海马在哺乳动物中枢神经系统的多种认知行为中具有重要功能,抑制性神经递质γ-氨基丁酸(GABA)及其A型受体(GABAA-R)在海马的功能形成中发挥重要作用,其接受来自奖赏相关脑区腹侧被盖区的投射[1]。脑区腹侧被盖区和海马之间的GABA能连接可能与奖赏相关的记忆和神经发生有关,在海马内还有很多GABA能中间神经元,它们也支配包括CA1和CA3等区域的神经元[1-3]。研究发现,GABA与海马干细胞的增殖和分化以及干细胞网络活动的控制有密切关系,海马齿状回颗粒细胞中GABAA-Rδ亚基的正常表达和功能建立对于构建和维持学习、记忆是必要的[4-5]。GABA能系统在海马功能中发挥显著调节作用,海马神经元中功能性GABAA-R对于海马的功能成熟是必需的。
神经发育在很大程度上依赖于功能性烟碱型乙酰胆碱受体(nAChR)的存在[6],其中α7-和β2-nAChR是两种主要亚基[7-8],后者通常与α3、α4等亚基结合共同发挥作用[9-10]。在海马发育过程中,GABAA-R正常功能的形成取决于不同种类的nAChR。报道显示,α7-nAChR的缺失将导致GABAA-R介导的氯离子平衡电位(EC1)发生正向偏移,并延缓GABAA-R通道的激活和失敏,进而使得海马体回路和功能发生进行性损伤[11]。在小鼠海马中间神经元研究中也发现,α7-nAChR参与调节GABAA-R的成熟[12]。而β2-nAChR是否也参与包括CA1和CA3等海马神经元中GABAA-R的功能成熟却仍然未知。有报道脊髓中的乙酰胆碱通过β2-nAChR调控GABA能的抑制作用,从而设定适宜的疼痛阈值[13],在小脑的研究中也发现β2-nAChR参与尼古丁诱发的GABA释放[14],提示β2-nAChR对GABA能系统存在调制作用。在海马CA1区域,β2-nAChR还参与介导尼古丁对兴奋性活动的增强作用,β2-nAChR还可以调节发育过程中的海马形成正常数量的树突棘[15-16],从而促进海马记忆的形成,其缺失可能导致阿尔茨海默病(AD)认知功能的减退[17]。但最近报道显示,将人类淀粉样前体蛋白通过病毒转染到海马齿状回后,相较于发生记忆损害的野生型小鼠,β2-nAChR基因敲除小鼠却未出现记忆缺失[18],提示β2-nAChR在AD的发病过程中可能具有更复杂的机制。因此,本研究采用穿孔膜片钳记录技术联合β2-KO小鼠,探索β2-nAChR是否参与海马CA1和CA3锥体神经元GABAA-R的功能成熟,这将为人类的学习记忆和AD等神经退行性疾病的研究提供重要的理论依据。
1 材料和方法 1.1 转基因动物杂合β2- KO小鼠购自Jackson Laboratories(Bar Harbor, ME, USA)。通过PCR鉴定它们的后代基因型,选择纯合的β2-nAChR基因敲除小鼠(β2-KO组)进行实验。野生型小鼠(WT组)小鼠5只,年龄17.4±0.7 d,β2- KO组4只,年龄18.3±1.3 d(两组小鼠年龄16~22 d),差异无统计学意义(P > 0.05)。
1.2 实验药品蝇蕈醇购自Sigma-Aldrich(St.Louis,MO),溶于蒸馏水,置于4 ℃冷藏备用。两性霉素B也购自SigmaAldrich,溶于二甲基亚砜(DMSO),置于-20 ℃冷冻备用。
1.3 CA1和CA3神经元的急性分离所有动物使用过程符合皖南医学院实验室动物饲养和使用规章条例。参考已有的程序制备海马冠状切片[19]。用异氟烷麻醉处死2~3周的WT组和β2-KO组小鼠并断头,快速取出脑组织并将其转移至冰水混合的人工脑脊液(ACSF)中持续1 min左右。ACSF配方为NaCl 124 mmol/L,KCl 5 mmol/L,NaHCO3 24 mmol/L,MgSO4 1.3 mmol/L,KH2PO4 1.2 mmol/L,CaCl2 2.4 mmol/L和glucose 10 mmol/L,ACSF持续充入95%O2+5%CO2的混合气体。使用振荡切片机Vibratome 1000 Plus(The Vibratome Company, St.Louis, MO, USA)连续切割含有海马的3~4片冠状切片(400 μm厚),并转移至室温(22±1 ℃)下ACSF中孵育1.5 h。