix和RNAase free water(TaKaRa);miR-199a-3p mimic和Smad1 siRNA(广州锐博);SDS-PAGE凝胶配置试剂盒(碧云天);Smad1抗体、p-Smad1抗体,Smad3抗体、p-Smad3抗体蛋白、GAPDH抗体、Col1a1抗体、Col3a1抗体(Protein Technology),α-平滑肌肌动蛋白(α- smooth muscle actin,α-SMA)抗体(Abcam);BCA蛋白定量试剂盒(Thermo);Marker(Fermentas);ECL发光液(Millipore);PVDF膜(Whatman);其他生化试剂均为进口分装或国产分析纯。
1.2 主要方法 1.2.1 原代分离培养小鼠心肌成纤维细胞和处理将出生3~4周SPF级C57BL/6小鼠心脏组织,以0.25% EDTA-胰蛋白酶消化法分离细胞。离心弃去上清液,沉淀用完全培养基(含10%胎牛血清及1×10-5 U/L青霉素和100 mg/L链霉素的DMEM/F12培养基)重悬浮,培养于25 cm2培养瓶中,置于37 ℃,5% CO2培养箱中培养。过夜,更换1次完全培养基。传代到P3代分别将100 nmol/L scramble对照、miR-199a-3p mimic和Smad1 siRNA转染到小鼠心肌成纤维细胞中,24 h后结束实验。
1.2.2 miR-199a-3p mimic和Smad1 siRNA瞬时转染至小鼠心肌成纤维细胞小鼠心肌成纤维细胞转染:按照脂质体Lipofectamine 2000产品说明书进行操作,取P2代心肌成纤维细胞接种于12孔板(细胞密度2×105/孔),待细胞贴壁过夜,弃去旧培养液用磷酸盐缓冲液(PBS)洗涤细胞2次,每孔加900 μL含1%胎牛血清的完全培养基;制备miR-199a-3p mimic和Smad1 siRNA(或miR-Negative Control,即Scramble)/脂质体复合物,12孔板每孔剂量用量如下:取1.6 μL脂质体至50 μL DMEM/F12培养基中充分混匀,室温静置5 min;同时每孔0.1 nmol scramble对照、miR-199a-3p mimic和Smad1 siRNA分别加至50 μL DMEM/F12培养基中充分混匀,室温静置5 min;用移液器分别取50 μL脂质体混合物至另外3个混合物中,充分混匀,室温静置20 min;用移液器分别取100 μL scramble对照、miR-199a-3p mimic和Smad1 siRNA/脂质体复合物至孔中,置于37 ℃,5% CO2培养箱中培养,转染24 h后结束实验。
1.2.3 荧光定量PCR检测Smad1以及纤维化标志物等基因表达情况用Trizol法提取心肌成纤维细胞总RNA。取1.0 μg总RNA,加入5×逆转录试剂4 μL(逆转录试剂盒),用oligo(dT)15和random primers逆转录出cDNA用于检测Smad1以及纤维化标志物的mRNA表达水平。取1.0 μg总RNA,用miR-199a-3p特异的RT引物逆转录出cDNA用于检测miR-199a-3p表达水平。分别用GAPDH和U6作为检测Smad1、纤维化标志物和miR-199a-3p表达水平的内参照。在vii A7 Quantitative PCR System(美国Applied Biosystems)进行PCR反应和结果分析。以2-∆∆Ct法计算Smad1,纤维化标志物和miR-199a-3p的相对表达水平。
1.2.4 Western blot法检测蛋白水平向处理后的小鼠心肌成纤维细胞,加入RIPA蛋白裂解液,冰上裂解,于4 ℃ 12 000r/min离心15min,取上清进行蛋白质定量后分装,加入4×上样缓冲液,70 ℃加热10min使蛋白质变性,然后进行聚丙烯酰胺凝胶电泳。用PVDF膜转膜,5%脱脂奶粉封闭2 h,分别用相应的抗体anti-Col1a1(1:1000)、anti-Col3a1(1:5000)、anti-α-SMA(1:5000)、anti-Smad1(1:1000)和anti-Smad3(1:1000)4℃孵育过夜。TBST洗膜,加入二抗(1:5000)室温孵育1 h。ECL发光试剂盒显影,以GAPDH(1:5000)为内参照,扫描灰度值并分析蛋白表达相对含量。
