2. 南方医科大学南方医院 心内科,广东 广州 510515
2. Department of Cardiology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China
动脉粥样硬化性心血管疾病(ASCVD)具有发病率和死亡率高的特点,急性血栓事件是致死、致残的最重要原因,及时开通闭塞血管、恢复组织血流再灌注是挽救濒死缺血组织和改善预后的关键[1-2]。介入治疗和药物溶栓是目前开通血管的主要手段,但因介入治疗对技术及器械要求高[3-4],药物溶栓具有血管开通率低、出血风险高等不足[5-6],二者临床使用受限。因此,需要寻找其他有效的再灌注治疗手段。
超声联合微泡有望成为现代再灌注治疗的重要补充。既往大量实验已证实超声联合微泡可有效溶解颈动脉[7-9]、大脑中动脉[10-12]、冠状动脉[13-15]及外周大动脉[16-18]等部位的血栓,从而减轻组织损伤和、改善预后。上述研究主要关注凝龄约3 h的新鲜血栓。然而,在临床实践中,部分患者由于各种原因导致就诊延误,血栓凝龄较大。虽有报道超声联合微泡对凝龄较大的血栓溶解能力下降[19-20],但该研究未进一步探索超声联合微泡治疗对组织缺血损伤及预后的改善作用。
本研究拟在大鼠髂总动脉血栓模型中探索超声联合微泡通过溶解不同凝龄动脉血栓挽救缺血组织的效率与血栓凝龄的关系,为超声联合微泡治疗动脉血栓性疾病时间窗选择提供理论依据。
1 材料和方法南方医院动物伦理委员会审阅并通过实验研究方案。
1.1 体外血栓制备 1.1.1 大鼠髂总动脉血栓模型的制备Sprague Dawley(SD)大鼠(69只,SPF级,雄性,250~300 g,南方医科大学实验动物中心)用3%戊巴比妥钠(Sigma Aldrich, USA)溶液腹腔注射,钝性分离右髂总主动脉,用浸泡10% FeCl3的滤纸覆盖,10 min后通过行二维超声(管腔内血栓影像)、彩色多普勒及脉冲多普勒超声(血流速度明显下降)证实血栓形成,9只(每组3只)模型分别于血栓形成后3、6及12 h注射10% KCl注射液处死,收集髂动脉血栓标本。
1.1.2 血栓组织学检查髂总动脉血栓制备后用10%中性甲醛固定,常规石蜡包埋切片,HE染色后光学显微镜(BX1, Olympus, Japan)拍照。
1.2 大鼠髂总动脉血栓模型溶栓实验参考既往实验[19-20],实验共分3组:3 h组、6 h组和12 h组,每组20只。脂质微泡(2×108/min,由南方医科大学药学院提供,平均粒径2.12±1.15 μm,浓度约2.11~ 4.24×109/mL)以微量输液泵经尾静脉缓慢注入。采用4V1C(频率2 MHz, MI 1.9)探头进行治疗,超声工作模式为工作10 s、间歇10 s(使微泡在治疗脉冲间歇充分填充)交替,治疗总时间为30 min。
1.3 大鼠髂总动脉二维及多普勒超声成像在治疗前及治疗后用超声仪(SeimensSequoia 512,15L8探头,频率7 MHz)采集大鼠髂总动脉二维及脉冲多普勒超声图像。血管再通率(%)=血管再通大鼠数量/每组模型总数量×100%。在血管再通动物中通过随机数字表法选取相同数量(血管再通率最低组动物数量)动物进行骨骼肌血流灌注、细胞损伤标记物及细胞凋亡率等指标分析,以评估超声联合微泡治疗开通血管后挽救缺血组织的效果差异。
1.4 对比超声(CEUS)图像采集使用Sequaio 512超声仪(Siemens, USA)、15L8探头(7MHz)分别于造模前即刻、溶栓治疗前即刻及溶栓治疗后即刻进行对比超声图像采集。将超声探头固定于大鼠后肢中部(膝关节与腹股沟中点连线的中点)短轴切面,二维模式选取满意切面后改用对比脉冲系列(CPS)技术,图像增益(-6dB)、机械指数(0.18)、动态范围(55dB)等参数在整个实验过程中保持不变。采用实时成像模式取图,脂质微泡(2×108/min)以微量输液泵经尾静脉缓慢注入,以高机械指数(1.9)破坏造影剂微泡后至造影剂再次充盈达到稳态。连续采集造影剂再充盈至稳态过程的图像(治疗前及治疗后6 h)。使用SequoiaACQ软件分析图像,记录平台期声强度值(AI)。
