2. 南昌大学第二附属医院 泌尿外科, 江西 南昌 330000;
3. 南昌大学第二附属医院 风湿免疫科, 江西 南昌 330000;
4. 南昌大学第二附属医院 江西分子医学重点实验室, 江西 南昌 330000
2. Department of Urology, Second Affiliated Hospital of Nanchang University, Nanchang 330000, China;
3. Department of Rheumatology, 4Jiangxi Key Laboratory of Molecular Medicine, Second Affiliated Hospital of Nanchang University, Nanchang 330000, China;
4. Jiangxi Key Laboratory of Molecular Medicine, Second Affiliated Hospital of Nanchang University, Nanchang 330000, China
原发性肝细胞癌简称肝癌,是我国乃至世界范围内最普遍的恶性肿瘤之一,而导致患者死亡的主要原因是肝癌的侵袭和转移[1]。尽管最近在HCC的临床检测和治疗方法方面取得了一些进展,但HCC根治性切除后的转移和复发率仍然很高,因此研究调控肝癌的侵袭迁移机制对于肝癌的临床治疗具有重要意义[2]。
真核翻译延伸因子1A1(eEF1A1)是真核细胞中高度保守的GTP结合蛋白,在蛋白质合成过程中起着重要作用[3-5]。eEF1A1参与肽链延伸、蛋白质翻译后修饰及细胞骨架调节等功能,同时也涉及调控细胞周期、生长和死亡[6-7]。最近研究也发现eEF1A1在乳腺癌、卵巢癌、肺癌和肝癌等多种肿瘤中高表达,并对肿瘤细胞增殖、凋亡以及肿瘤细胞侵袭和转移等方面发挥重要作用[5, 8-10]。但eEF1A1是否参与肝癌侵袭转移及其作用机制仍不清楚。NI1/RPN12结合蛋白1同源物(NOBl)是由NOBl基因编码的一个多功能的蛋白质,其参与核糖体小亚基和26s蛋白酶体的生物合成[11]。细胞中的NOB1表达发生改变时,会直接影响细胞内核糖体和蛋白酶体的生物合成[12]。而核糖体和蛋白酶体发生改变,与肿瘤细胞的侵袭、迁移、增殖及凋亡密切相关[13-14]。研究报道在肺癌、卵巢癌和骨肉瘤等多种肿瘤中,NOB1能够被mircoRNA调控从而影响肿瘤的增殖、侵袭和转移[15-17]。然而,NOBl是否参与肝癌的侵袭和转移及其调控机制未见报道。因此,本研究主要探讨eEF1A1和NOB1两者在肝癌侵袭转移过程中的作用及其相关机制。
1 材料和方法 1.1 主要材料与试剂ABI 7900实时定量PCR仪(ABI),Bio-Rad电泳仪(Bio-Rad),Thermo Scientific Sorvall ST16R台式离心机、CO2培养箱(Themor),实时无标记细胞分析仪(ACEA Biosciences);Amersham Imager 600自动化学发光凝胶成像分析仪(GE)。
肝癌细胞MHCC97h、HCCLM03、Hep3B、SMMC7721和正常肝细胞HL-7702(中国科学院典型培养物保藏委员会细胞库);DMEM培养基、胎牛血清(Gibco),质粒转染试剂盒LipofectAMINE® 3000(含P3000TM试剂及LipofectAMINE® 3000试剂)、RNA提取试剂TRIzol-Reagent(Invitrogen);BCA法蛋白测定试剂盒(碧云天);质粒提取试剂盒、RNA逆转录试剂盒及大肠杆菌DH5α感受态细胞(大连宝生物);eEF1A1、NOB1和内参一抗(Proteintech);辣根过氧化物酶标记的羊抗兔及羊抗鼠二抗(中杉金桥生物);Transwell侵袭小室(BD);蛋白提取试剂盒(普利莱);pcDNA5- eEF1A1过表达质粒和pcDNA5-NOB1过表达质粒委托上海吉凯基因构建。
