脑白质病变(WML)与老年痴呆、脑卒中、残疾等相关,尤其是中重度WML导致的认知和运动功能障碍是不可逆的,加重了社会医疗体系的负担。既往研究多侧重于探讨WML发病与否的危险因素,但轻度WML无明显临床症状,一般不易被发现,况且年龄是不可干预的因素。因此,寻找中重度WML危险因素,尤其是可干预的危险因素,具有重要意义。载脂蛋白B(ApoB)和载脂蛋白AI (ApoAI)是动脉硬化和心血管事件的良好预测指标[1-2]。有研究发现ApoAI与痴呆之间存在负相关关系,ApoB与痴呆存在正相关关系[3-4]。另有研究发现ApoAI是WML的独立危险因素,但仅阐明ApoAI通过炎症机制与WML相关。目前暂无研究报道ApoB及ApoB/ApoAI与WML的关系及作用机制。然而已有研究表明ApoB和ApoB/ApoAI不仅与机体的炎症水平相关,并且与血管病变及脑血流灌注相关[5-7],因此,将ApoAI、ApoB及其比值结合起来探讨其与WML的关系有助于寻找影响WML发展的危险因素。本研究采用回顾性分析方法,探讨在中老年WML患者中,血清ApoAI、ApoB及其比值与WML严重程度的相关性。
1 资料和方法 1.1 一般资料回顾性收集2015年1月~2017年12月南方医科大学第三附属医院神经内科连续入院、符合以下标准的患者648例。纳入标准:(1)年龄≥50岁;(2)完善头颅MRI检查(包括T1 WI、T2 WI、T2-FLAIR序列),证实存在WML者;(3)临床病历资料齐全,住院期间完善血脂、血尿酸、血同型半胱氨酸等检查。排除标准:(1)非血管源性的(多发性硬化、脑白质营养不良等);(2)急性卒中患者;(3)肿瘤,痴呆,精神病患者;(4)入院前1月内规律使用降脂药物的患者。
1.2 分析指标 1.2.1 临床资料包括姓名、性别、年龄、高血压、糖尿病,冠心病、既往卒中史、吸烟。
1.2.2 血液指标所有入组患者于入院后次日清晨采集空腹静脉血,送至我院临床检验科完善血脂、血尿酸、血同型半胱氨酸等检查。收集指标包括ApoAI和ApoB,总胆固醇(TC)、甘油三脂(TG)、高密度脂蛋白胆固醇(HDL-C)和低密度脂蛋白胆固醇(LDL-C)。
1.2.3 影像学检查所有入组患者于住院期间完善头颅MRI检查(包括T1 WI、T2 WI、T2-FLAIR序列),由2位临床经验丰富的神经科医生采用双盲法根据Fazekas量表[8]对所有入组患者进行WML评分,有异议者由第3位神经科医生再次评估后决定。将脑室旁和深部白质病变分开评分。脑室旁白质病变评分,1分:帽状或者铅笔样薄层病变;2分:病变呈光滑的晕圈;3分:不规则的脑室旁高信号,延伸到深部白质。深部白质病变评分,1分:点状病变;2分:病变开始融合;3分:病变大面积融合。两部分的得分相加计算总分。总分 < 3为轻度组,总分≥3分为中重度组。
1.3 统计学方法采用SPSS 22.0统计软件进行分析。呈正态分布的计量资料以均数±标准差表示,组间比较采用两独立样本t检验。计数资料以百分数表示,组间比较采用卡方检验。采用多因素logistic回归分析血清ApoAI、ApoB及ApoB/ApoAI水平与脑白质变性严重程度的相关性。P < 0.05认为差异有统计学意义。
2 结果 2.1 临床资料比较本研究共纳入轻度WML患者386例(59.6%),中重度WML患者262例(40.4%)。与轻度WML组比较,中重度WML组患病年龄更大(P < 0.001),男性比例更高(P=0.044),高血压、糖尿病、冠心病、既往卒中史的患病率更高(P < 0.05),同型半胱氨酸、ApoB/ApoAI水平更高(P < 0.001),而HDL-C、ApoAI水平较低(P < 0.001)。两组的ApoB、尿酸、TC、TG、LDL-C水平、吸烟情况差异无统计学意义(P > 0.05,表 1)。
| 表 1 轻度WML组与中重度WML组患者的临床资料比较 Table 1 Comparison of the clinical data between mild WML group and moderate to severe WML group |