之后,将切片在31 ℃下用链霉蛋白酶(1 mg/6 mL; Calbiochem, San Diego, CA, USA)消化25~45 min。在酶处理后,在体视显微镜下(Meiji Techno Co.,Ltd.,Tokyo,Japan),参考小鼠脑图谱[20]区分海马区,用自制的抛光针头在CA1和CA3细胞线上分别冲压取出微小组织块,并将每个组织块转移至直径35 mm的培养皿中,培养皿预先充满用100%O2氧饱和的标准外液,配方:NaCl 150 mmol/L,KCl 5 mmol/L,MgCl2 1 mmol/L,CaCl2 2 mmol/L,glucose 10 mmol/L,HEPES 10 mmol/L。使用Tris-base将标准溶液的pH调节至7.4[21]。在倒置显微镜(Olympus IX70, Tokyo, Japan)的协助下,使用火抛光的微巴斯德移液管机械分离获得的微小海马组织块。具有良好形态的急性分离的神经元通常在30 min内粘附于培养皿的底部,然后开始电生理实验。
1.4 穿孔膜片钳记录室温下,在分离的海马神经元上进行穿孔膜片钳记录[19, 22-23]。首先,将含有分离的CA1或CA3神经元的培养皿分别放置在倒置显微镜的载物台上,给予标准外液连续灌流。通过两步电极拉制仪(P-830; Narishige)制备玻璃记录电极(1.5 mm×100 mm; Narishige,Greenvale,NY,USA),在充以电极内液后电极电阻为3~5 MΩ,电极内液配方为potassium gluconate 140 mmol/L,KCl 10 mmol/L,MgCl2 5 mmol/L和HEPES 10 mmol/L,用Tris-base将pH调节至7.2。使用程控的药物灌流系统(SF-77B Perfusion Fast Step; Warner Instruments, Hamden, CT, USA)来加速溶液交换速率,蝇蕈醇灌流4 s,间隔2 min,这样可获得稳定的GABAA-R反应。使用Axopatch 200B放大器(Molecular Devices, Sunnyvale, CA, USA)记录神经元在不同钳制电位(VH)时的GABAA-R介导的电流。对于穿孔膜片钳记录,将含有两性霉素B的原液(DMSO中40 mg/mL)用上述电极内液稀释至终浓度(200 μg/mL)。在形成高阻抗封接后10 min内接入电阻(Ra)通常降至 < 60 MΩ,实验只接受 < 60 MΩ的Ra。使用pClamp 9.2(Molecular Devices, Sunnyvale, CA, USA)软件进行数据采集和分析。
1.5 统计与分析所有统计数据均以均数±标准差表示。应用pClamp 9.2软件进行数据分析,测定电流的幅度、上升时间和衰减时间等。将在不同VH时,蝇蕈醇诱导的峰电流相对于各自对照(把VH=-60 mV时的峰电流幅度设定为100%)进行归一化处理,并且将归一化值用于统计分析和绘图。根据每例细胞的峰电流幅度和对应的VH,通过电流-电压(Ⅰ-Ⅴ)曲线的线性拟合,获得蝇蕈醇诱导电流的平衡电位(EMus)。并使用Origin 5.0(OriginLab Corp., Northampton, MA)等软件依据结果绘图。使用Student's t检验进行两组平均值的比较。P < 0.05为差异有统计学意义。
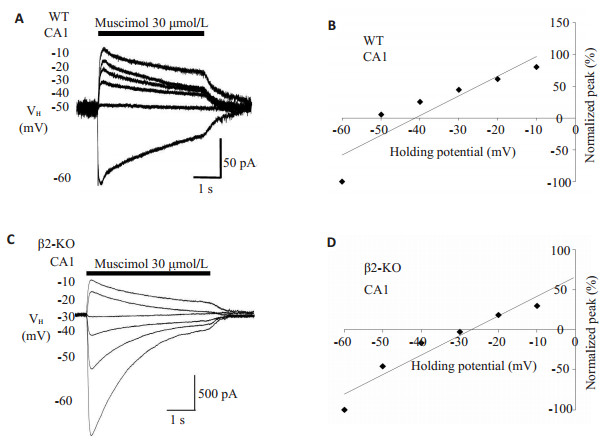
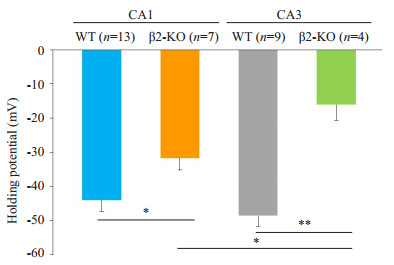
