2. 南方医科大学第五附属医院 病理科,广东 广州 510900
2. Department of Pathology, the Fifth Affiliated Hospital, Southern Medical University, Guangzhou 510900, China
急性肝损伤的主要特征是大量肝细胞死亡,如损伤的肝细胞未得到及时的清除,会进一步影响细胞环境稳定,容易进展成暴发性肝衰竭。但其确切发病机制仍未完全阐明,临床上尚缺乏有效的预防和治疗手段,病死率极高。因此,在肝脏发生急性损伤后,如何维持细胞环境稳定并阻止肝细胞大量死亡,已成为基础和临床防治该疾病研究的难点和热点。
我们的研究表明[1],西红花酸能在急性肝损伤早期有效促进肝细胞生长和抑制细胞死亡,从而阻止肝细胞大量死亡,但其作用机制尚不十分明确。自噬在急性肝损伤早期能清除有害物质、保护肝细胞免受攻击等发挥着重要的作用[2]。那么西红花酸是否通过影响细胞的自噬,而阻止细胞大量死亡?本研究通过建立肝损伤细胞模型,探讨西红花酸对肝细胞损伤的自噬影响,为急性肝损伤发病机制及防治研究提供新思路。
1 材料和方法 1.1 实验动物7~9周龄健康雄性Wistar大鼠,体质量(200±10)g,购自南方医科大学实验动物中心,动物合格证号:SCXK(粤)2011-0015,在室温20±2 ℃环境下饲养,实验前适应环境饲养1周。
1.2 实验试剂DMEM、10%胎牛血清(Hyclone),3-甲基腺嘌呤(3-MA)、西红花酸、脂多糖和D-氨基半乳糖胺(Sigma),丙氨酸转氨酶(ALT)、天冬氨酸转氨酶(AST)和乳酸脱氢酶(LDH)试剂(建成生物),p62、GAPDH、LC3、SIRT1单克隆抗体(Bio Vision)。
1.3 实验方法 1.3.1 大鼠肝细胞的分离和培养无菌条件下取出实验Wistar大鼠肝脏,轻轻撕去肝包膜后,以生理盐水清洗后用小剪刀剪成细组织块。移入用PBS缓冲液配置的0.25%胰蛋白酶中,4 ℃冷消化6~8 h后,然后以200目筛网研磨,用一定量的PBS缓冲液吹打成为单个细胞悬液。置25 mL离心管中,1000 r/min离心5 min,弃上清液,反复离心3次。沉淀为肝实质细胞,再以含10%胎牛血清的DMEM培养液悬浮细胞,分离的肝细胞经0.4%台盼蓝拒染色计数细胞活力大于90%。调整细胞密度为1×108/L,接种于24孔板中,置于37 ℃、5%CO2培养箱中培养。本实验经医院伦理委员会批准,动物处置方法符合动物伦理学标准。
1.3.2 实验分组及处理将细胞分为对照组、损伤组、西红花酸组、3MA组和西红花酸+3MA组。肝细胞培养24 h后,吸弃上清液,更换培养液;损伤组、西红花酸组和3MA组加入脂多糖1 mg/L+D-氨基半乳糖胺60 mg/L(用10%胎牛血清溶解),制成肝细胞损伤模型;西红花酸组加入西红花酸500 mg/L,3MA组加入自噬抑制剂3MA10 mg/L,西红花酸+3MA组加入西红花酸500 mg/L和3MA10 mg/L,继续培养12 h。