1.2.5 双荧光素酶报告实验验证miR-199a-3p与Smad1 3'-UTR的结合作用参照已报道方法[15]分别构建包含miR-199a-3p潜在结合序列的Smad1 3'UTR重组萤光素酶报告质粒pGL3-Smad1-107-126,pGL3- Smad1-521-545及包含结合序列突变的重组质粒pGL3-Smad1-107-126-MUT,pGL3-Smad1-521-545- MUT。利用HEK293细胞,将HEK293细胞(细胞密度约为1×105每孔/12孔板)转染1.5 μg重组荧光素酶报告质粒,100 nmol/L miR-199a-3p mimic以及10 ng pRLTK(表达海肾荧光素酶的内参照质粒)。转染24 h后,测定萤火虫荧光素酶(FL)及海肾荧光素酶(RL)强度,两种荧光强度比值(FL/RL)变化可反映miR-199a-3p与Smad1 3'UTR结合的能力。
1.3 统计学处理用SPSS 20.0统计软件进行分析。计量资料以均数±标准差表示,两组间比较采用t检验;多组间比较采用单因素方差分析,并用Bonferroni校正的t检验进行组间两两比较。P < 0.05为差异有统计学意义。
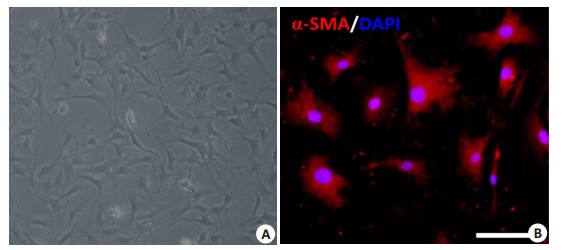
2 结果 2.1 小鼠心肌成纤维细胞形态和鉴定从C57BL/6小鼠心肌组织中分离出的成纤维细胞呈球状。过夜后更换培养基,可见细胞完全贴壁,但形态较小。3 d后,成纤维细胞完全伸展开,形态呈偏平梭形,能看清晰的细胞轮廓。通过细胞免疫荧光法,用α- SMA对P3代成纤维细胞进行标记鉴定。本研究采用P3代心肌成纤维细胞进行细胞学实验。
|
图 1 原代分离小鼠心肌成纤维细胞形态和鉴定 Figure 1 Morphology and identification of cardiac fibroblasts by immunofluorescence assay. A: Mouse cardiac fibroblasts under optical microscope; B: Expression of α-SMA in the cultured cardiac fibroblasts. Scale bar: 100 μm. |
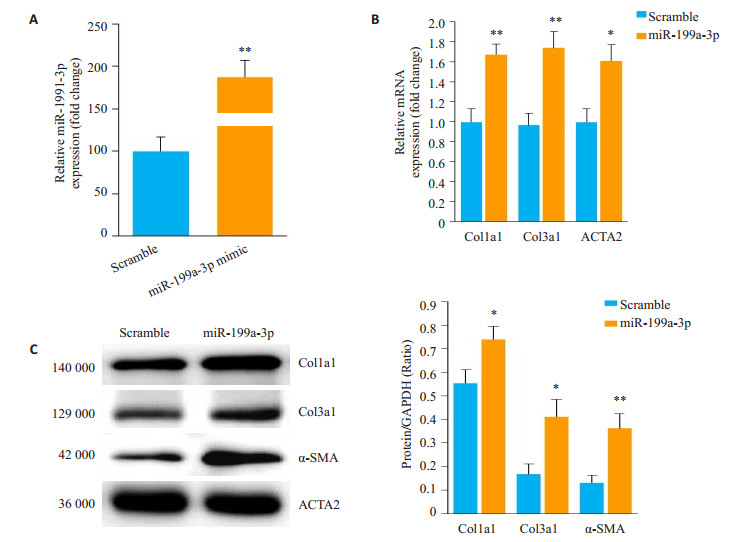
利用体外合成miR-199a-3p mimic,转染C57BL/6小鼠来源的成纤维细胞后,检测纤维化相关基因和蛋白表达情况。RT- qPCR结果显示,转染miR-199a-3p mimic的小鼠心肌成纤维细胞中miR-199a-3p水平显著升高(图 2A),纤维化相关基因Col1a1、Col3a1和ACTA2表达明显增强(图 2B)。Western blot检测显示,miR-199a-3p mimic过表达的小鼠心肌成纤维细胞中,纤维化相关蛋白Col1a1、Col3a1和α-SMA表达明显增强(图 2C)。
|
图 2 miR-199a-3p对纤维化相关基因的调控作用 Figure 2 Effect of miR-199a-3p on the expression of fibrosis-related genes in cardiac fibroblasts. A: Level of miR- 199a-3p after transfection with miR-199a-3p mimic; B: Upregulated mRNA levels of fibrosis-related genes in mouse cardiac fibroblasts transfected with miR-199a-3p mimic; C: Up-regulated protein levels of fibrosis-related genes in mouse cardiac fibroblasts transfected with miR-199a-3p mimic. *P < < 0.05, **P < 0.01 vs Scramble (n=3). |
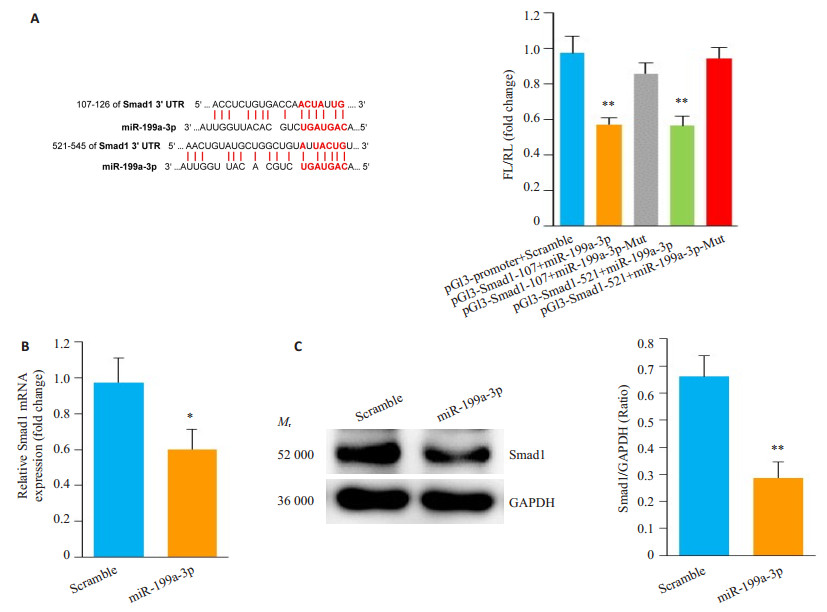