2 结果 2.1 海马锥体神经元GABAA-R介导电流的平衡电位比较 2.1.1 海马CA1锥体神经元的GABAA-R介导电流平衡电位首先,为测试含β2的nAChR在CA1细胞中GABAA-R的发育中是否发挥作用,对于急性分离的CA1锥体细胞灌流GABAA-R选择性激动剂蝇蕈醇(30 μmol/ L),以诱发GABAA-R介导的电流,并通过改变VH获得一系列电流。当VH设定在-60~-10 mV,蝇蕈醇在WT小鼠的CA1神经元诱导出大小不一的电流(图 1A),通过Ⅰ-Ⅴ曲线的线性拟合,得到该例WT小鼠CA1神经元的EMus为-41.3 mV(图 1B)。取自5只WT小鼠共13例CA1细胞,EMus为-44.1±3.3 mV(图 3)。β2-KO CA1神经元中蝇蕈醇诱导电流发生了明显变化(图 1C),β2-KO CA1神经元的EMus为-27.0 mV(图 1D)。4只β2- KO小鼠的7个CA1细胞的EMus为-31.7±3.5 mV,与WT小鼠相比差异有统计学意义(P < 0.05,图 3)。
|
图 1 野生型和β2-KO小鼠海马CA1神经元上GABAA-R平衡电位的比较 Figure 1 Comparisons on the equilibrium potential (EMus) mediated by GABAA-Rs in the hippocampal CA1 neurons between WT and β2-KO mice. A, C: Typical currents induced by muscimol, the GABAA-R selective agonist, were recorded in a WT CA1(A) and a β2-KO CA1 (C) neuron respectively at the different holding potentials (VHs) indicated on the left of each current curve; B, D: Amplitude of peak current and matched VH were plotted to yield the EMus from a linear fit of the I-V curve for each cell corresponding to A and C. In D, the regression line was extra plotted to positive direction. |
|
图 3 野生型和β2-KO小鼠海马CA1和CA3神经元上GABAA-R平衡电位的比较 Figure 3 Bar chart summarizing the difference of the equilibrium potentials (EMuss) mediated by GABAA-Rs in the hippocampus CA1 and CA3 neurons obtained from the WT and β2-KO mice. Cell numbers were marked in the corresponding brackets above each bar. *P < 0.05, **P < 0.01. |
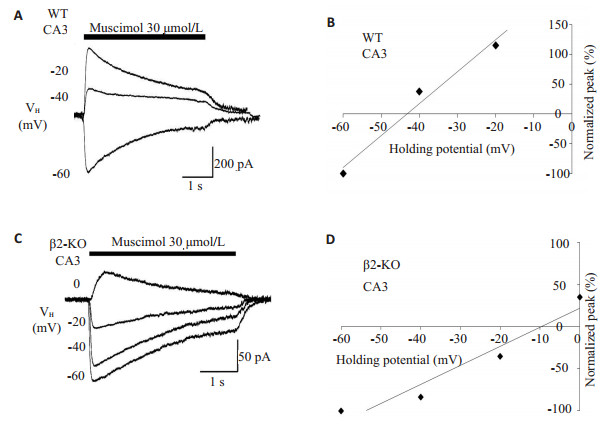
蝇蕈醇在WT和β2-KO的CA3细胞诱导的电流表现出一致的结果。图 2A和C分别为1例WT和1例β2- KO CA3神经元在不同VH时的GABAA-R介导电流的代表性记录。分别对应于图 2A,C,图 2B,D显示的EMus分别为-43.2和-9.7 mV。总体而言,取自4只β2-KO小鼠的CA3细胞(n=4)的EMus低于5只WT共9例CA3的EMus(-16.1±4.6 mV vs -48.6±3.2 mV,P < 0.01,图 3)。
|