1.5 骨骼肌损伤标记物(Mb、CK)治疗后6 h处经颈静脉抽血(3%柠檬酸钠抗凝),4 ℃离心机中离心(2500 r/min)15 min,取上清液。采用大鼠肌红蛋白(Mb)、肌酸激酶(CK)ELISA试剂盒(武汉华美公司,中国)进行检测,严格按照试剂盒说明书来进行操作。用酶标仪中进行吸光度分析(波长450 nm),每孔重复两次取平均值。
1.6 骨骼肌细胞凋亡检测治疗后6 h处死大鼠,取大腿骨骼肌常规进行石蜡包埋及超薄切片(4 μm),采用TMR-Red调亡试剂盒(Roche)根据说明书进行骨骼肌细胞凋亡检测,红色表示调亡细胞核,蓝色(DAPI)表示全部细胞核,随机选取10个高倍视野计算骨骼肌细胞凋亡率(调亡细胞核/全部细胞核)。
1.7 统计学分析采用SPSS 19.0软件进行统计分析。计数资料以率表示,通过χ2检验进行统计学分析。计量资料用均数±标准差表示,以完全随机设计资料的单因素方差分析(One-WayANOVA)分析并进行事后检验(如果满足方差齐性用Bonferroni进行检验,方差不齐则用Dunnett's T3检验),P < 0.05为差异有统计学意义。
2 结果 2.1 髂总动脉血栓组织学特点髂总动脉血栓以血小板纤维蛋白网结构为主,血栓收缩程度与凝龄密切相关,凝龄越长,血栓收缩程度越明显,具体表现为纤维蛋白网致密(图 1)。
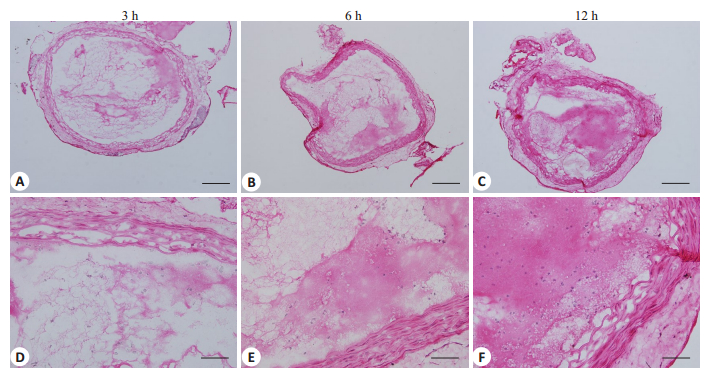
|
图 1 大鼠髂总动脉血栓模型组织学特点 Figure 1 Histological observation of the thrombi in the common iliac artery of the rat models. HE staining showed that the thrombi in the common iliac artery was composed mainly of cross-linked platelet-fibrin meshwork, which became denser as the time after thrombosis prolonged. Upper panel: original magnification: × 100, scale bar: 50 μm; lower panel: ×400, scale bar: 12.5 μm. |
超声联合微泡治疗后6 h组血管再通率(40%,8/ 20)低于3 h组(80%,16/20)(P=0.01),12 h组血管再通率(25%,5/20)低于3 h组(80%,16/20)(P < 0.001),差异具有统计学意义(图 2A~B)。
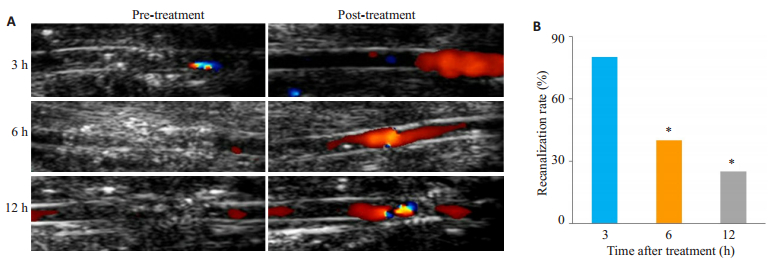
|
图 2 大鼠髂总动脉血栓模型超声联合微泡溶栓效果 Figure 2 Thrombolytic effect of combined ultrasound and microbubble treatment in the rat models of common iliac artery thrombosis. A: Representative 2D and color Doppler images of the common iliac artery before and after treatment, showing artery recanalization after the treatment; B: Quantification of the recanalization rate of the common iliac artery after the treatment. (*P < 0.01 vs 3 h group; 6 h group vs 3 h group, P=0.01). |
AI值反映骨骼肌微血管血容量,超声联合微泡治疗前,3 h组(1.96±1.08 IU)、6 h组(3.02±1.07 IU)和12 h组(2.46±1.31 IU)AI值差异无统计学意义(n=5, P > 0.05)。治疗后,6 h组AI值(8.84±1.05 IU)低于3 h组(11.08±1.16 IU)(n=5, P=0.012),12 h组AI值低于3 h组(n=5, P < 0.001),12 h组AI值低于6 h组(n=5, P= 0.038),差异具有统计学意义(图 3A~B)。