将单因素分析结果中P < 0.1的变量作为自变量纳入二分类logistic回归模型中。ApoAI及ApoB/ApoAI作连续型变量资料纳入多因素logistic回归模型中,调整相关混杂因素后发现年龄、高血压、既往卒中史、ApoB/ApoAI是中重度WML的独立危险因素(P < 0.05),ApoAI是中重度WML的独立保护因素(P < 0.001,表 2)。
| 表 2 中重度WML独立危险因素的多因素logistic回归分析 Table 2 Multivariate logistic regression analysis of the factors associated with moderate to severe WML |
分别将ApoAI、ApoB/ApoAI以四分位数法分为4个等级,作为等级变量进入logistic回归方程。ApoAI的4个等级分别为:≤0.96,0.96~1.15,1.15~1.38,≥1.38 g/L;ApoB/ApoAI比值的4个等级分别为:≤0.58,0.58~ 0.73,0.73~0.91,≥0.91 g/L。调整混杂因素后发现,血清ApoAI水平降低、ApoB/ApoAI水平升高有加重WML的风险。在WML患者中,最高水平等级者罹患中重度WML的风险为最低水平等级者的0.1和7.8倍(表 3)。
| 表 3 中重度WML独立危险因素的多因素logistic回归分析 Table 3 Multivariate logistic regression analysis of the factors associated with moderate to severe WML |
以ApoAI的上四分位数(p75)为截点分为低ApoAI(≥1.38 g/L)、高ApoAI(< 0.138 g/L);以ApoB/ ApoAI的下四分位数(p25)为截点分为低ApoB/ApoAI (≤0.58 g/L)、高ApoB/ApoAI (> 0.58 g/L)。再根据血清ApoAI及ApoB/ApoAI的高低将所有研究对象分为4组:(1)高ApoAI、高ApoB/ApoAI组;(2)高ApoAI、低ApoB/ApoAI组;(3)低ApoAI、高ApoB/ApoAI组;(4)低ApoAI、低ApoB/ApoAI组。通过比较发现,高ApoAI、低ApoB/ApoAI组的中重度WML的发病率最低(n=11, 10.9%),低于其他3组(P < 0.05,图 1)。
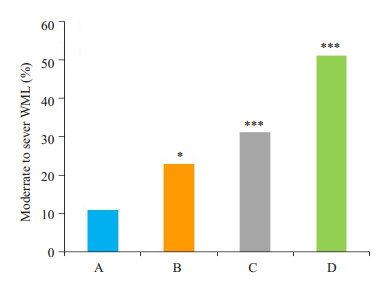
|
图 1 血清ApoAI联合ApoB/ApoAI水平对患者WML严重程度的影响 Figure 1 Association of ApoAI combined with ApoB/AI ratio with the severity of brain white matter lesions. *P < 0.05, ***P < 0.001 vs high ApoAI and low ApoB/AI group. A: High ApoAI level with low ApoB/ApoAI; B: High ApoAI level with high ApoB/ApoAI; C: Low ApoAI level with low ApoB/ApoAI; D: Low ApoAI level with high ApoB/ ApoAI. |
本研究是在中老年人群中探索血清ApoAI、ApoB及其比值与WML严重程度的相关性。通过回顾性分析648例WML患者的临床资料及血脂情况,调整混杂因素后发现年龄、高血压、既往卒中史是WML的独立危险因素,这与既往的研究结果一致[9-10]。另外,本研究发现ApoB与WML严重程度没有相关性,而血清ApoAI、ApoB/ApoAI水平与WML严重程度相关。通过多因素logistic回归分析显示,调整了混杂因素后发现血清ApoAI水平降低及ApoB/ApoAI水平增高有加重WML的风险。通过比较发现,低ApoAI、高ApoB/ApoAI组的中重度WML的发病率最高,二者相结合更有助于预测WML的发生发展。