特异性针对eEF1A1基因的shRNA-1(靶点序列为CCAGGACACAGAGACTTTA)、shRNA-2(靶点序列为GCTCCAGTCAACGTTACAA)以及特异性针对NOB1基因的shRNA(靶点序列为GGAGAAACCCTT TGCCCAA)和阴性对照shRNA(靶点序列为TTC TCCGAACGTGTCACGT)均由上海吉凯基因构建。
1.2 实验方法 1.2.1 细胞培养及转染肝癌细胞株在37 ℃、5% CO2条件的恒温箱中含10%胎牛血清的DMEM培养基中呈贴壁培养。将对数生长期的细胞接种于10 cm培养皿中,待细胞密度达到60%~70%时,按新型脂质体Lipofectamine® 3000转染试剂(含P300TM试剂及LipofectAMINE® 3000试剂)说明用不含10%胎牛血清的DMEM培养液进行转染,于转染6~8 h后更换含有10%胎牛血清的DMEM培养液中继续培养,48 h后质粒高表达期提取细胞的RNA和总蛋白检验转染效果。
1.2.2 实时荧光定量PCR检测肝癌细胞中eEF1A1和NOB1的mRNA的表达收集培养48 h后的肝癌细胞(MHCC97h、HCCLM3、Hep3B和SMMC7721细胞)和HL-7702正常肝细胞;分别收集sheEF1A1-1干扰组、sheEF1A1-2干扰组、shNC对照组和pcDNA3.1- eEF1A1过表达组、pcDNA3.1-vector对照组的MHCC97和HCCLM3细胞。采用TRIzol法抽提各组细胞的总RNA,紫外分光光度计检测并计算总RNA纯度并定量,电泳检测总RNA的完整性。按TaKaRa PrimeScriptTM RT reagent Kit with gDNA Eraser说明书提供的流程进行反转录制备cDNA,并以此为模板,采用SYBR Premix Ex TaqTM试剂盒在ABI PRISM7500自动荧光PCR仪进行荧光定量PCR检测eEF1A1和NOB1以及内参GAPDH的mRNA表达水平,每个样品均设置3个复孔,设定阈值,测定平均的Ct值。引物序列示于表 1中。
| 表 1 正向和反向引物的序列 Table 1 Sequence of forward and reverse primers |
提取各组肝癌细胞(MHCC97h、HCCLM3、Hep3B和SMMC7721细胞)和HL-7702正常肝细胞的总蛋白;为了观察干扰或过表达eEF1A1后NOB1的蛋白表达变化,分别提取sheEF1A1-1干扰组、sheEF1A1-2干扰组、shNC对照组和pcDNA3.1- eEF1A1过表达组、pcDNA3.1-vector对照组的MHCC97和HCCLM3细胞的总蛋白,并通过BCA法测定蛋白浓度,取等量50 μg蛋白质样品煮沸10 min后行10% SDS-PAGE电泳,转膜,然后分别结合对应的eEF1A1、NOB1、Tubulin及辣根过氧化物酶标记的二抗,最后利用化学发光凝胶成像分析仪观察eEF1A1及NOB1的蛋白表达情况。
1.2.4 Transwell侵袭实验在肝癌细胞中干扰或过表达eEF1A1的表达,使用Transwell实验分析HCC细胞的侵袭转移能力。取对数期生长的sheEF1A1-1干扰组、sheEF1A1-2干扰组、shNC对照组和pcDNA3.1-eEF1A1过表达组、pcDNA3.1- vector对照组的MHCC97h和HCCLM3细胞,经胰酶消化后按2×104细胞数加入每个小室,用无血清的培养液进行培养,放置于含有10%的FBS的培养液的24孔板上,5% CO2、37 ℃孵育24 h,甲醛固定10~20 min,用0.1%结晶紫染色20 min,用棉签轻轻擦掉上层未迁移细胞,用PBS洗3遍。显微镜下观察细胞,计数。