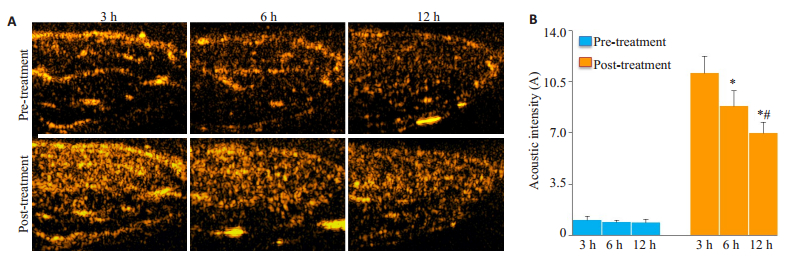
|
图 3 大鼠髂总动脉血栓模型超声联合微泡溶栓后后肢骨骼肌微循环血流灌注 Figure 3 Microvascular perfusion in the hindlimbs of the rat models after combined ultrasound and microbubble treatment. A: Representative contrast ultrasound images of the hindlimbs before and after treatment, showing decreased microvascular perfusion as the time after thrombosis prolonged; B: Quantification of microvascular blood volume of the rat hindlimbs before and after treatment. (*: 12 h group vs 3 h group, P < 0.01; 6 h group vs 3 h group, P=0.012; #: 12 h group vs 6 h group, P=0.038). |
超声联合微泡治疗后,6 h组骨骼肌细胞凋亡率(20.40%±0.51%)高于3 h组(12.43%±0.15%)(n=5,P < 0.0001),12 h组骨骼肌细胞凋亡率(27.51%±0.43%)高于3 h组(n=5, P < 0.0001),12 h组骨骼肌细胞凋亡率高于6 h组(n=5, P < 0.0001),差异具有统计学意义(图 4A~B)。
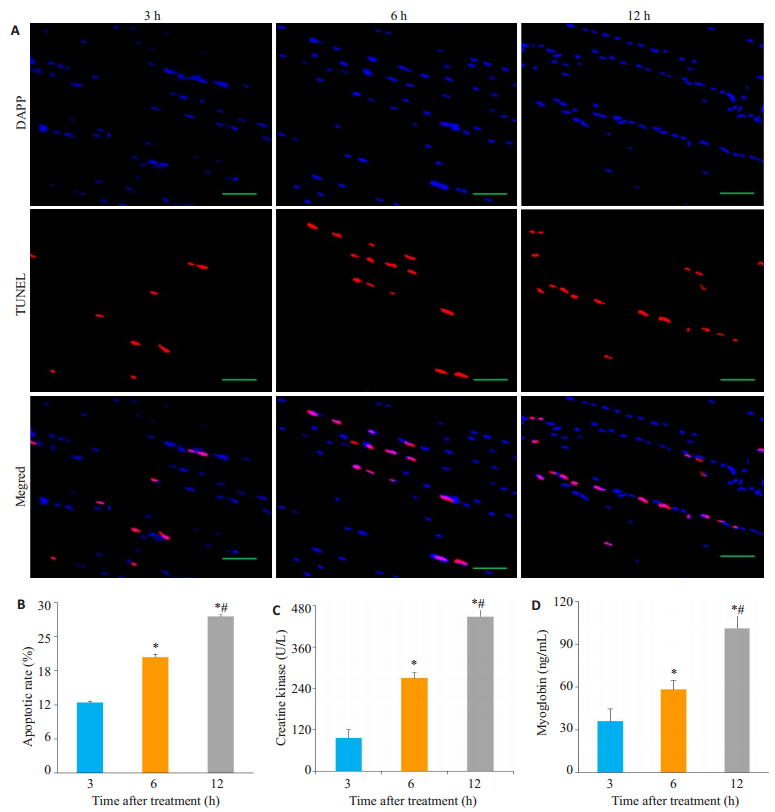
|
图 4 大鼠髂总动脉血栓模型超声联合微泡溶栓后后肢骨骼肌损伤情况 Figure 4 Apoptotic rate of skeletal muscle cells and serum levels of skeletal muscle injury markers in the rat models after ultrasound and microbubble treatment. A. TUNEL staining of the hindlimb of the rats after treatment, showing increased apoptotic rate of skeletal muscle cells the time after thrombosis prolonged; B. Quantification of apoptotic rate of skeletal muscle cells of the hindlimb after treatment; C: Serum levels of creatine kinase (CK) after treatment; D: Serum level of myoglobin (Mb) after treatment. (*: 12 h group & 6 h group vs 3 h group; #: 12 h group vs 6 h group, P < 0.0001). |
超声联合微泡治疗后,6 h组CK水平(269.99± 17.66)高于3 h组(97.02±22.90 U/L)(n=5, P < 0.0001),12 h组CK水平(448.05±20.06)高于3 h组(n=5, P < 0.0001),12 h组CK水平高于6 h组(n=5, P < 0.0001),差异具有统计学意义(图 4C)。
