2. 中山大学中山医学院病理生理学教研室,广东 广州 510080
2. Department of Pathophysiology, Zhongshan Medical School, Sun Yat-Sen University, Guangzhou 510080, China
血管钙化是指钙磷矿物质异位沉积于动脉管壁,常见于动脉粥样硬化、高血压、糖尿病和慢性肾病晚期患者。血管钙化是心血管疾病发生率和死亡率增加的独立危险因素[1-2]。近些年来的大量研究表明血管钙化与骨生成过程类似,可受基因表达的主动调控[3-4]。研究表明氧化应激是促进血管钙化的重要因素[5-7]。氧化型低密度脂蛋白(Ox-LDL)可促进动脉粥样硬化和血管钙化的发生发展[8-9]。Ox-LDL通过增加血管平滑肌细胞氧化应激,提高碱性磷酸酶(ALP)的活性,从而促进血管平滑肌细胞成骨样分化和钙化[10-12]。
Toll样受体4(TLR4)属于模式识别受体家族成员,在动脉粥样斑块处出现高表达,可调节炎症和动脉粥样硬化的发生[13-14]。研究表明Ox-LDL可上调巨噬细胞和血管平滑肌细胞TLR4的表达[15-16]。我们前期研究发现:Ox-LDL可通过上调TLR4的表达,促进血管平滑肌细胞成骨样分化和钙化[17]。目前尚未有干预TLR4信号通路抑制血管钙化的药物。
槲皮素是一种天然黄酮类化合物, 具有清除自由基,抑制脂质过氧化,下调活性氧(ROS)介导的下游信号通路,如核转录因子(NF-κB)等作用[18]。然而槲皮素对Ox-LDL诱导的血管平滑肌细胞钙化未见报道。本研究通过建立血管平滑肌细胞钙化体外模型,研究槲皮素对Ox-LDL诱导的血管平滑肌细胞钙化的作用,阐明其作用机制是否与调控TLR4的表达有关,为血管钙化的防治提供新思路。
1 材料和方法 1.1 材料与试剂人血管平滑肌细胞(ATCC);细胞培养试剂、TRIzol、TLR4 siRNA、Lipofectamine 3000转染试剂(Life technology);槲皮素、p-nitrophenylphosphate、茜素红、β-甘油磷酸(BGP)(Sigma);活性氧试剂盒和SOD试剂盒(Beyotime);PrimeScript RT、SYBR green试剂盒(TaKaRa Biotechnology);BCA蛋白定量试剂盒(Pierce);Western blot发光试剂盒(Millipore)。
1.2 细胞培养将人血管平滑肌细胞置于含10% FBS的DMEM培养基中培养,每周换培养液3次。细胞长满后传代,用含有10 mmol/L的β-甘油磷酸培养基处理血管平滑肌细胞,加入Ox-LDL(50 μg/mL)诱导细胞钙化,以native LDL(N-LDL)处理细胞作为实验对照。为了研究槲皮素对Ox-LDL诱导的细胞钙化的影响,在Ox-LDL处理细胞的基础上,用不同浓度的槲皮素(10、20、50 μmol/L)处理细胞。
1.3 Ox-LDL的制备将EDTA加入健康人的血标本中抗凝,离心后收集上层血浆。加入密度为1.33 g/mL的KBr溶液,将血浆的密度调整为1.019 g/mL,4 ℃超高速(100 000 r/min)离心5 h。将上层VLDL弃掉,继续加入高密度KBr溶液,将血浆的密度调整至1.063 g/mL,然后4 ℃超高速(100 000 r/min)离心5 h,收集上层黄色的native LDL,将5 μmol/L CuSO4加入native LDL中即可获得Ox-LDL。