1.2.5 实时无标记动态细胞分析技术(RTCA)在肝癌细胞中干扰或过表达eEF1A1的表达,使用RTCA实验分析HCC细胞的侵袭转移能力。MHCC97h和HCCLM3细胞分组如下:sheEF1A1- 1干扰组、sheEF1A1-2干扰组和shNC对照组,以及pcDNA3.1- eEF1A1过表达组和pcDNA3.1-vector对照组。参照Gao等[14]的方法,将165 µL含有10% FBS(细胞培养基)的DMEM加入到CIM-plate的下室中,在上室孔中加入100 µL混合均匀的无血清各分组细胞悬液,细胞密度为5×104/mL,加好后的CIM板置于超净台中室温放置30 min后放到培养箱中的RTCA仪器上以进行连续阻抗记录。通过每15 min连续阻抗记录测量的CI值反映了细胞侵袭迁移能力。
1.3 统计学分析采用SPSS 17.0和GraphPad Prism7统计软件对相应的实验结果数据进行统计学分析,数据以均数±标准差表示,组间差异比较采用独立样本t检验,P < 0.05为差异有统计学意义。
2 结果 2.1 肝癌细胞中eEF1A1和NOB1 mRNA和蛋白的表达RT-PCR和Western blot结果显示4种肝癌细胞(MHCC97h、HCCLM3、SMMC7721和Hep3B)中eEF1A1和NOB1的mRNA和蛋白表达明显高于正常肝脏细胞(P < 0.01,图 1),并且两者之间的表达呈正相关。
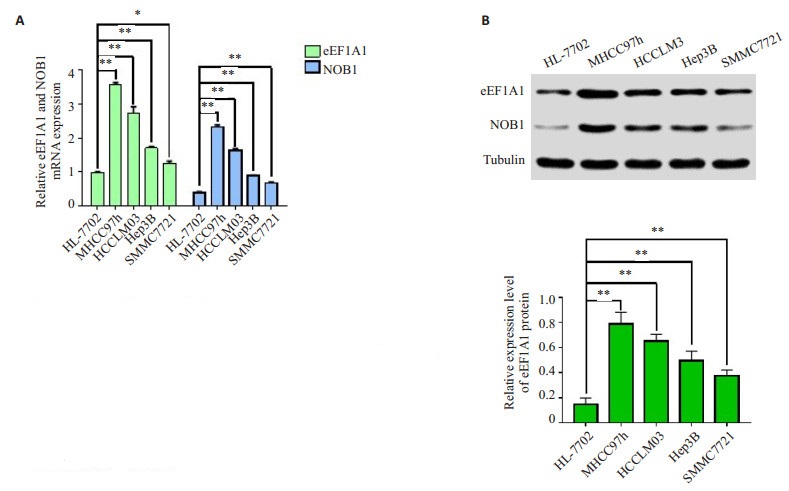
|
图 1 eEF1A1和NOB1在肝癌细胞系(MHCC97h、HCCLM3、Hep3B和SMMC7721)中表达上调 Figure 1 Expressions of eEF1A1 and NOB1 were up-regulated in HCC cell lines MHCC97h, HCCLM3, Hep3B and SMMC7721. A: qRT-PCR results showing up-regulation of eEF1A1 and NOB1 mRNA in the 4 HCC cells compared with normal liver cell line HL- 7702; B: Western blotting showing up-regulation of eEF1A1 and NOB1 proteins in the 4 HCC cells compared with normal liver cell line HL-7702. **P < 0.01. |
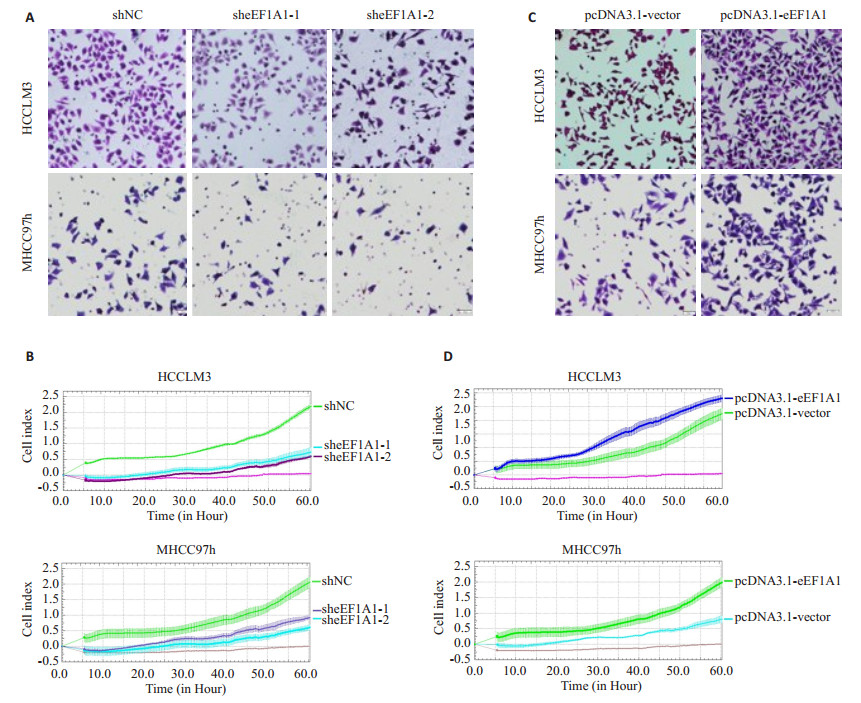
|
图 2 调控eEF1A1的表达影响肝癌细胞MHCC97h和HCCLM3的侵袭和迁移能力 Figure 2 Effect of modulating eEF1A1 expression on invasion and migration ability of HCCLM3 and MHCC97h cells. A, B: Transwell and RTCA assays of HCCLM3 and MHCC97h cells with stable eEF1A1 knockdown; C, D: Transwell and RTCA assays of HCCLM3 and MHCC97h cells with overexpression of eEF1A1. All data are shown as Mean±SD. **P < 0.01. |