超声联合微泡治疗后,6 h组Mb水平(58.36±6.17)高于3 h组(36.01±8.80 ng/mL)(n=5, P=0.003),12 h组Mb水平(101.29±8.92)高于3 h组(n=5, P < 0.0001),12 h组Mb水平高于6 h组(n=5,P < 0.0001),差异具有统计学意义(图 4D)。
3 讨论本研究发现超声联合微泡溶解凝龄较大(6、12 h)的髂总动脉血栓的效率较凝龄较小的(3 h)低;超声联合微泡改善骨骼肌缺血损伤的效果随血栓凝龄的增加而减弱,提示超声联合微泡治疗越早进行,临床获益可能越大。
既往大量研究已证实超声联合微泡通过空化效应可有效溶解大动脉新鲜血栓[5-18](凝龄约3 h)、改善组织微循环血流灌注及组织缺血损伤,但有关超声联合微泡对较大凝龄血栓溶栓效果的研究较少。近期有研究报道超声联合微泡溶解颈总动脉血栓的能力与血栓凝龄密切相关,溶栓效率随血栓凝龄的增加而下降,这可能与血栓致密程度增加有关[19-20]。本研究观察了超声联合微泡对不同凝龄的髂总动脉血栓的溶栓效率,发现超声联合微泡溶解凝龄较大血栓的效率低于凝龄较小的血栓,与我们的前期研究结果[19]具有一致性。值得注意的是,我们的前期研究并未探索溶栓后组织损伤及预后改善情况,而减轻组织缺血损伤、改善预后是溶解动脉血栓的根本目的。在本研究中,我们发现随着血栓凝龄的增加,超声联合微泡治疗后骨骼肌微循环血流灌注下降、骨骼肌损伤标记物及骨骼肌细胞凋亡率升高,这些指标均提示骨骼肌缺血损伤加重。上述研究发现提示超声联合微泡溶解动脉血栓及挽救缺血组织的效率随着血栓凝龄的增加而下降。
既往关于介入治疗及药物溶栓治疗的大量研究证实,时间是挽救缺血组织、改善动脉血栓性疾病预后的关键[21-23],其原因主要表现在两个方面:首先,再灌注治疗主要针对的是梗死周边区的缺血濒死组织,随着缺血时间延长,该部分组织出现坏死、调亡[24-25],因此,闭塞血管开通时间越早,组织损伤越少,预后越好。其次,随着血栓形成时间延长,血栓变得致密,血管再通率下降[26-27]。本研究结果与既往关于介入治疗及药物溶栓治疗的研究发现类似,超声联合微泡溶栓治疗进行越早,血管再通率越高,缺血组织损伤程度越轻。本研究为临床进行超声联合微泡治疗动脉血栓性疾病的时间窗选择提供了理论依据。
本研究探索超声联合微泡溶栓后挽救缺血组织效率与血栓凝龄的关系,实验设立3 h组、6 h组和12 h组共3组,虽然也能说明实验假设,但组数相对偏少,如增加实验组数,将能使实验结论更具说服力。并且本实验仅使用声学造影法定量骨骼肌血容量,虽然这种方法也是测定血容量的经典方法,若能结合PET-CT或微球法等方法定量骨骼肌血容量,实验数据将会更加准确。
综上所述,超声联合微泡溶解血栓后挽救缺血组织的效果随着血栓凝龄的增高而降低。
| [1] |
Hartley A, Marshall DC, Salciccioli JD, et al. Trends in mortality from ischemic heart disease and cerebrovascular disease in Europe: 1980 to 2009[J].
Circulation, 2016, 133(20): 1916-26.
DOI: 10.1161/CIRCULATIONAHA.115.018931. |
| [2] |
Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of t[J].
Eur Heart J, 2016, 37(3): 267.
DOI: 10.1093/eurheartj/ehv320. |
| [3] |
Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction[J].
N Engl J Med, 2013, 368(15): 1379-87.
DOI: 10.1056/NEJMoa1301092. |
| [4] |
Dorn F, Stehle S, Lockau H, et al. Endovascular treatment of acute intracerebral artery occlusions with the solitaire stent: single-centre experience with 108 recanalization procedures[J].