1.3.3 肝细胞损伤指标检测采用ELISA检测各组培养的上清液,首先用抗大鼠ALT、AST和LDH单克隆抗体(单抗)包被酶标板;加入生物素化的大鼠ALT、AST和LDH二抗以及由辣根过氧化物酶标记的链亲和素;最后加入显色液显色、终止液终止反应,在波长450 nm处读取吸光度(A)。ALT、AST和LDH的浓度与A值成正比,根据样品的A值可在标准曲线上得知ALT、AST和LDH的浓度。
1.3.4 免疫荧光染色观察自噬各组细胞用4%多聚甲醛固定15 min,在室温下用3%山羊血清封闭20 min,加入LC3抗体4 ℃孵育过夜,第2天细胞用PBS清洗3次,然后加入二抗37 ℃孵育1 h之后,用PBS清洗2次,在荧光显微镜下观察染色细胞LC3荧光点。
1.3.5 透射电子显微镜观察自噬各组细胞用PBS清洗2次,用2.5%戊二醛4 ℃固定过夜,0.1%二甲胂酸钠缓冲液洗涤后,1%锇酸固定3 h,用PBS清洗细胞3次,酒精梯度脱水,转移到1:1混合的无水丙酮中20 min,Spurr树脂包埋剂过夜。最后,标本放置在含包埋剂的胶囊中,70 ℃加热约9 h,用切片机超薄切片(70 nm),切片标本分别用醋酸双氧铀和柠檬酸铅染色10 min,用水冲洗后透射电子显微镜观察。
1.3.6 Western blot检测肝细胞中LC3、p62、SIRT1表达收集各组细胞,用细胞裂解液提取细胞的总蛋白,用BCA法测定蛋白浓度,调整每孔上样量为20 μg,经10% SDS-PAGE凝胶电泳后,凝胶蛋白转移至硝酸纤维素膜。5%脱脂牛奶封闭1 h后,以GAPDH(1:1000)、LC3(1:1500)、p62(1:1000)、SIRT1(1:1000)一抗置4 ℃孵育过夜。第二天PBST洗涤3次后,以兔二抗(1: 5000)常温孵育1 h。PBST洗涤3次,ECL化学发光法显像。
1.3.7 统计学方法采用SPSS 20软件进行统计分析,计量数据用均数±标准差表示,组间比较用方差分析。P < 0.05为差异有统计学意义。
2 结果 2.1 西红花酸可减轻肝细胞的损伤肝细胞经损伤处理后,ALT、AST和LDH水平均较对照组明显升高,说明经损伤处理后肝细胞损伤造模成功;经自噬抑制剂3MA处理后3MA组与西红花酸+ 3MA组ALT、AST、LDH水平达到最高,说明经抑制自噬后,肝细胞损伤加重;而经西红花酸处理后ALT、AST、LDH水平比损伤组明显低(表 1)。
| 表 1 各组肝细胞损伤后ALT、AST和LDH水平比较 Table 1 Comparison ofALT, AST andLDHlevels after hepatocyte injury in each group (Mean±SD, U/L) |
荧光倒置显微镜可观察标记的LC3荧光,从而观察细胞自噬情况。损伤处理后肝细胞LC3荧光明显比对照组多。与损伤组相比,西红花酸组的LC3荧光最多(P < 0.05);经自噬抑制剂3MA处理后3MA组与西红花酸+3MA组,LC3荧光比损伤组少(P < 0.05,图 1)。
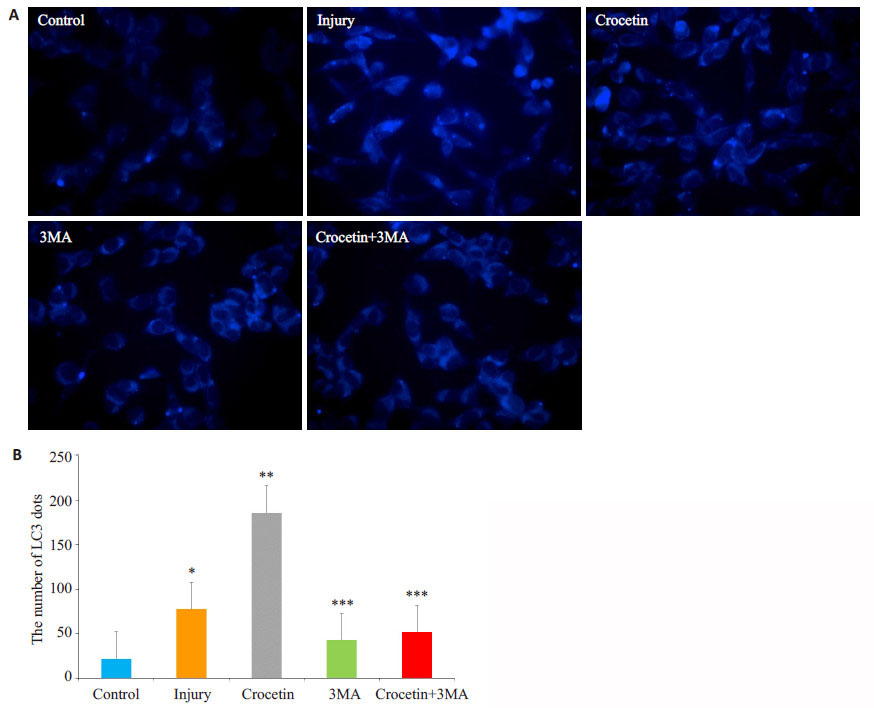
|