1.4 TLR4 siRNA转染血管平滑肌细胞参考公司试剂说明,将3~6代的血管平滑肌细胞接种到6孔板,细胞密度为2 × 105/孔,然后用Lipofectamine 3000转染试剂将TLR4 siRNA或Scrambled siRNA(对照)转染血管平滑肌细胞。用荧光定量qPCR检测细胞的TLR4表达水平。
1.5 测定细胞钙化用茜素红染色检测血管平滑肌细胞钙化沉积。去除培养液后,用PBS溶液洗细胞3次,4%的甲醛室温下固定细胞10 min,然后加入2%茜素红(pH 4.2)溶液染色5 min,去离子水清除残余颜色,相差显微镜下拍摄染色的细胞。采用邻甲酚酞络合剂的方法测定细胞钙离子浓度[19]。
1.6 ALP活性0.1% Triton X-100裂解细胞后抽提蛋白,用BCA法定量蛋白。在细胞裂解样品中加入pnitrophenylphosphate(p-NPP)反应底物,37 ℃孵育反应15 min,无色的底物p-NPP在ALP的作用下变成黄色的p-nitrophenol,使用NaOH终止反应后用分光光度计检测溶液吸光度A450 nm。
1.7 荧光定量qPCR参照Invitrogen试剂公司的使用说明,用TRIzol试剂提取血管平滑肌细胞的总RNA。用PrimeScript RT将1 μg mRNA反转录为cDNA,然后用SYBR green试剂盒配制成20 μL PCR反应体系,在StepOne Real Time PCR仪上进行荧光定量PCR。实验所用的引物如下:β- actin (forward): CTCTTCCAGCCTTCCTTCCT, β-actin (reverse): AGCACTGTGTTGGCGTACAG; Msx2 (forward): TGGATGCAGGAACCCGG, Msx2 (reverse): AGGGCTCATATGTCTTGGCG; BMP2 (forward): GCTAGACCTGTATCGCAGGC, BMP2 (reverse): AAACTCCTCCGTGGGGATAG; Osterix (forward): TAATGGGCTCCTTTCACCTG, Osterix (reverse): CACTGGGCAGACAGTCAGAA; SM22α (forward): AACAGCCTGTACCCTGATGG, SM22α (reverse): CGGTAGTGCCCATCATTCTT; SMA (forward): CCGGGAGAAAATGACTCAAA, SMA (reverse): GAAGGAATAGCCACGCTCAG; TLR4 (forward): AGAACTGCAGGTGCTGGATT, TLR4 (reverse): AAACTCTGGATGGGGTTTCC。以β- actin作为内参,△△Ct的方法计算基因mRNA表达的相对量。