在肝癌细胞中干扰eEF1A1的表达后,Transwell实验结果显示:HCCLM3和MHCC97h细胞中sheEF1A1-1组和sheEF1A1-2组细胞的侵袭迁移能力较shNC组明显下降(P < 0.01,图 4A);另外,RTCA实验结果同样显示:细胞中shNC组的侵袭迁移能力要强于sheEF1A1-1组和sheEF1A1-2组的侵袭迁移能力(P < 0.01,图 4B)。
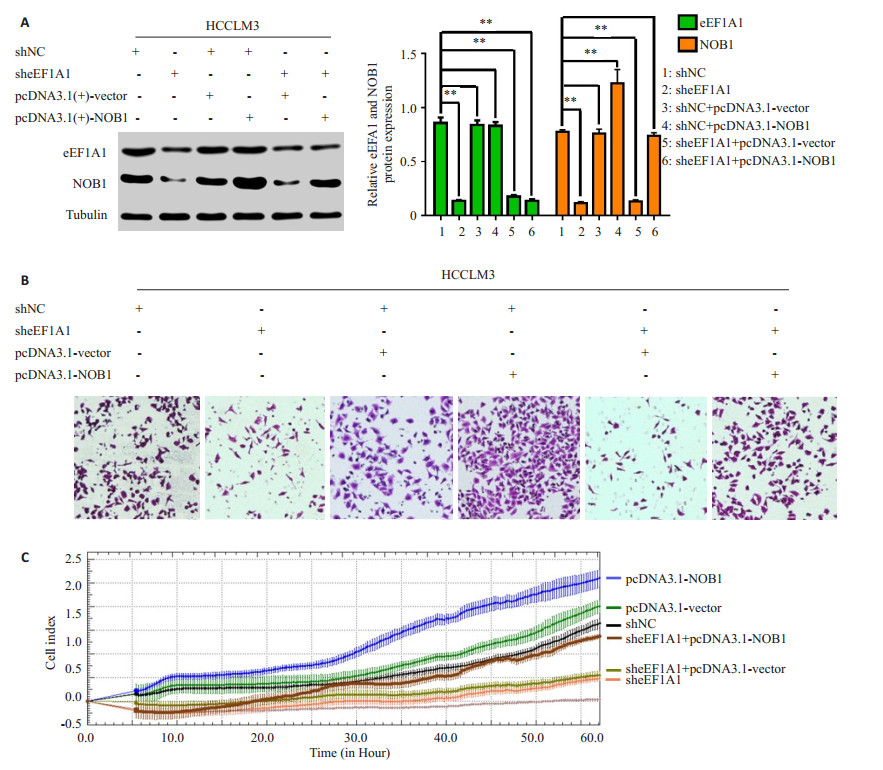
|
图 4 干扰eEF1A1通过下调NOB1的表达抑制肝癌细胞的侵袭迁移能力 Figure 4 Stable knockdown of eEF1A1 inhibits HCC cell invasion and metastasis by reducing NOB1 expression. A: Western blotting showing that the interference of eEF1A1 down-regulated NOB1 expression, and up-regulation of NOB1 attenuated the loss of NOB1 expression in HCCLM3-sheEF1A1 cells; B-C: Transwell and RTCA assays showing that up-regulation of NOB1 significantly rescued the cell migration and invasion in HCCLM3-sheEF1A1 cells. All data are shown as Mean±SD. **P < 0.01. |
我们进一步在肝癌细胞中过表达eEF1A1后,Transwell实验结果显示:HCCLM3和MHCC97h细胞中eEF1A1的过表达明显增强了HCC细胞的侵袭迁移能力(55.00±4.36 vs 94.00±4.00;39.67±3.51 vs 68.33± 2.52;P < 0.01,图 4C)。另外,RTCA实验结果同样显示:在肝癌细胞中过表达eEF1A1后侵袭迁移能力明显强于pcDNA3.1-vector组(P < 0.01,图 4D)。
2.3 eEF1A1调控NOB1的mRNA和蛋白表达荧光定量PCR结果显示:在HCCLM3和MHCC97h细胞中干扰eEF1A1表达(eEF1A1-shRNA-1组和eEF1A1-shRNA-2组),eEF1A1的mRNA表达水平明显下调,同时NOB1的mRNA表达水平也明显下调(图 3A)。Western blot结果显示:在HCCLM3和MHCC97h细胞中干扰eEF1A1表达(eEF1A1-shRNA-1组和eEF1A1-shRNA-2组),eEF1A1蛋白表达下降的同时明显下调NOB1的蛋白表达水平(图 3A)。
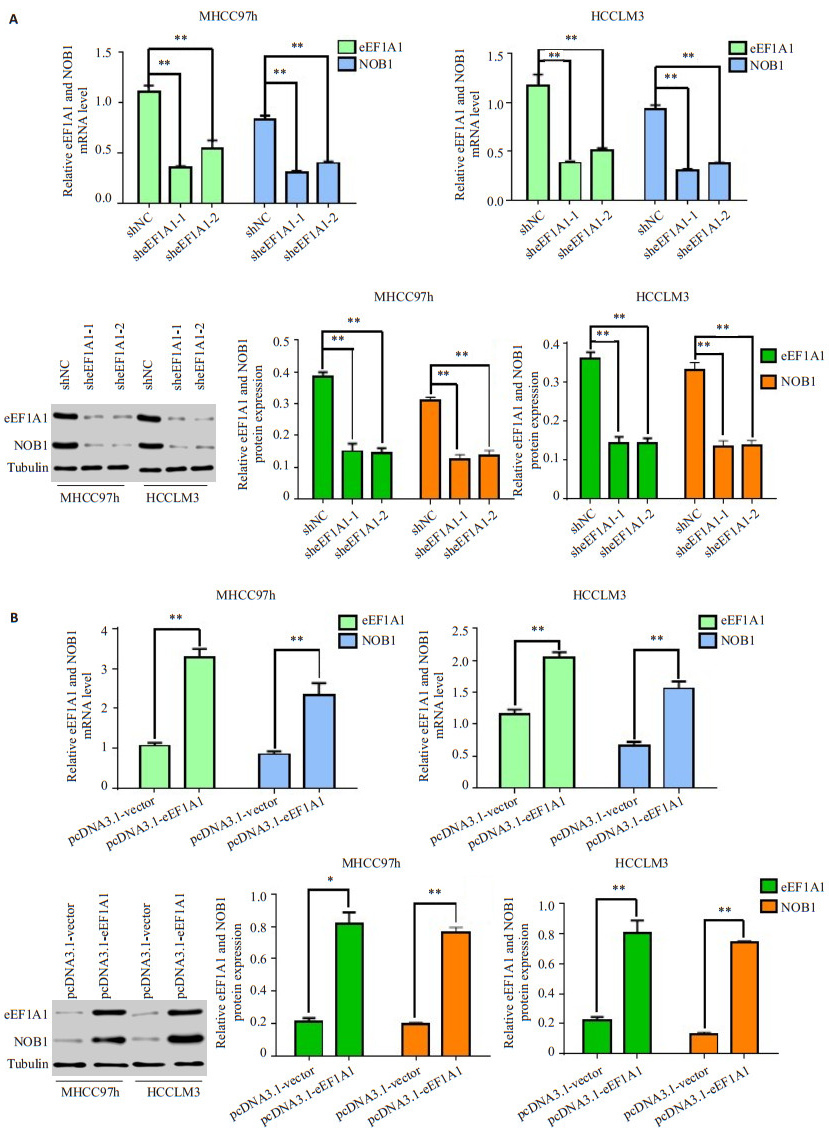
|