WML的发病机制主要慢性低灌注缺氧、氧化应激和炎症反应、内皮功能紊乱、血脑屏障破坏、β淀粉样物质沉积、基因多态性、胶质增生等有关[11]。既往对临床常用的血脂指标与WML相关性研究的结果并不统一,有研究显示TC和LDL-C升高和是WML的独立危险因素[12],而这个结论并未被其他研究证实[13],在本研究中并未发现TC、LDL-C与WML存在相关性。有研究指出HDL-C降低是WML的独立危险因素[14],但在另一项研究中却发现TG升高是WML的独立危险因素,HDL-C降低并不是WML的独立危险因素[15]。本研究也并未发现TG、HDL-C与WML严重程度相关。越来越多的研究表明,载脂蛋白比目前临床中常使用的传统脂质参数更能准确预测心血管疾病的风[16-18]。
ApoAI是高密度脂蛋白的主要载脂蛋白,具有清除胆固醇和防止周围组织脂质沉积的功能,参与胆固醇从外周到肝脏的逆向转运过程,而且具有抗氧化和抗炎作用。相反,ApoB是低密度脂蛋白和极低密度脂蛋白的主要脂蛋白成分,可刺激动脉平滑肌增值并进入内膜下层,具有致动脉粥样硬化的作用。因此,ApoB/ApoAI代表促动脉粥样硬化和抗动脉粥样硬化之间的平衡关系。
血清ApoAI、ApoB/ApoAI水平与WML的病理机制尚不完全清楚,而目前认为可能的机制包括:ApoAI具有抗氧化、抗炎症反应的作用。ApoAI通过对APT结盒转运子AI产生作用而抑制白细胞介素-6等炎性因子释放[19],避免炎症因反应造成脑白质损伤。ApoAI可与β淀粉样蛋白结合,避免β淀粉样蛋白的神经活性介导脑白质发生病变[20]。此外,ApoAI可以直接避免小血管发生动脉瘤,防止脑白质发生病变[21]。ApoB/ApoAI与氧化应激与炎症水平呈正相关[22],当血清ApoB/ApoAI水平增加时,氧化应激与炎症产生众多的炎症因子(如C反应蛋白、白细胞介素-6、肿瘤坏死因子-α、单核细胞趋化蛋白-1、细胞间粘附分子-1和血管粘附分子-1等)均与动脉粥样硬化炎症过程相关,它们可以通过不同的生理功能作用于动脉粥样硬化发展的不同阶段,相互作用,相互交联,形成复杂的网络,扩大炎症的级联反应。而临床研究和动物实验都已证明炎症反应是WML的重要发病之一[23-25]。血清ApoB/ApoAI水平增加时,动脉发生粥样硬化改变[26],血管反应减弱甚至消失。有研究发现脑血管自身调节受损,增加了血压波动期间脑白质区域受损的易感性[5]。此外,Brisset等[6]研究证明了脑内大血管硬化程度与白质区域血流量减少有关。这些研究都证明了动脉粥样硬化后,低灌注引起脑白质变性。另外,动脉硬化过程中的炎症因子(如肿瘤坏死因子-α)刺激血管内皮发生损伤性改变,内皮功能障碍与脑白质变性相关[27]。血管内皮细胞的损伤也改变了血脑屏障通透性,BBB通透性的改变也与脑白质疏松症和腔隙性脑梗死相关[28]。ApoB/ApoAI升高时,颈动脉内中膜增厚、硬化狭窄[29],颈动脉粥样硬化与WML的发生有关[30]。
目前对于WML的预防和治疗仍缺少有效的手段,临床上主要为控制血压、血糖、戒烟、戒酒、纠正认知障碍等药物治疗,然而效果有限。因为WML是一个慢性系统性炎症反应的过程,但目前对抗炎症过程的预治的研究尚少。根据本研究的结果,旨在升高ApoAI和(或)降低ApoB/ApoAI的治疗方案在延缓WML进展的方面可能具有潜在的益处。最近有研究表明,依折麦布/辛伐他汀(10/20 mg)和阿托伐汀(20 mg)均可降低ApoB/ApoAI[31],另一项研究则表明辛伐他汀可以延缓WML的进展[32],提示上述药物在WML防止中的作用,但需更多的研究来证实。
本研究存在一定的局限性:本研究是采用Fazekas量表对患者WML严重程度分级,不同观察者可能存在误差。本研究为回顾性研究,入选的研究对象是以医院神经内科为基础的患者组成,可能比以社区为基础的患者具有更多的血管危险因素,未来的研究需要进一步排除相关影响因素,在一般人群中验证本研究的结果。
| [1] |
Leslie M. To help save the heart, is it time to retire cholesterol tests[J].