1.8 ROS含量和SOD活性检测采用活性氧检测试剂盒检测ROS含量,去除培养液,用PBS溶液洗细胞后加入荧光染料DCFH-DA,然后用PBS清洗细胞,检测荧光强度。SOD测定采用水溶性四氮唑(WST-1)法,按试剂盒说明书操作。
1.9 统计学分析数据采用SPSS20.0软件分析,正态分布的计量资料结果以均数±标准差表示,两组间比较采用t检验,两组以上的比较采用单因素方差分析,P < 0.05为差异有统计学意义。
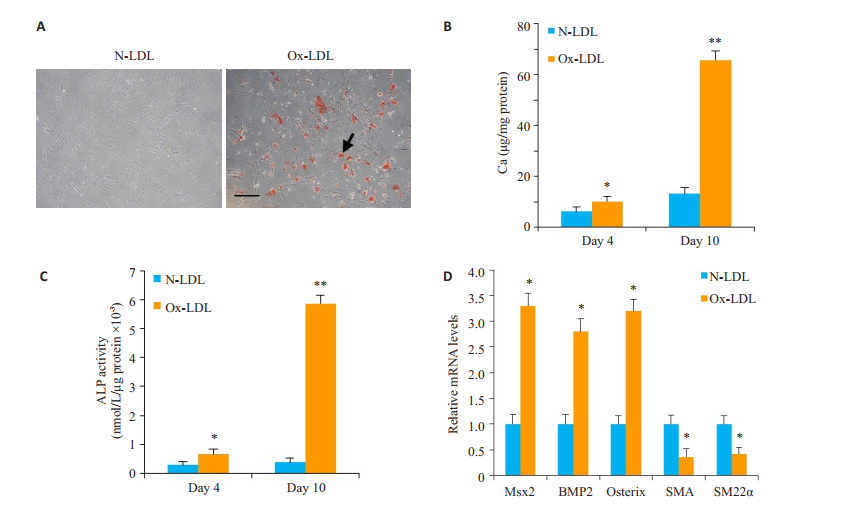
2 结果 2.1 Ox-LDL促进血管平滑肌细胞钙化采用茜红素染色检测细胞钙化,与N-LDL对照组比较,Ox-LDL(50 μg/mL)能明显促进血管平滑肌细胞钙化(图 1A)。此外,钙离子浓度检测结果显示:相比较对照组,Ox-LDL可明显升高细胞钙离子浓度(P < 0.05,图 1B)。荧光定量qPCR结果表明:Ox-LDL明显提高ALP的活性(P < 0.05,图 1C),上调血管平滑肌细胞Msx2、BMP2、Osterix mRNA的表达水平,下调血管平滑肌收缩蛋白SMA和SM22α mRNA的表达水平(P < 0.05,图 1D)。
|
图 1 Ox-LDL对血管平滑肌细胞钙化的影响 Figure 1 Effect of Ox-LDL on calcification of human VSMCs (n=3). A: Calcification was detected by Alizarin red staining. B: Calcium content in the cells; C: ALP activity in the cells; D: mRNA expressions of Msx2, BMP2, Osterix, SMA and SM22α detected by qPCR at day 10. Compared with N-LDL. Scale bar=100 μm. (*P < 0.05, **P < 0.01 vs control). |
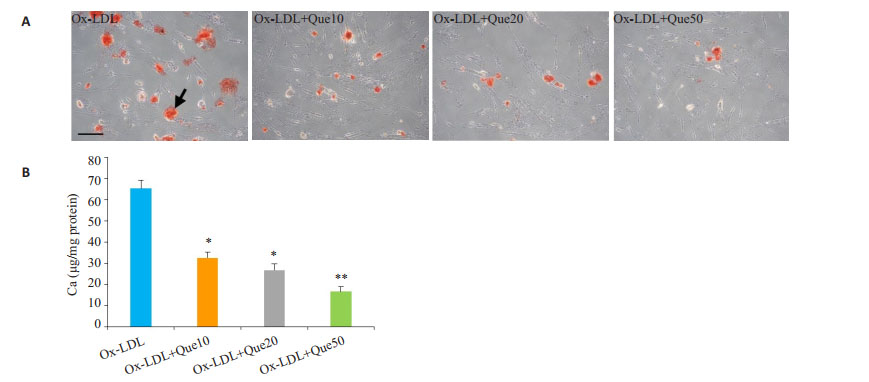
与Ox-LDL组比较,槲皮素组的血管平滑肌细胞钙化明显减轻(P < 0.05,图 2A)。槲皮素可降低细胞钙离子浓度(P < 0.05,图 2B)。
|
图 2 槲皮素对Ox-LDL诱导的血管平滑肌细胞钙化的作用 Figure 2 Effect of quercetin on Ox-LDL-induced human VSMC calcification (n=3). A: Cell calcification detected by Alizarin red staining. B: Calcium contents in the cells. Compared with Ox-LDL, Scale bar=50 μm. (*P < 0.05, **P < 0.01 vs control). |
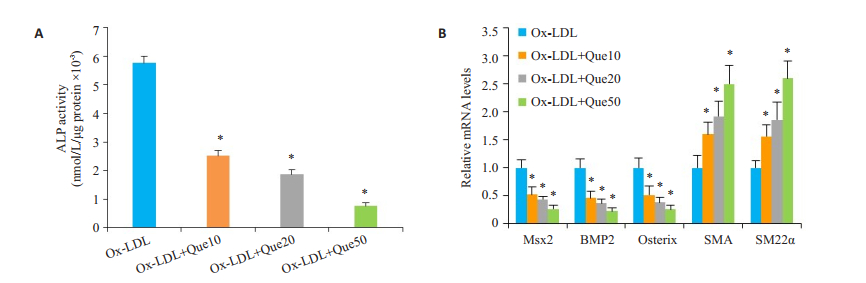
槲皮素可降低ALP活性(P < 0.05,图 3A),下调Msx2、BMP2、Osterix mRNA的表达水平,上调收缩蛋白SMA和SM22α mRNA的表达水平(P < 0.05,图 3B)。