图 1 各组肝细胞LC3荧光表达情况 Figure 1 Expression of LC3 in the hepatocytes in different groups detected by immunofluorescence assay. A: Immunofluorescence staining in each group; B: Quantitative analysis of LC3 expression in the cells in each group. *P < 0.05 vs control; **P < 0.05 vs control and Injury groups; ***P < 0.05 vs control, injury and crocetin groups. |
透射电子显微镜可用来观察自噬体的超微结构,经损伤处理后肝细胞自噬体形成增加。与损伤组相比,西红花酸组的自噬体增加明显;经自噬抑制剂3MA处理后,3MA组与西红花酸+3MA组自噬体形成明显抑制(图 2)。
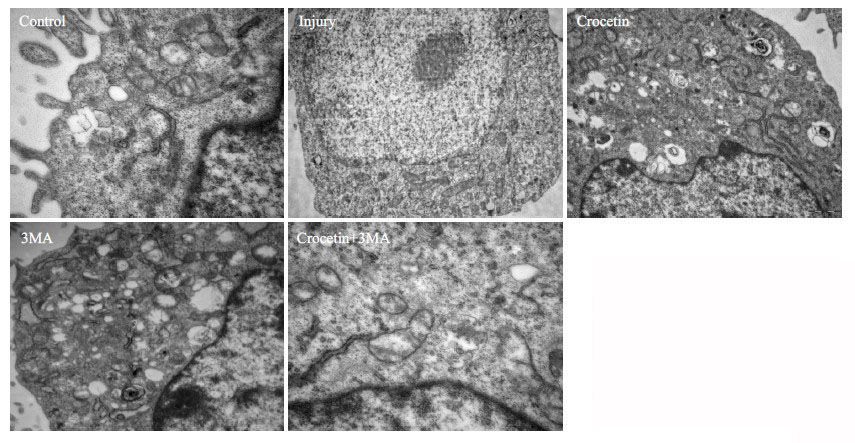
|
图 2 透射电子显微镜观察各组肝细胞中的自噬体 Figure 2 Autophagy in rat hepatocytes in each group observed by transmission electron microscope (Original magnification: ×10 000). |
与对照组相比,损伤组和西红花酸组均可见LC3和SIRT1表达上调,p62表达下调(P < 0.05);而经西红花酸处理后,LC3和SIRT1表达最高,p62表达最低(P < 0.05);经自噬抑制剂3MA处理后3MA组与西红花酸+ 3MA组,LC3和SIRT1表达最低,p62表达最高(P < 0.05,图 3)。
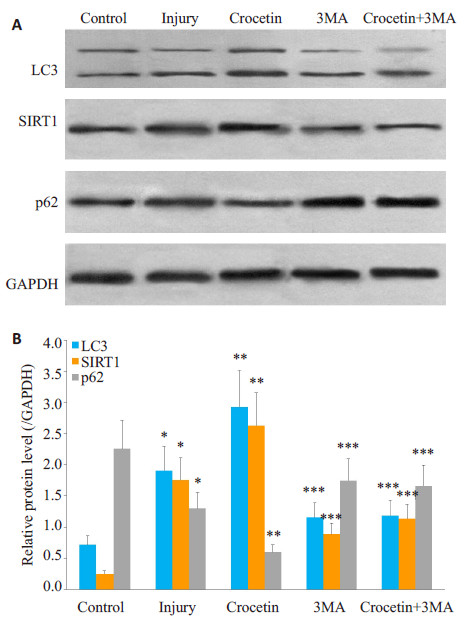
|
图 3 LC3、SIRT1、p62在各组肝细胞中的表达 Figure 3 Expression of LC3, SIRT1, and p62 in rat hepatocytes in each group detected by Western blotting. A: Western blots of LC3, SIRT1, and p62 in each group; B: Quantitative analysis of LC3, SIRT1, and p62 expressions. *P < 0.05 vs control; **P < 0.05 vs control and injury groups; ***P < 0.05 vs control, injury and crocetin groups. |