Cerebrovasc Dis, 2012, 34(1): 70-7.
DOI: 10.1159/000338903. |
| [5] |
Pinto DS, Frederick PD, Chakrabarti AK, et al. Benefit of transferring ST-segment-elevation myocardial infarction patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase[J].
Circulation, 2011, 124(23): 2512-21.
DOI: 10.1161/CIRCULATIONAHA.111.018549. |
| [6] |
Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length[J].
Stroke, 2011, 42(6): 1775-7.
DOI: 10.1161/STROKEAHA.110.609693. |
| [7] |
Porter TR, Xie F, Lof J, et al. The thrombolytic effect of diagnostic ultrasound-induced microbubble cavitation in acute carotid thromboembolism[J].
Invest Radiol, 2017, 52(8): 477-81.
DOI: 10.1097/RLI.0000000000000369. |
| [8] |
Wang X, Gkanatsas Y, Palasubramaniam J, et al. Thrombus-Targeted theranostic microbubbles: a new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis[J].
Theranostics, 2016, 6(5): 726-38.
DOI: 10.7150/thno.14514. |
| [9] |
Tomkins AJ, Hood RJ, Pepperall D, et al. Thrombolytic recanalization of carotid arteries is highly dependent on degree of stenosis, despite sonothrombolysis[J].
J Am Heart Assoc, 2016, 5(2): pii: e002716.
|
| [10] |
Gao S, Zhang Y, Wu J, et al. Improvements in cerebral blood flow and recanalization rates with transcranial diagnostic ultrasound and intravenous microbubbles after acute cerebral emboli[J].
Invest Radiol, 2014, 49(9): 593-600.
DOI: 10.1097/RLI.0000000000000059. |
| [11] |
Culp WC, Flores R, Brown AT, et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke[J].
Stroke, 2011, 42(8): 2280-5.
DOI: 10.1161/STROKEAHA.110.607150. |
| [12] |
Perren F, Loulidi J, Poglia D, et al. Microbubble potentiated transcranial duplex ultrasound enhances Ⅳ thrombolysis in acute stroke[J].