图 3 肝癌细胞中eEF1A1调控NOB1的mRNA和蛋白表达 Figure 3 eEF1A1 regulates NOB1 mRNA and protein expressions in HCC cells A: qRT- PCR and Western blotting for detecting eEF1A1 and NOB1 expression in MHCC97h and HCCLM3 cell transfected with shNC vector or sheEF1A1 plasmid; B: qRT-PCR and Western blotting for detecting eEF1A1 and NOB1 expression in MHCC97h and HCCLM3 cell transfected with pcDNA3.1 vector or pcDNA3.1-eEF1A1 plasmid. All data are shown as Mean±SD. *P < 0.05, **P < 0.01. |
荧光定量PCR结果显示:HCCLM3和MHCC97h细胞中过表达eEF1A1,eEF1A1的mRNA表达增加的同时显著增加NOB1的mRNA表达(图 3B)。Western blot结果显示:HCCLM3和MHCC97h细胞中过表达eEF1A1,使eEF1A1蛋白表达增加,同时NOB1的蛋白表达也明显增加(图 3B)。
2.4 eEF1A1调节肝癌细胞的侵袭和转移依赖于NOB1干扰eEF1A1后,eEF1A1表达下降的同时下调NOB1表达(图 4A)和抑制肝癌细胞侵袭迁移能力(P < 0.01,图 4B和C);然而在稳定低表达eEF1A1的HCCLM3细胞中过表达NOB1组中,eEF1A1的表达仍然下调(P < 0.01,图 4A),但NOB1的过表达可以上调由干扰eEF1A1导致下调的NOB1表达(P < 0.01,图 4A)和恢复由干扰eEF1A1导致抑制的肝癌细胞侵袭迁移能力(P均 < 0.01,图 4B和C)。
过表达eEF1A1后,eEF1A1表达增加(P < 0.01,图 4A)的同时上调NOB1表达(图 5A),并促进肝癌细胞侵袭迁移(P < 0.01,图 5B和C);然而在稳定过表达eEF1A1的肿瘤细胞中干扰NOB1组中,eEF1A1的表达上调(P < 0.01,图 4A),但NOB1表达的干扰可以下调由eEF1A1过表达导致升高的NOB1表达(P < 0.01,图 5A)和抑制由eEF1A1过表达导致增高的肝癌细胞侵袭迁移能力(图 5B和C)。
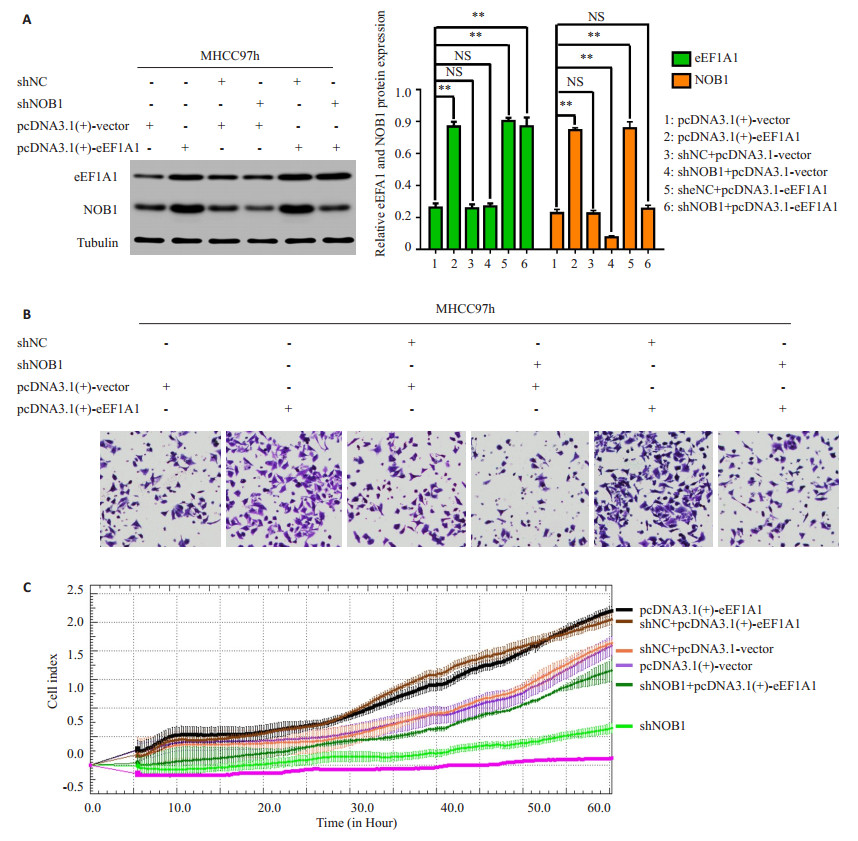
|
图 5 过表达eEF1A1通过上调NOB1的表达促进肝癌细胞的侵袭迁移能力 Figure 5 Overexpression of eEF1A1 promotes HCC cell invasion and metastasis by upregulating NOB1 expression. A: Western blot assays showed that the overexpression of eEF1A1 upregulated NOB1 expression and the knockdown of NOB1 dramatically inhibited the increase of NOB1 expression in MHCC97h-eEF1A1 cell. B-C: Transwell and RTCA assays showed that NOB1 inhibition reduced eEF1A1-enhanced cell migration and invasion. All data are shown as Mean±SD. **P < 0.01. |
HCC是全球癌症相关死亡的第3大原因,它的高死亡率主要与手术切除后的肿瘤浸润、转移和肿瘤复发有关[18]。尽管最近在HCC的临床检测和治疗方法方面取得了一些进展,但HCC根治性切除后的转移和复发率仍然很高[19-20]。了解HCC转移的分子机制对于HCC患者治疗策略的发展具有重要意义[21]。