自噬是指从粗面内质网的无核糖体附着区脱落的双层膜包裹部分胞质和细胞内需降解的细胞器、蛋白质等成分形成自噬体,并与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物,以实现细胞本身的代谢需要和某些细胞器的更新[2-5]。自噬现象广泛存在于真核细胞,是生物在其发育、老化过程中都存在的净化自身多余或受损细胞器的共同机制,生命体借此维持蛋白代谢平衡及细胞环境稳定,这一过程在细胞清除废物、结构重建、生长发育中起重要作用[6-11]。自噬通过降解异常物质,参与了对机体多系统的保护。
我们既往的研究表明[1, 12-13]:如急性肝组织损伤未得到及时处理,会产生一系列连锁反应,出现肝细胞坏死和凋亡,导致肝细胞过度死亡,最终可出现急性肝功能衰竭。诸多研究表明[14-19],自噬在肝脏的生理功能及各种肝脏疾病如酒精性肝病、病毒性肝炎、肝癌及急性肝损伤中发挥着重要作用,自噬水平的变化影响着肝脏生理功能和疾病的发生。本实验中肝细胞损伤后ALT、AST和LDH水平均较正常组明显升高,表明通过脂多糖+ D-氨基半乳糖胺成功诱导了体外肝细胞的损伤,而西红花酸可减轻肝细胞的损伤。西红花酸是通过什么机制减轻受伤的肝细胞呢?通过荧光倒置显微镜和透射电子显微镜观察细胞发现,经损伤处理后肝细胞自噬增加,而西红花酸组比损伤组的自噬体明显增加,说明西红花酸可以促进肝细胞损伤的自噬。由此我们可发现,西红花酸诱导自噬增加的肝细胞损伤减轻,而自噬抑制的肝细胞损伤加重,表明细胞自噬具有保护肝细胞的作用。
作为代谢传感器和转录调节功能的组蛋白脱乙酰酶sirtuin 1(SIRT1),它在机体细胞周期、细胞衰老、凋亡和自噬等方面的调节中发挥重要作用,是组织细胞自我保护的一种重要分子[20-22]。有研究表明[23],SIRT1在巨噬细胞内通过降低炎症相关因子诱导型一氧化氮合酶和细胞间黏附因子1的表达及活性,从而抑制炎症反应。因此,提高SIRT1的表达和活性可以作为一个调节炎症反应、保护机体的新手段。最近的研究表明[24],通过SIRT1接受LC3结合Atg7和其它元素,LC3脱乙酰化参与自噬,这表明SIRT1可以调节自噬的诱导。LC3-Ⅱ和p62是自噬必不可少的标记物[25-27],LC3-Ⅰ通过水解蛋白的裂解转化成LC3-Ⅱ已视为哺乳动物自噬的标志,而p62在自噬体中主要介导元素的补充[28-30]。我们的实验发现,肝细胞损伤后LC3和SIRT1表达上调、p62表达下调,而西红花酸处理后LC3和SIRT1表达最高、p62表达最低,经自噬抑制剂3MA处理后3MA组与西红花酸+3MA组,LC3和SIRT1表达最低、p62表达最高。由此表明,西红花酸可能通过上调SIRT1,进而调节LC3和p62的表达,影响肝细胞损伤的自噬通路,进而起到保护肝细胞的作用。
综上所述,西红花酸可能通过上调SIRT1和LC3、下调p62,而激活了肝细胞损伤的自噬通路,增加自噬能清除有害物质,减轻脂多糖+D-氨基半乳糖胺诱导的肝细胞损伤,从而对肝细胞起到保护作用。而西红花酸调节的具体通路是什么?通过哪些机制调节SIRI1、LC3和p62,仍需更进一步的科学实验来分析和阐明,可为临床中医中药治疗肝损伤提供新的治疗方法和实验依据。
| [1] |
高珂, 郭宏兴, 刘亮明, 等. 西红花酸对百草枯中毒大鼠的肝保护效应[J].