|
图 3 槲皮素对Ox-LDL诱导的血管平滑肌细胞成骨样分化的影响。 Figure 3 Effect of quercetin on human VSMC osteogenic differentiation induced by Ox-LDL (n=3). A: ALP activity in the cells; B: mRNA expressions of Msx2, BMP2, Osterix, SMA and SM22α assessed by qPCR. Compared with Ox-LDL (*P < 0.05 vs control). |
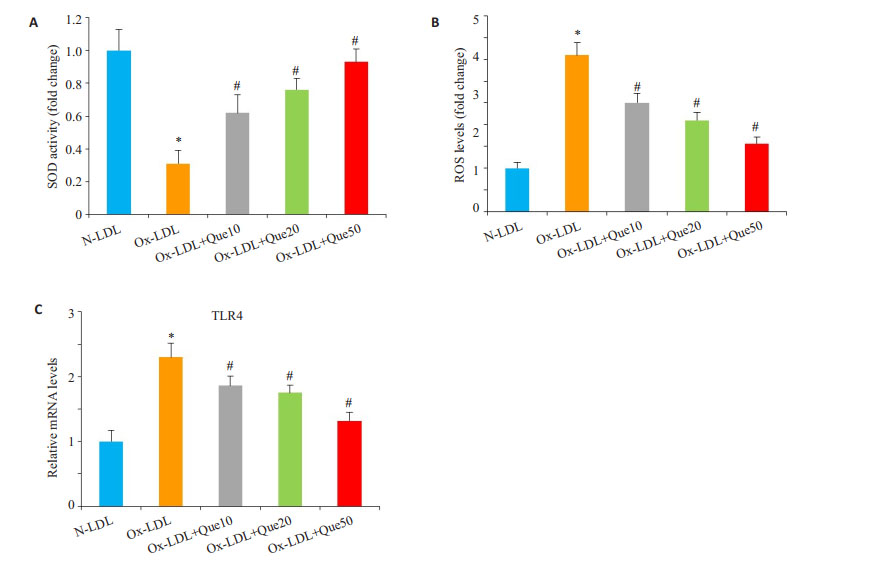
Ox-LDL可降低SOD的活性,而槲皮素能明显提高SOD的活性(P < 0.05,图 4A);Ox-LDL可提高氧化活性物质ROS的含量,使用槲皮素能降低ROS的水平(P < 0.05,图 4B);此外,Ox-LDL可上调TLR4的表达水平,槲皮素能下调TLR4的表达水平(P < 0.05,图 4C)。
|
图 4 槲皮素对血管平滑肌细胞ROS/TLR4信号的影响 Figure 4 Effect of quercetin on ROS/TLR4 signaling in human VSMCs (n=3). Human VSMCs were treated with Quercetin in the presence of Ox-LDL for 10 days. A: SOD activity in the cells; B: ROS levels in the cells; C: TLR4 mRNA expression detected by qPCR. Compared with N-LDL, *P < 0.05; Compared with Ox-LDL, #P < 0.05. |
与对照组Scrambled siRNA比较,转染TLR4 siRNA的细胞TLR4表达水平下降90%(P < 0.05,图 5A)。TLR4 siRNA能明显抑制血管平滑肌细胞钙化(P < 0.05,图 5B),降低ALP的活性(P < 0.05,图 5C),下调Msx2、BMP2和Osterix mRNA的表达,以及上调SMA和SM22α mRNA的表达(P < 0.05,图 5D)。
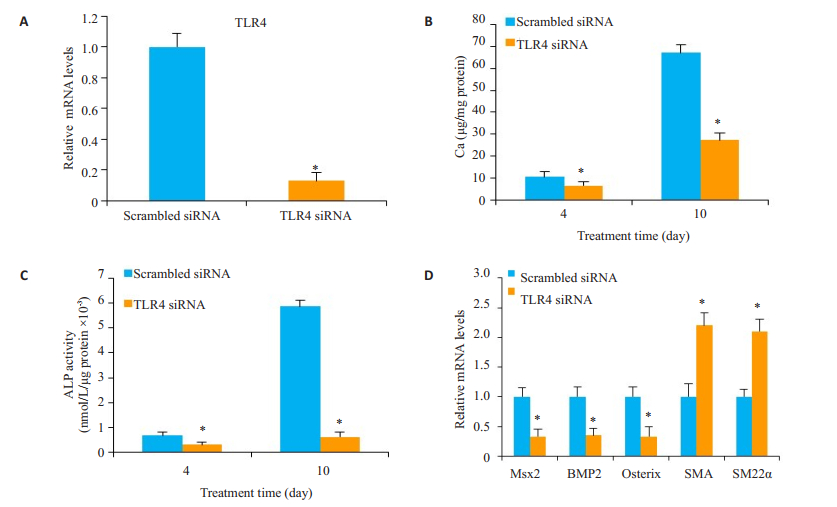
|
图 5 敲低TLR4对Ox-LDL诱导的平滑肌细胞钙化的影响 Figure 5 Effect of TLR4 siRNA on calcification of human VSMCs (n=3). Human VSMCs tansfected with TLR4 siRNA were treated with calcifying medium for 10 days. A: TLR4 mRNA expression analyzed by qRT-PCR on day 2; B: Calcium contents in the cells; C: ALP activity in the cells; D: mRNA expression of Msx2, BMP2, Osterix, SMA and SM22α assessed by qPCR at day 10. Compared with Scrambled siRNA, *P < 0.05. |