图 2 野生型和β2-KO小鼠CA3神经元上GABAA-R平衡电位的差异 Figure 2 Differences in the equilibrium potential (EMus) mediated by GABAA-Rs in the hippocampus CA3 neurons obtained from the WT and β2- KO mice. A, C: Representative currents induced by muscimol were recorded at the indicated VHs in a WT (A) and a β2-KO (C) neuron; B, D: Peak amplitude of the GABAA-R-mediated currents as a function of voltage in a WT (B) and a β2-KO (D) neuron as in A and C. The regression line was extra plotted to positive direction in D. |
WT小鼠CA3和CA1神经元的EMus差异无统计学意义,但β2-KO小鼠CA3和CA1神经元的EMus差异显著(P < 0.05,图 3)。此外,β2-KO的CA1和CA3神经元的Ⅰ~ Ⅴ曲线明显向去极化方向偏移(图 1C~D,图 2C~D,图 3)。
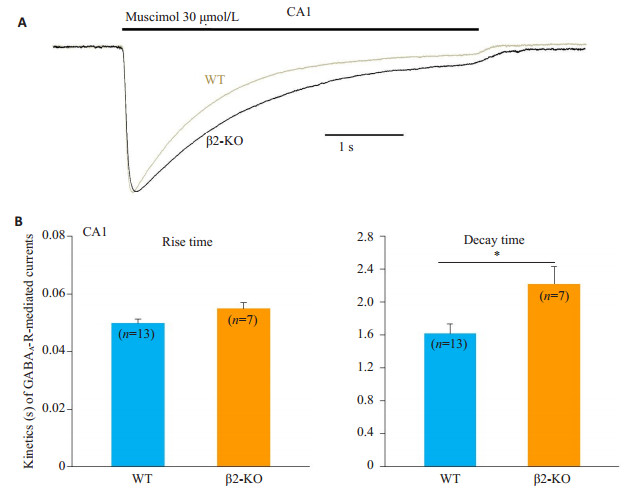
2.2 海马锥体神经元GABAA-R介导电流的动力学比较 2.2.1 海马CA1神经元的GABAA-R介导电流动力学为深入分析β2-nAChR在GABAA-R发育中的作用,本研究对WT和β2-KO小鼠GABAA-R介导电流在动力学上进行了比较。蝇蕈醇在1例WT和1例β2-KO的CA1细胞上分别诱导出GABA电流,两个电流达峰前的部分几乎完全重叠(图 4A),在受体通道激活动力学上WT和β2-KO小鼠无显著差异(图 4B),其上升时间分别为0.050±0.001 s和0.055±0.002 s(P > 0.05),但在电流达到峰值后,β2-KO受体通道的失敏显著减慢,其衰减时间为2.2±0.2 s,WT组衰减时间为1.6±0.1 s,差异具有统计学意义(P < 0.05)。
|
图 4 野生型和β2-KO小鼠CA1神经元上GABAA-R介导电流的动力学比较 Figure 4 Comparisons of kinetics of GABAA-R-mediated currents in CA1 neurons of WT and β2-KO mice. A: Representative currents induced by muscimol were recorded in the CA1 neurons respective from WT (gray) and β2-KO (black) mice; B: Quantification of rise time (left) and decay time (right) of the GABAA-R-mediated currents as in A. Cell numbers are marked inside the bars. *P < 0.05. |
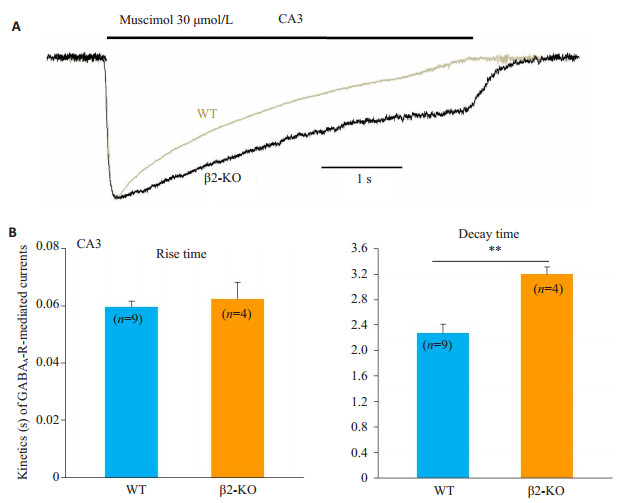
蝇蕈醇分别在1例WT和1例β2-KO的CA3细胞上诱发出典型GABA电流(图 5A),这两个电流在达峰前完全吻合,但在受体通道失敏过程中,β2-KO的电流明显减慢(图 5B),WT和β2-KO的上升时间分别为0.060± 0.002 s和0.062±0.006 s(P > 0.05),衰减时间分别为2.3± 0.1 s和3.2±0.1 s(P < 0.01)。
|
图 5 野生型和β2-KO小鼠CA3神经元上GABAA-R介导电流的动力学差异 Figure 5 Differences in kinetics of GABAA-R-mediated currents in CA3 neurons between WT and β2- KO mice. A: Typical currents induced by muscimol were recorded in the CA3 neurons respective from WT (gray) and β2-KO (black) mice; B: Quantification of rise time (left) and decay time (right) of the GABAA-R-mediated currents as in A. Cell numbers are marked inside the bars. **P < 0.01. |