eEF1A1是一种触发蛋白质翻译延伸的启动蛋白,并参与控制细胞生物学行为[6, 23-26]。我们前期研究发现eEF1A1在多种癌细胞中高表达并影响肿瘤细胞的增殖[6, 27],最近的研究也发现eEF1A1在胃癌细胞的侵袭迁移过程中发挥作用[28],但对肝癌细胞侵袭迁移是否也存在影响,目前未见相关报道。在本研究中,我们发现eEF1A1在肝癌细胞中高表达,降低eEF1A1的表达可以显著抑制肝癌细胞侵袭迁移,而过表达eEF1A1可以促进肝癌细胞侵袭迁移。这些结果提示eEF1A1在肝癌细胞的侵袭迁移中起重要作用。
NOB1是一个多功能的蛋白质,它与肿瘤的发生、发展、增殖和侵袭迁移密切相关[11-12, 29-31]。研究发现NOBl能够被miR145下调,从而抑制肺癌细胞的侵袭迁移;另外,miR-215也能够通过抑制NOB1的表达进而抑制卵巢癌细胞的侵袭迁移[15, 17],但NOB1与肝癌细胞的侵袭迁移能力的关系及其相关调控机制仍不清楚。本研究发现NOB1在肝癌细胞中高表达,并与肝癌细胞的侵袭迁移能力的相关。
为了进一步探讨eEF1A1和NOB1调控肝癌细胞的侵袭迁移是否有关联,以及相互间调控机制。我们通过检测4种不同肝癌细胞中eEF1A1和NOB1 mRNA和蛋白表达水平证实了eEF1A1和NOB1的表达在肝癌细胞中呈正相关。研究结果显示干扰eEF1A1导致NOB1表达下降和肝癌细胞侵袭迁移能力的抑制。另外,研究还发现在稳定低表达eEF1A1的肝癌细胞系中过表达NOBl导致NOB1表达的上调和肝癌细胞侵袭迁移能力的恢复;然而在稳定过表达eEF1A1的肝癌细胞系中降低NOB1导致NOB1表达的下调和肝癌细胞侵袭迁移能力的抑制。这些结果表明eEF1A1是通过调控NOBl的表达,从而进一步影响肝癌细胞侵袭迁移,即eEF1A1调控肝癌细胞侵袭迁移依赖于NOBl的表达。
综上所述,本研究证实在原发性肝癌细胞中eEF1A1能够调控NOB1的表达,从而影响肝癌细胞侵袭和迁移,为原发性肝癌的治疗发现临床诊治新靶点提供了理论基础。
| [1] |
Forner A, Reig M, Bruix J. Hepatocellular carcinoma[J].
Lancet, 2018, 391(10127): 1301-14.
DOI: 10.1016/S0140-6736(18)30010-2. |
| [2] |
Yuan RF, Wang K, Hu JW, et al. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying beta-catenin degradation[J].
Cancer Res, 2014, 74(18): 5287-300.
|
| [3] |
Becker M, Kuhse J, Kirsch J. Effects of two elongation factor 1A isoforms on the formation of gephyrin clusters at inhibitory synapses in hippocampal neurons[J].
Histochem Cell Biol, 2013, 140(6): 603-9.
DOI: 10.1007/s00418-013-1122-9. |
| [4] |
Liu HZ, Ding J, Chen F, et al. Increased expression of elongation factor-1 alpha is significantly correlated with poor prognosis of human prostate cancer[J].