中华危重病急救医学, 2016, 28(10): 876-80.
DOI: 10.3760/cma.j.issn.2095-4352.2016.10.003. |
| [2] |
Długońska H. Autophagy as a Universal intracellular process. A comment on the 2016 nobel prize in physiology or medicine[J].
Ann Parasitol, 2017, 63(3): 153-7.
|
| [3] |
Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, et al. Is autophagy associated with diabetes mellitus and its complications? A review[J].
EXCLI J, 2018, 17: 709-20.
|
| [4] |
Kuma A, Komatsu M, Mizushima N. Autophagy-monitoring and autophagy-deficient mice[J].
Autophagy, 2017, 13(10): 1619-28.
DOI: 10.1080/15548627.2017.1343770. |
| [5] |
Shao M, Shen YE, Sun HJ, et al. Protectiveness of artesunate given prior ischemic cerebral infarction is mediated by increased autophagy[J].
Front Neurol, 2018, 9: 634.
DOI: 10.3389/fneur.2018.00634. |
| [6] |
Grizotte-Lake M, Vaishnava S. Autophagy: suicide prevention hotline for the gut epithelium[J].
Cell Host Microbe, 2018, 23(2): 147-8.
DOI: 10.1016/j.chom.2018.01.015. |
| [7] |
Bednarczyk M, Muc-Wierzgon M, Waniczek D, et al. Autophagyrelated gene expression in colorectal cancer patients[J].
J Biol Regul Homeost Agents, 2017, 31(4): 923-7.
|
| [8] |
Karabiyik C, Lee MJ, Rubinsztein DC. Autophagy impairment in Parkinson's disease[J].
Essays Biochem, 2017, 61(6): 711-20.
DOI: 10.1042/EBC20170023. |
| [9] |
Mcewan DG. Host-pathogen interactions and subversion of autophagy[J].
Essays Biochem, 2017, 61(6): 687-97.
DOI: 10.1042/EBC20170058. |
| [10] |
Motegi S, Fujiwara C, Yamazaki S, et al. Possible contribution of autophagy in pyogenic granuloma[J].
J Dermatol, 2018, 45(9): 1145-6.
DOI: 10.1111/jde.2018.45.issue-9. |
| [11] |
Nozawa T. Selective autophagy mechanism against group a streptococcus infection[J].
Nihon Saikingaku Zasshi, 2018, 73(3): 193-9.
DOI: 10.3412/jsb.73.193. |
| [12] |
郭宏兴, 高珂, 罗亮, 等. 早期百草枯中毒大鼠的急性肝损伤研究[J].
中华危重病急救医学, 2014, 26(6): 374-8.
DOI: 10.3760/cma.j.issn.2095-4352.2014.06.002. |
| [13] |
郭宏兴, 刘亮明, 张吉翔, 等. 大鼠内毒素性急性肝损伤后肝细胞凋亡与炎性因子的表达[J].
中华传染病杂志, 2008, 26(7): 415-9.
DOI: 10.3321/j.issn:1000-6680.2008.07.010. |
| [14] |
Gao K, Liu FQ, Guo HX, et al. miR-224 suppresses HBV replication posttranscriptionally through inhibiting SIRT1-mediated autophagy[J].