Science, 2017, 358(6368): 1237-8.
DOI: 10.1126/science.358.6368.1237. |
| [2] |
O'donnell MJ, Chin SL, Rangarajan SA, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study[J].
Lancet, 2016, 388(146): 761-75.
|
| [3] |
Saczynski JS, White L, Peila RL, et al. The relation between apolipoprotein A-I and dementia: the Honolulu-Asia aging study[J].
Am J Epidemiol, 2007, 165(9): 985-92.
DOI: 10.1093/aje/kwm027. |
| [4] |
Song F, Poljak A, Crawford J, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals[J].
PLoS One, 2012, 7(6): e34078-84.
DOI: 10.1371/journal.pone.0034078. |
| [5] |
Matsushita K, Kuriyama Y, Nagatsuka K, et al. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients[J].
Hypertension, 1994, 23(5): 565-8.
DOI: 10.1161/01.HYP.23.5.565. |
| [6] |
Kobari M, Meyer JS, Ichijo M, et al. Leukoaraiosis: correlation of Mr and CT findings with blood flow, atrophy, and cognition[J].
Am J Neuroradiol, 1990, 11(2): 273-81.
|
| [7] |
Tian YF, Zhou YP, Zhong CK, et al. C-reactive Protein Level, Apolipoprotein B-to-apolipoprotein A-1 ratio, and risks of ischemic stroke and coronary heart disease among Inner Mongolians in China[J].
Biomed Environ Sci, 2016, 29(7): 467-74.
|
| [8] |
Fazekas F, Chawluk JB, Alavi A, et al. Mr signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging[J].
Am J Roentgenol, 1987, 149(2): 351-6.
DOI: 10.2214/ajr.149.2.351. |
| [9] |
Guan J, Yan C, Gao Q, et al. Analysis of risk factors in patients with leukoaraiosis[J].
Medicine (Baltimore), 2017, 96(8): e6153-8.
DOI: 10.1097/MD.0000000000006153. |
| [10] |
Godin O, Tzourio C, Maillard PA, et al. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes the Three-City (3C)-Dijon magnetic resonance imaging study[J].
Circulation, 2011, 123(3): 266-73.
DOI: 10.1161/CIRCULATIONAHA.110.961052. |
| [11] |
Iadecola C. The pathobiology of vascular dementia[J].
Neuron, 2013, 80(4): 844-66.
DOI: 10.1016/j.neuron.2013.10.008. |
| [12] |
Murray AD, Staff RT, Shenkin SD, et al. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly People[J].
Radiology, 2005, 237(1): 251-7.
DOI: 10.1148/radiol.2371041496. |
| [13] |
Jimenez-Conde J, Biffi A, Rahman R, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke[J].
Stroke, 2010, 41(3): 437-42.
DOI: 10.1161/STROKEAHA.109.563502. |
| [14] |
Crisby M, Bronge L, Wahlund LO. Low levels of high density lipoprotein increase the severity of cerebral white matter changes: implications for prevention and treatment of cerebrovascular diseases[J].
Curr Alzheimer Res, 2010, 7(6): 534-9.
DOI: 10.2174/156720510792231694. |
| [15] |
Park K, Yasuda N, Toyonaga S, et al. Significant association between leukoaraiosis and metabolic syndrome in healthy subjects[J].
Neurology, 2007, 69(10): 974-8.
DOI: 10.1212/01.wnl.0000266562.54684.bf. |
| [16] |
Kaneva AM, Potolitsyna NN, Bojko ER, et al. The apolipoprotein B/apolipoprotein A-I ratio as a potential marker of plasma atherogenicity[J].