本实验着眼于β2-nAChR是否参与海马CA1和CA3锥体神经元中GABAA-R的发育,使用穿孔膜片钳记录技术联合β2-KO小鼠开展研究,发现与对照组(WT小鼠)相比,β2-KO的CA1和CA3神经元的EMus明显偏向去极化方向,并且GABAA-R通道失敏显著滞后,表明β2-nAChR与CA1和CA3中GABAA-R的功能成熟可能存在密切联系。已知GABAA-R与Cl-通道偶联,本研究应用的EMus即为EC1。有研究显示在海马齿状回α7- nAChR-KO(α7-KO)导致EC1显著去极化[11],与本研究结果一致,β2-KO小鼠的CA1和CA3神经元的EMus均显著低于各对照组,提示β2-nAChR与α7-nAChR在促进GABAA-R功能成熟上可能具有相似作用。但α7-KO既影响GABAA-R通道的激活,也延缓其失敏,与该文的研究结果又不尽相同,提示β2- nAChR在调节GABAA-R功能成熟方面,与α7-nAChR可能存在差异性作用。此外,本研究还发现,β2-nAChR对CA3神经元GABAA-R的功能成熟比对CA1神经元具有更强的影响,说明β2-nAChR在神经发育中可能存在不均衡的作用。
研究结果提示,无论对于CA1还是CA3神经元,β 2-nAChR都可能具有调节GABAA-R成熟的能力,也表明β2-nAChR可能参与海马回路和功能的构建,β2- nAChR缺陷或许会导致海马神经进行性损伤,进而产生学习和记忆功能障碍。本研究认为β2-nAChR在神经发育初期可能具有维持GABA能兴奋性/去极化作用,并适时终止兴奋作用并转变为抑制功能,这将有助于与兴奋性系统如谷氨酸能和胆碱能系统之间建立平衡关系。早期的兴奋/去极化功能对于出生后早期和成年时期的神经元在中枢神经系统的发育和整合是必需的[24-26],而本研究也提示CA1和CA3神经元可能需要β 2-nAChR参与信号传导,从而促进神经元正常发育和整合,以及促使细胞内外Cl-浓度梯度的形成,并最终实现GABAA-R介导的抑制性电流。
海马功能的发挥取决于神经的正常发育和整合。切除神经前体细胞将导致成年神经元的损伤,使得海马某些依赖性学习和记忆功能缺失[27-28]。通过正常发育和成熟的神经元在成年期具有许多关键功能,包括编码时间、地点整合[29]、增强先前记忆[30]以及实现记忆从海马到大脑皮层的传递[31]等。此外,成年期健康的神经元能够在减少成瘾行为和降低复发率方面发挥重要作用[32]。因此,神经系统的正常发育对于成年期的作用是不言而喻的,但是神经退行性疾病却给人类的认知功能带来严重危害。AD是一种具有较明显家族遗传性的神经退行性疾病,存在一系列认知功能障碍,尤其表现在学习记忆方面。与AD发病直接相关的是疾病过程中逐渐累积的β-淀粉样肽[33],现阶段很多研究发现β-淀粉样肽抑制海马神经元中的α7-nAChR功能[34-36],但鲜有对β2-nAChR功能影响的研究。已知海马神经元中具有正常功能的GABAA-R有助于海马成熟和维持学习、记忆功能[5]。本研究推测在AD患者婴幼年时期,机体内微量的β-淀粉样肽或许已经存在,并对β2-nAChR产生轻微抑制作用,导致GABAA-R发育上出现瑕疵,进而逐步影响远期的学习、记忆能力。有关这方面的假设,需要进一步开展研究。
总之,本研究结果提示β2-nAChR可能通过促进GABAA-R的功能成熟,实现海马CA1和CA3神经元的健康发育,从而形成并改善海马依赖性的学习、记忆功能,现阶段的工作将为进一步研究学习记忆和神经退行性疾病提供重要的理论依据。
| [1] |
Ntamati NR, Lüscher C. VTA projection neurons releasing GABA and glutamate in the dentate gyrus[J].