本文基于Targetscan-Vert (www.targetscan.org)的序列分析提示,Smad1的3'UTR的107-126和521-545区域有miR-199a-3p的潜在结合位点。双荧光素酶报告基因实验结果显示,转染pGL3-Smad1-107和pGL3- Smad1-521质粒的HEK293细胞中,荧光素酶活性较对照组明显下降,而转染含突变结合序列的质粒pGL3- Smad1-MUT则对荧光素酶的活性无明显影响。以上结果证明,miR-199a-3p能够特异与Smad1 3'UTR的107-126和521-545位点结合,从而抑制结合位点上的萤火虫荧光素酶的表达(图 3A)。
|
图 3 miR-199a-3p靶基因鉴定 Figure 3 Identification of Smad1 as the potential target gene of miR-199a-3p. A: The predicted miR-199a-3p seed sequence matches to the sequence in the 3'UTR of Smad1 mRNA. The seed sequence of miR- 199a- 3p is CAGUAGU, and the complementary nucleotide sequences are highlighted in red; B: Smad1 mRNA expression in cardiac fibroblasts transfected with miR- 199a- 3p mimic; C: Smad1 protein expression in cardiac fibroblasts transfected with miR-199a-3p mimic, *P < 0.05, **P < 0.01, vs Scramble group (n=3). |
RT-qPCR结果显示,利用小鼠心肌成纤维细胞,转染miR-199a-3p mimic,Smad1 mRNA水平显著降低(图 3B)。同样转染处理小鼠心肌成纤维细胞,Western blot结果证明,相对于Scramble组,miR-199a-3p转染组中Smad1蛋白表达显著性下调(图 3C)。
2.4 miR-199a-3p靶向Smad1并激活Smad3信号通路Western blot结果显示,过表达miR-199a-3p和沉默Smad1,均可以降低Smad1蛋白表达,降低磷酸化Smad1表达水平,上调纤维化相关蛋白Col1a1、Col3a1和α-SMA表达。同时发现,磷酸化Smad3蛋白水平显著性增加。
3 讨论miR-199a-3p和miR-199a-5p是来自共同miR-199a stem-loop前体的序列不同的2个miRNA;RNA测序结果提示细胞中miR-199a-3p水平高于miR-199a-5p(www.mirbase.org)。研究表明,TGF-β1可下调人肺成纤维细胞的肝细胞生长因子表达,肝细胞生长因子被证实是miR-199a-3p作用靶基因并介导miR-199a-3p的促肺成纤维细胞的纤维化表型[16]。在肺气道囊性纤维化的研究发现,囊性纤维化的病人miR-199a-3p的表达水平下调。过表达人支气管上皮细胞miR-199a-3p实验证明,miR-199a-3p可降低了IKkβ蛋白的表达,抑制NF-κB信号活性,减少IL-8的分泌,抑制肺气道囊性纤维化进程[17]。miR-199a-3p对心肌缺血再灌注损伤有保护作用,凋亡相关基因ddit 4和ing4是miR-199a-3p的作用靶点,ddit 4和ing4在过表达miR-199a-3p的心肌细胞中表达降低,并介导miR-199a-3p抑制心肌细胞凋亡的作用[18]。有研究发现外周血中miR-199a-3p水平可能与心力衰竭发病相关[19]。而目前关于miR-199a-3p在心肌纤维化中可能的作用和机制尚不明确。