J Thromb Thrombolysis, 2008, 25(2): 219-23.
DOI: 10.1007/s11239-007-0044-6. |
| [13] |
Mathias W, Tsutsui JM, Tavares BG, et al. Diagnostic ultrasound impulses improve microvascular flow in patients with STEMI receiving intravenous microbubbles[J].
J Am Coll Cardiol, 2016, 67(21): 2506-15.
DOI: 10.1016/j.jacc.2016.03.542. |
| [14] |
Slikkerveer J, Kleijn SA, Appelman Y, et al. Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: pilot of the Sonolysis study[J].
Ultrasound Med Biol, 2012, 38(2): 247-52.
DOI: 10.1016/j.ultrasmedbio.2011.11.001. |
| [15] |
Xie F, Lof J, Matsunaga T, et al. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions[J].
Circulation, 2009, 119(10): 1378-85.
DOI: 10.1161/CIRCULATIONAHA.108.825067. |
| [16] |
Wang B, Wang L, Zhou XB, et al. Thrombolysis effect of a novel targeted microbubble with low-frequency ultrasound in vivo[J].
Thromb Haemost, 2008, 100(2): 356-61.
|
| [17] |
Zhu Y, Guan L, Mu Y. Combined low-frequency ultrasound and urokinase-containing microbubbles in treatment of femoral artery thrombosis in a rabbit model[J].
PLoS One, 2016, 11(12): e0168909.
DOI: 10.1371/journal.pone.0168909. |
| [18] |
Culp WC, Porter TR, Mccowan TC, et al. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs[J].
J Vasc Interv Radiol, 2003, 14(3): 343-7.
DOI: 10.1097/01.RVI.0000058409.01661.B4. |
| [19] |
王世飞, 景远文, 李海瑞, 等. 超声联合微泡对不同凝龄富血小板血栓和富红细胞血栓的溶栓效果[J].
中国医学影像技术, 2017, 33(6): 832-7.
|
| [20] |
Sutton JT, Ivancevich NM, Perrin SR, et al. Clot retraction affects the extent of ultrasound-enhanced thrombolysis in an ex vivo porcine thrombosis model[J].
Ultrasound Med Biol, 2013, 39(5): 813-24.
DOI: 10.1016/j.ultrasmedbio.2012.12.008. |
| [21] |
Menon V, Pearte CA, Buller CE, et al. Lack of benefit from percutaneous intervention of persistently occluded infarct arteries after the acute phase of myocardial infarction is time independent: insights from occluded artery trial[J].
Eur Heart J, 2009, 30(2): 183-91.
|
| [22] |
Ndrepepa G, Kastrati A, Mehilli J, et al. Mechanical reperfusion and long-term mortality in patients with acute myocardial infarction presenting 12 to 48 hours from onset of symptoms[J].
JAMA, 2009, 301(5): 487-8.
DOI: 10.1001/jama.2009.32. |
| [23] |
Adlbrecht C, Huber K, Reynolds HR, et al. Effects of timing, location and definition of reinfarction on mortality in patients with totally occluded infarct related arteries late after myocardial infarction[J].
Int J Cardiol, 2014, 174(1): 90-5.
DOI: 10.1016/j.ijcard.2014.03.149. |
| [24] |
Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction[J].
Am Heart J, 2014, 167(5): 637-45.
DOI: 10.1016/j.ahj.2014.01.015. |
| [25] |
Braunwald E. Clinical efforts to reduce myocardial infarct size--the next step[J].
J Cardiovasc Pharmacol Ther, 2011, 16(3/4): 349-53.
|
| [26] |
Kramer MC, Van Der Wal AC, Koch KT, et al. Presence of older thrombus is an Independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention[J].
Circulation, 2008, 118(18): 1810-6.
DOI: 10.1161/CIRCULATIONAHA.108.780734. |
| [27] |
Li X, Kramer MC, Damman P, et al. Older coronary thrombus is an Independent predictor of 1-year mortality in acute myocardial infarction[J].
Eur J Clin Invest, 2016, 46(6): 501-10.
DOI: 10.1111/eci.2016.46.issue-6. |
 2018, Vol. 38
2018, Vol. 38