Eneuro, 2016, 3(4): 116-37.
|
| [2] |
Zheng F, Khanna S. Intra-hippocampal tonic inhibition influences formalin pain-induced pyramidal cell suppression, but not excitation in dorsal field CA1 of rat[J].
Brain Res Bull, 2008, 77(6): 374-81.
DOI: 10.1016/j.brainresbull.2008.09.004. |
| [3] |
Rambousek L, Kleteckova L, Kubesova A, et al. Rat intrahippocampal NMDA infusion induces cell-specific damage and changes in expression of NMDA and GABA(A) receptor subunits[J].
Neuropharmacology, 2016, 105(13): 594-606.
|
| [4] |
Sibbe M, Kulik A. GABAergic regulation of adult hippocampal neurogenesis[J].
Mol Neurobiol, 2017, 54(7): 5497-510.
DOI: 10.1007/s12035-016-0072-3. |
| [5] |
Lee V, Mackenzie G, Hooper A, et al. Reduced tonic inhibition in the dentate gyrus contributes to chronic stress-induced impairments in learning and memory[J].
Hippocampus, 2016, 26(10): 1276-90.
DOI: 10.1002/hipo.v26.10. |
| [6] |
Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: From basic science to therapeutics[J].
Pharmacol Ther, 2013, 137(1): 22-54.
DOI: 10.1016/j.pharmthera.2012.08.012. |
| [7] |
Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb[J].
Genes Cells, 2006, 11(10): 1145-59.
DOI: 10.1111/gtc.2006.11.issue-10. |
| [8] |
Ide Y, Fujiyama F, Okamoto-Furuta K, et al. Rapid integration of young newborn dentate gyrus granule cells in the adult hippocampal circuitry[J].
Eur J Neurosci, 2008, 28(12): 2381-92.
DOI: 10.1111/ejn.2008.28.issue-12. |
| [9] |
King MD, Long T, Andersen T, et al. Genetic algorithm managed peptide mutant screening:optimizing peptide ligands for targeted receptor binding[J].
J Chem Inf Model, 2016, 56(12): 2378-87.
DOI: 10.1021/acs.jcim.6b00095. |
| [10] |
Lange-Asschenfeldt C, Schäble S, Suvorava T, et al. Effects of varenicline on alpha4-containing nicotinic acetylcholine receptor expression and cognitive performance in mice[J].
Neuropharmacology, 2016, 107(21): 100-10.
|
| [11] |
Campbell NR, Fernandes CC, Halff AW, et al. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus[J].
J Neurosci, 2010, 30(26): 8734-44.
DOI: 10.1523/JNEUROSCI.0931-10.2010. |
| [12] |
Liu ZP, Zhang JM, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development[J].
Biochem Pharmacol, 2007, 74(8): 1112-9.
DOI: 10.1016/j.bcp.2007.05.022. |
| [13] |
Yalcin I, Charlet A, Cordero-Erausquin M, et al. Nociceptive thresholds are controlled through spinal beta(2)-subunit-containing nicotinic acetylcholine receptors[J].
Pain, 2011, 152(9): 2131-7.
DOI: 10.1016/j.pain.2011.05.022. |
| [14] |
Pereira Silva JD, Campos DV, Nogueira-Bechara FM, et al. Altered release and uptake of gamma-aminobutyric acid in the cerebellum of dystrophin-deficient mice[J].
Neurochem Int, 2018, 85(6): 1-14.
|
| [15] |
Nakauchi S, Sumikawa K. Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice[J].
Eur J Neurosci, 2012, 35(9): 1381-95.
DOI: 10.1111/ejn.2012.35.issue-9. |
| [16] |
Lozada AF, Wang XL, Gounko NV, et al. Induction of dendritic spines by beta 2-containing nicotinic receptors[J].
J Neurosci, 2012, 32(24): 8391-400.