我们证实miR-199a-3p可促进小鼠心肌成纤维细胞中纤维化相关基因表达,进而明确miR-199a-3p具有促进心肌纤维化的作用。双荧光素酶报告基因实验提示miR-199a-3p能和Smad1 3'UTR特异性结合,可在mRNA和蛋白水平抑制心肌成纤维细胞中Smad1表达,说明miR-199a-3p在转录水平抑制Smad1表达。进一步证实miR-199a-3p和Smad1 siRNA可一致性地激活Smad3信号通路,促进心肌成纤维细胞纤维化相关基因表达。因此,本文结果证实Smad1是miR-199a-3p的靶基因,参与介导了miR-199a-3p促进心肌成纤维细胞中纤维化相关基因的表达,进而发挥促进心肌纤维化作用。本研究结果与以往在软骨细胞[20]和前列腺癌细胞[21]中证实Smad1介导miR-199a-3p发挥生物学作用的报道一致。
TGF-β/Smad3信号通路与心肌纤维化的发生和发展密切相关[22-24]。过表达miR-199a-3p的小鼠心肌纤维中Smad1和p-Smad1水平降低,但p-Smad3水平升高,进而促进纤维化相关基因表达。本文结果与以往p-Smad1发挥负调控p-Smad3作用的报道方面一致[25]。
综上所述,本文阐明miR-199a-3p具有促进心肌成纤维细胞中纤维化相关基因表达的作用。证实Smad1是miR-199a-3p的作用靶基因,并介导miR-199a-3p激活Smad3信号通路来增加纤维化相关基因表达。在后续研究中,我们将在整体动物水平,进一步明确miR- 199a-3p对Smad1表达和对心肌纤维化的调控作用。
| [1] |
Thum T. Noncoding RNAs and myocardial fibrosis[J].
Nat Rev Cardiol, 2014, 11(11): 655-63.
DOI: 10.1038/nrcardio.2014.125. |
| [2] |
Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis[J].
Circ Res, 2015, 116(7): 1269-76.
DOI: 10.1161/CIRCRESAHA.116.305381. |
| [3] |
Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure[J].
Heart Fail Rev, 2014, 19(2): 173-85.
DOI: 10.1007/s10741-012-9365-4. |
| [4] |
Thannickal VJ, Zhou Y, Gaggar A, et al. Fibrosis: ultimate and proximate causes[J].
J Clin Invest, 2014, 124(11): 4673-7.
DOI: 10.1172/JCI74368. |
| [5] |
Vasan RS, Benjamin EJ. Diastolic heart failure--no time to relax[J].
N Engl J Med, 2001, 344(1): 56-9.
DOI: 10.1056/NEJM200101043440111. |
| [6] |
Jugdutt B I. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways[J].
Curr Drug Targets Cardiovasc Haematol Disord, 2003, 3(1): 1-30.
DOI: 10.2174/1568006033337276. |
| [7] |
Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling[J].
Pharmacol Ther, 2009, 123(2): 255-78.
DOI: 10.1016/j.pharmthera.2009.05.002. |
| [8] |
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function[J].
Cell, 2004, 116(2): 281-97.
DOI: 10.1016/S0092-8674(04)00045-5. |
| [9] |
Li L, Huang W, Li K, et al. Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway[J].