Scand J Urol Nephrol, 2010, 44(5): 277-83.
DOI: 10.3109/00365599.2010.492787. |
| [5] |
Hamrita B, Nasr HB, Hammann P, et al. An elongation factor-like protein (EF-Tu) elicits a humoral response in infiltrating ductal breast carcinomas: an immunoproteomics investigation[J].
Clin Biochem, 2011, 44(13): 1097-104.
DOI: 10.1016/j.clinbiochem.2011.06.005. |
| [6] |
Liu X, Chen L, Ge J, et al. The ubiquitin-like protein FAT10 stabilizes eEF1A1 expression to promote tumor proliferation in a complex manner[J].
Cancer Res, 2016, 76(16): 4897-907.
|
| [7] |
Koiwai K, Maezawa S, Hayano T, et al. BPOZ-2 directly binds to eEF1A1 to promote eEF1A1 ubiquitylation and degradation and prevent translation[J].
Genes Cells, 2008, 13(6): 593-607.
DOI: 10.1111/j.1365-2443.2008.01191.x. |
| [8] |
Abbas W, Kumar A, Herbein G. The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections[J].
Front Oncol, 2015, 5: 75.
|
| [9] |
Kobayashi Y, Yonehara S. Novel cell death by downregulation of eEF1A1 expression in tetraploids[J].
Cell Death Differ, 2009, 16(1): 139-50.
|
| [10] |
Zhu G, Yan W, He HC, et al. Inhibition of proliferation, invasion, and migration of prostate cancer cells by downregulating elongation factor-1alpha expression[J].
Mol Med, 2009, 15(11/12): 363-70.
|
| [11] |
Zhang Y, Ni J, Zhou G, et al. Cloning, expression and characterization of the human NOB1 gene[J].
Mol Biol Rep, 2005, 32(3): 185-9.
DOI: 10.1007/s11033-005-3141-7. |
| [12] |
Veith T, Martin R, Wurm JP, et al. Structural and functional analysis of the archaeal endonuclease Nob1[J].
Nucleic Acids Res, 2012, 40(7): 3259-74.