Dis Markers, 2015, 25(7): 591454-67.
|
| [17] |
Lawler PR, Akinkuolie AO, Ridker PM, et al. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women[J].
Clin Chem, 2017, 63(4): 870-9.
DOI: 10.1373/clinchem.2016.264515. |
| [18] |
Ryoo JH, Park SK, Hong HP, et al. Clinical significance of serum apolipoproteins as a predictor of coronary heart disease risk in Korean men[J].
Clin Endocrinol (Oxf), 2016, 84(1): 63-71.
DOI: 10.1111/cen.12843. |
| [19] |
Tang C, Liu Y, Kessler PS, et al. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor[J].
J Biol Chem, 2009, 284(47): 32336-43.
DOI: 10.1074/jbc.M109.047472. |
| [20] |
Paula-Lima AC, Tricerri MA, Brito-Moreira J, et al. Human apolipoprotein A-I binds amyloid-beta and prevents Abeta-induced neurotoxicity[J].
Int J Biochem Cell Biol, 2009, 41(6): 1361-70.
DOI: 10.1016/j.biocel.2008.12.003. |
| [21] |
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges[J].
Lancet Neurol, 2010, 9(7): 689-701.
DOI: 10.1016/S1474-4422(10)70104-6. |
| [22] |
Emoto T, Sawada T, Morimoto N, et al. The apolipoprotein B/A1 ratio is associated with reactive Oxygen metabolites and endothelial dysfunction in statin-treated patients with coronary artery disease[J].
J Atheroscler Thromb, 2013, 20(7): 623-9.
DOI: 10.5551/jat.16824. |
| [23] |
Fornage M, Chiang YA, O'meara ES, et al. Biomarkers of inflammation and MRI-Defined small vessel disease of the brain: the cardiovascular health study[J].
Stroke, 2008, 39(7): 1952-9.
DOI: 10.1161/STROKEAHA.107.508135. |
| [24] |
Satizabal CL, Zhu YC, Mazoyer B, et al. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study[J].
Neurology, 2012, 78(10): 720-7.
DOI: 10.1212/WNL.0b013e318248e50f. |
| [25] |
Jalal FY, Yang Y, Thompson J, et al. Myelin loss associated with neuroinflammation in hypertensive rats[J].
Stroke, 2012, 43(4): 1115-22.
DOI: 10.1161/STROKEAHA.111.643080. |
| [26] |
Kim MK, Ahn CW, Kang S, et al. Association between Apolipoprotein B/Apolipoprotein A-1 and arterial stiffness in metabolic syndrome[J].
Clin Chim Acta, 2014, 43(7): 115-9.
|
| [27] |
Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration[J].
Lancet Neurol, 2013, 12(8): 822-38.
DOI: 10.1016/S1474-4422(13)70124-8. |
| [28] |
Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease[J].
Stroke, 2011, 42(11): 3323-8.
DOI: 10.1161/STROKEAHA.110.608257. |
| [29] |
Huang F, Yang Z, Xu B, et al. Both serum apolipoprotein B and the apolipoprotein B/apolipoprotein A-I ratio are associated with carotid intima-media thickness[J].
PLoS One, 2013, 8(1): e54628-34.
DOI: 10.1371/journal.pone.0054628. |
| [30] |
Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment the framingham study[J].
Stroke, 2009, 40(5): 1590-6.
DOI: 10.1161/STROKEAHA.108.535245. |
| [31] |
Lee JH, Kang HJ, Kim HS, et al. Effects of ezetimibe/simvastatin 10/20 mg vs. atorvastatin 20 mg on apolipoprotein B/apolipoprotein A1 in Korean patients with type 2 diabetes mellitus: results of a randomized controlled trial[J].
Am J Cardiovasc Drugs, 2013, 13(5): 343-51.
DOI: 10.1007/s40256-013-0031-6. |
| [32] |
Mok VC, Lam WW, Fan YH, et al. Effects of statins on the progression of cerebral white matter lesion: Post hoc analysis of the ROCAS (regression of cerebral artery stenosis) study[J].
J Neurol, 2009, 256(5): 750-7.
DOI: 10.1007/s00415-009-5008-7. |
 2018, Vol. 38
2018, Vol. 38