Oncotarget, 2015, 6(41): 43605-19.
|
| [10] |
Tao L, Bei Y, Chen P, et al. Crucial Role of miR-433 in regulating cardiac fibrosis[J].
Theranostics, 2016, 6(12): 2068-2083.
DOI: 10.7150/thno.15007. |
| [11] |
Zhou Y, Deng L, Zhao D, et al. MicroRNA-503 promotes angiotensin Ⅱ-induced cardiac fibrosis by targeting apelin-13[J].
J Cell Mol Med, 2016, 20(3): 495-505.
DOI: 10.1111/jcmm.2016.20.issue-3. |
| [12] |
Wiegman EM, Blaese MA, Loeffler H, et al. TGFβ-1 dependent fast stimulation of ATM and p53 phosphorylation following exposure to ionizing radiation does not involve TGFβ-receptor I signalling[J].
Radiother Oncol, 2007, 83(3): 289-95.
DOI: 10.1016/j.radonc.2007.05.013. |
| [13] |
Kim SH, Lee SH, Choi YL, et al. Extensive alteration in the expression profiles of TGFB pathway signaling components and TP53 is observed along the gastric dysplasia-carcinoma sequence[J].
Histol Histopathol, 2008, 23(12): 1439-52.
|
| [14] |
Chen J, Lin YE, Xie F, et al. Expression of bone morphogenetic protein 7 in lung cancer and its biological impact on lung cancer cells[J].
Anticancer Res, 2010, 30(4): 1113-20.
|
| [15] |
Shan ZX, Lin QX, Deng CY, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes[J].
FEBS Lett, 2010, 584(16): 3592-600.
DOI: 10.1016/j.febslet.2010.07.027. |
| [16] |
Park K, Teoh JP, Wang Y, et al. Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury[J].
Am J Physiol Heart Circ Physiol, 2016, 311(2): H371-83.
DOI: 10.1152/ajpheart.00807.2015. |
| [17] |
Vegter EL, Schmitter D, Hagemeijer Y, et al. Use of biomarkers to establish potential role and function of circulating microRNAs in acute heart failure[J].
Int J Cardiol, 2016, 224: 231-9.
DOI: 10.1016/j.ijcard.2016.09.010. |
| [18] |
Bardin P, Marchal Duval E, Sonneville F, et al. Small RNA and transcriptome sequencing reveal the role of miR-199a-3p in inflammatory processes in cystic fibrosis airways[J].
J Pathol, 2018, 245(4): 410-20.
DOI: 10.1002/path.2018.245.issue-4. |
| [19] |
Mungunsukh O, Day RM. Transforming growth factorβ1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism[J].
Mol Biol Cell, 2013, 24(13): 2088-97.
DOI: 10.1091/mbc.e13-01-0017. |
| [20] |
Lin EA, Kong L, Bai XH, et al. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1[J].
J Biol Chem, 2009, 284(17): 11326-35.
DOI: 10.1074/jbc.M807709200. |
| [21] |
Feng Q, Zheng J, Gan W, et al. MiR-199a-3p suppresses proliferation and invasion of prostate cancer cells by targeting Smad1[J].
Oncotarget, 2017, 8(32): 52465-73.
|
| [22] |
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, et al. TGFbeta signaling in vascular fibrosis[J].
Cardiovasc Res, 2007, 74(2): 196-206.
DOI: 10.1016/j.cardiores.2007.02.008. |
| [23] |
Zhu XY, Daghini E, Rodriguez-Porcel M, et al. Redox-sensitive myocardial remodeling and dysfunction in swine diet-induced experimental hypercholesterolemia[J].
Atherosclerosis, 2007, 193(1): 62-9.
DOI: 10.1016/j.atherosclerosis.2006.08.035. |
| [24] |
Wang W, Huang XR, Canlas E, et al. Essential role of Smad3 in angiotensin Ⅱ-induced vascular fibrosis[J].
Circ Res, 2006, 98(8): 1032-9.
DOI: 10.1161/01.RES.0000218782.52610.dc. |
| [25] |
Chen X, Xu J, Jiang B, Liu D. Bone morphogenetic protein-7 antagonizes myocardial fibrosis induced by atrial fibrillation by restraining transforming growth factor-β (TGF-β)/Smads signaling[J].
Med Sci Monit, 2016, 22: 3457-68.
DOI: 10.12659/MSM.897560. |
 2018, Vol. 38
2018, Vol. 38