DOI: 10.1093/nar/gkr1186. |
| [13] |
Li W, Liu M, Feng Y, et al. Downregulated miR-646 in clear cell renal carcinoma correlated with tumour metastasis by targeting the nin one binding protein (NOB1) J][J].
Br J Cancer, 2014, 111(6): 1188-200.
|
| [14] |
Gao X, Wang J, Bai W, et al. NOB1 silencing inhibits the growth and metastasis of laryngeal cancer cells through the regulation of JNK signaling pathway[J].
Oncol Rep, 2016, 35(6): 3313-20.
|
| [15] |
Lin Y, Jin Y, Xu T, et al. MicroRNA-215 targets NOB1 and inhibits growth and invasion of epithelial ovarian cancer[J].
Am J Transl Res, 2017, 9(2): 466-77.
|
| [16] |
Wang J, Cao L, Wu J, et al. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma[J].
Int J Oncol, 2018, 52(1): 77-88.
|
| [17] |
Liu K, Chen H, You Q, et al. miR-145 inhibits human non-smallcell lung cancer growth by dual-targeting RIOK2 and NOB1[J].
Int J Oncol, 2018, 53(1): 257-65.
|
| [18] |
El-Serag HB. Rudolph K L.hepatocellular carcinoma: epidemiology and molecular carcinogenesis[J].
Gastroenterology, 2007, 132(7): 2557-76.
DOI: 10.1053/j.gastro.2007.04.061. |
| [19] |
Yuan Y, Wang J, Li J, et al. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma[J].
Clin Cancer Res, 2006, 12(22): 6687-95.
DOI: 10.1158/1078-0432.CCR-06-0921. |
| [20] |
Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma[J].
Gastroenterology, 2011, 140(5): 1501-12.
DOI: 10.1053/j.gastro.2011.02.006. |
| [21] |
Huang D, Du X, Yuan R, et al. Rock2 promotes the invasion and metastasis of hepatocellular carcinoma by modifying MMP2 ubiquitination and degradation[J].
Biochem Biophys Res Commun, 2014, 453(1): 49-56.
DOI: 10.1016/j.bbrc.2014.09.061. |
| [22] |
Scaggiante B, Dapas B, Grassi G, et al. Interaction of G-rich GT oligonucleotides with nuclear-associated eEF1A is correlated with their antiproliferative effect in haematopoietic human cancer cell lines[J].
FEBS J, 2006, 273(7): 1350-61.
|
| [23] |
Schulz I, Engel C, Niestroj AJ, et al. A non-canonical function of eukaryotic elongation factor 1A1: regulation of interleukin-6 expression[J].
Biochim Biophys Acta, 2014, 1843(5): 965-75.
DOI: 10.1016/j.bbamcr.2014.01.022. |
| [24] |
Qi HY, Ning L, Yu ZY, et al. Proteomic identification of eEF1A1 as a molecular target of curcumol for suppressing metastasis of MDAMB-231 cells[J].
J Agric Food Chem, 2017, 65(14): 3074-82.
DOI: 10.1021/acs.jafc.7b00573. |
| [25] |
Talapatra S, Wagner JD, Thompson CB. Elongation factor-1 alpha is a selective regulator of growth factor withdrawal and ER stressinduced apoptosis[J].
Cell Death Differ, 2002, 9(8): 856-61.
|
| [26] |
Lamberti A, Longo O, Marra M, et al. C-Raf antagonizes apoptosis induced by IFN-alpha in human lung cancer cells by phosphorylation and increase of the intracellular content of elongation factor 1A[J].
Cell Death Differ, 2007, 14(5): 952-62.
|
| [27] |
Li X, Li J, Li F. P21 activated kinase 4 binds translation elongation factor eEF1A1 to promote gastric cancer cell migration and invasion[J].
Oncol Rep, 2017, 37(5): 2857-64.
DOI: 10.3892/or.2017.5543. |
| [28] |
Fatica A, Oeffinger M, Dlakić M, et al. Nob1p is required for cleavage of the 3' end of 18S rRNA[J].
Mol Cell Biol, 2003, 23(5): 1798-807.
DOI: 10.1128/MCB.23.5.1798-1807.2003. |
| [29] |
Yin JR, Wang JC, Jiang Y, et al. Downregulation of NOB1 inhibits proliferation and promotes apoptosis in human oral squamous cell carcinoma[J].
Oncol Rep, 2015, 34(6): 3077-87.
DOI: 10.3892/or.2015.4271. |
| [30] |
Li Y, Ma C, Qian M, et al. Downregulation of NOB1 suppresses the proliferation and tumor growth of non-small cell lung cancer in vitro and in vivo[J].
Oncol Rep, 2014, 31(3): 1271-6.
DOI: 10.3892/or.2014.2991. |
 2018, Vol. 38
2018, Vol. 38