DOI: 10.1523/JNEUROSCI.6247-11.2012. |
| [17] |
Bell KA, Shim H, Chen CK, et al. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain alpha 4 and beta 2 subunits[J].
Neuropharmacology, 2011, 61(8): 1379-88.
DOI: 10.1016/j.neuropharm.2011.08.024. |
| [18] |
Lombardo S, Catteau J, Besson M, et al. A role for β2*nicotinic receptors in a model of local amyloid pathology induced in dentate gyrus[J].
Neurobiol Aging, 2016, 46(9): 221-34.
|
| [19] |
Wang K, Zheng C, Wu C, et al. alpha-Chloralose diminishes gamma oscillations in rat hippocampal slices[J].
Neurosci Lett, 2008, 441(1): 66-71.
DOI: 10.1016/j.neulet.2008.06.014. |
| [20] |
Paxinos G, Franklin K.
The mouse brain in stereotaxic coordinates[M]. Elsevier: New York, 2001.
|
| [21] |
Yang KC, Jin GZ, Wu J. Mysterious alpha 6-containing nAChRs: function, pharmacology, and pathophysiology[J].
Acta Pharmacol Sin, 2009, 30(6): 740-51.
DOI: 10.1038/aps.2009.63. |
| [22] |
Wu J, Kuo YP, George AA, et al. Beta-amyloid directly inhibits human alpha 4 beta 2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells[J].
J Biol Chem, 2004, 279(36): 37842-51.
DOI: 10.1074/jbc.M400335200. |
| [23] |
Yang KC, Hu J, Lucero L, et al. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons[J].
J Physiol, 2009, 587(2): 345-61.
DOI: 10.1113/jphysiol.2008.162743. |
| [24] |
Tozuka Y, Fukuda S, Namba T, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells[J].
Neuron, 2005, 47(6): 803-15.
DOI: 10.1016/j.neuron.2005.08.023. |
| [25] |
Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain[J].
Nature, 2006, 439(7076): 589-93.
DOI: 10.1038/nature04404. |
| [26] |
Cancedda L, Fiumelli H, Chen K, et al. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo[J].
J Neurosci, 2007, 27(19): 5224-35.
DOI: 10.1523/JNEUROSCI.5169-06.2007. |
| [27] |
Snyder JS, Hong NS, Mcdonald R, et al. A role for adult neurogenesis in spatial long-term memory[J].
Neuroscience, 2005, 130(4): 843-52.
DOI: 10.1016/j.neuroscience.2004.10.009. |
| [28] |
Winocur G, Wojtowicz JM, Sekeres M, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function[J].
Hippocampus, 2006, 16(3): 296-304.
DOI: 10.1002/(ISSN)1098-1063. |
| [29] |
Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories[J].
Nat Neurosci, 2006, 9(6): 723-7.
DOI: 10.1038/nn1707. |
| [30] |
Trouche S, Bontempi B, Roullet P, et al. Recruitment of adultgenerated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory[J].
Proc Natl Acad Sci USA, 2009, 106(14): 5919-24.
DOI: 10.1073/pnas.0811054106. |
| [31] |
Kitamura T, Saitoh Y, Takashima N, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory[J].
Cell, 2009, 139(4): 814-27.
DOI: 10.1016/j.cell.2009.10.020. |
| [32] |
Noonan MA, Bulin SE, Fuller DC, et al. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction[J].
J Neurosci, 2010, 30(1): 304-15.
DOI: 10.1523/JNEUROSCI.4256-09.2010. |
| [33] |
Selkoe DJ. Normal and abnormal biology of the β-amyloid precursor protein[J].
Annu Rev Neurosci, 1994, 17(11): 489-517.
|
| [34] |
Liu Q, Kawai H, Berg DK. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons[J].
Proc Natl Acad Sci USA, 2001, 98(8): 4734-9.
DOI: 10.1073/pnas.081553598. |
| [35] |
Pettit DL, Shao Z, Yakel JL. β-Amyloid1-42 peptide directly modulates nicotinic receptors in the rat hippocampal slice[J].
J Neurosci, 2001, 21(1): 1-5.
|
| [36] |
Dougherty JJ, Wu JL, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex[J].
J Neurosci, 2003, 23(17): 6740-7.
DOI: 10.1523/JNEUROSCI.23-17-06740.2003. |
 2018, Vol. 38
2018, Vol. 38