Int J Clin Exp Pathol, 2018, 11(1): 189-98.
|
| [15] |
Zhang X, Zhang PH, Gao JL, et al. Autophagy dysregulation caused by ApoM deficiency plays an important role in liver lipid metabolic disorder[J].
Biochem Biophys Res Commun, 2018, 495(4): 2643-8.
DOI: 10.1016/j.bbrc.2017.12.148. |
| [16] |
Kan CY, Liu AD, Fang HS, et al. Induction of autophagy reduces ischemia/reperfusion injury in steatotic rat livers[J].
J Surg Res, 2017, 216(1): 207-18.
|
| [17] |
Chun SK, Lee S, Yang MJ, et al. Exercise-induced autophagy in fatty liver disease[J].
Exerc Sport Sci Rev, 2017, 45(3): 181-6.
DOI: 10.1249/JES.0000000000000116. |
| [18] |
Wang KW. Autophagy and apoptosis in liver injury[J].
Cell Cycle, 2015, 14(11): 1631-42.
DOI: 10.1080/15384101.2015.1038685. |
| [19] |
Jiang Z, Bo L, Meng Y, et al. Overexpression of homeodomaininteracting protein kinase 2(HIPK2) attenuates sepsis-mediated liver injury by restoring autophagy[J].
Cell Death Dis, 2018, 9(9): 847.
DOI: 10.1038/s41419-018-0838-9. |
| [20] |
Kim MG, Kim DH, Lee HR, et al. Sirtuin inhibition leads to autophagy and apoptosis in porcine preimplantation blastocysts[J].
Biochem Biophys Res Commun, 2017, 488(4): 603-8.
DOI: 10.1016/j.bbrc.2017.05.087. |
| [21] |
Wang WR, Li TT, Jing T, et al. SIRT1 regulates the inflammatory response of vascular adventitial fibroblasts through autophagy and related signaling pathway[J].
Cell Physiol Biochem, 2017, 41(2): 569-82.
DOI: 10.1159/000457878. |
| [22] |
Li J, Jz D, Ren YL, et al. Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages[J].
Exp Ther Med, 2018, 16(3): 2593-9.
|
| [23] |
Li Y, Yang X, He Y, et al. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells[J].
Immunobiology, 2017, 222(3): 552-61.
DOI: 10.1016/j.imbio.2016.11.002. |
| [24] |
Huang R, Liu W. Identifying an essential role of nuclear LC3 for autophagy[J].
Autophagy, 2015, 11(5): 852-3.
DOI: 10.1080/15548627.2015.1038016. |
| [25] |
Sil P, Muse G, Martinez J. A ravenous defense: canonical and noncanonical autophagy in immunity[J].
Curr Opin Immunol, 2018, 50(1): 21-31.
|
| [26] |
Huang R, Xu Y, Wan W, et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation[J].
Mol Cell, 2015, 57(3): 456-66.
DOI: 10.1016/j.molcel.2014.12.013. |
| [27] |
Wu B, Yu L, Wang YS, et al. Aldehyde dehydrogenase 2 activation in aged heart improves the autophagy by reducing the carbonyl modification on SIRT1[J].
Oncotarget, 2016, 7(3): 2175-88.
|
| [28] |
Zheng SL, Han F, Shi YX, et al. Single- Prolonged-stress-induced changes in autophagy-related proteins beclin-1, LC3, and p62 in the medial prefrontal cortex of rats with post-traumatic stress disorder[J].
J Mol Neurosci, 2017, 62(1): 43-54.
DOI: 10.1007/s12031-017-0909-x. |
| [29] |
Berezowska S, Galván JA. Immunohistochemical detection of the autophagy markers LC3 and p62/SQSTM1 in formalin-fixed and paraffin-embedded tissue[J].
Methods Mol Biol, 2017, 1560: 189-94.
DOI: 10.1007/978-1-4939-6788-9. |
| [30] |
Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy[J].
Int J Biochem Cell Biol, 2004, 36(12): 2503-18.
DOI: 10.1016/j.biocel.2004.05.009. |
 2018, Vol. 38
2018, Vol. 38