血管钙化的发生受到钙化因子的调控,其过程与骨生成的过程类似。在钙化的血管可检测到高水平表达的骨相关蛋白如Runx2、Msx2、Osterix和BMP2等,而血管平滑肌细胞收缩蛋白的表达下调[20-22]。本研究报道槲皮素可通过抑制ROS/TLR4信号通路减轻Ox-LDL诱导的血管平滑肌钙化,为血管钙化的防治提供了新的线索。
Ox-LDL诱导细胞钙化的关键是细胞氧化应激,抑制氧化应激可明显改善细胞钙化[23-24]。动物实验也证明了氧化应激参与了血管钙化的调控过程[5]。本实验表明:Ox-LDL可提高血管平滑肌细胞ROS的水平,降低SOD活性,增强细胞氧化应激,从而促进细胞成骨样分化和钙化。已有研究报道Ox-LDL能促进血管平滑肌细胞TLR4的表达水平上调[17, 25]。我们以前的研究表明Ox-LDL可通过上调TLR4的表达水平参与血管平滑肌细胞钙化的调控[17]。本研究也证实:Ox-LDL能明显上调血管平滑肌细胞的TLR4的表达水平,敲低TLR4的表达水平可明显抑制细胞钙化,进一步证实了TLR4介导了Ox-LDL诱导的血管平滑肌细胞钙化。
槲皮素可通过清除氧自由基,抑制脂质过氧化发挥其对心血管疾病的保护作用[26-27]。氧化应激可通过上调成骨样分化转录因子促进血管钙化[28]。然而槲皮素对Ox-LDL诱导的血管钙化的作用,尤其是确切的机制尚不清楚。本研究发现:槲皮素可明显抑制Ox-LDL诱导的氧化应激,下调TLR4的表达水平,减轻Ox-LDL诱导的细胞钙化和抑制血管平滑肌细胞成骨样分化。这些结果说明:槲皮素可通过调控ROS/TLR4信号抑制血管平滑肌细胞钙化。
综上所述,Ox-LDL作用于血管平滑肌细胞后激活ROS/TLR4信号通路,促进血管平滑肌细胞成骨样分化和钙化。槲皮素可通过阻断ROS/TLR4信号通路抑制血管平滑肌细胞钙化。体内动物实验有望进一步证实槲皮素对血管钙化的抑制作用。
| [1] |
Sallam T, Cheng H, Demer LL, et al. Regulatory circuits controlling vascular cell calcification[J].
Cell Mol Life Sci, 2013, 70(17): 3187-97.
DOI: 10.1007/s00018-012-1231-y. |
| [2] |
Bastos Gonçalves F, Voûte MT, Hoeks SE, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis[J].
Heart, 2012, 98(13): 988-94.
DOI: 10.1136/heartjnl-2011-301464. |
| [3] |
Cai Z, Ding Y, Zhang M, et al. Ablation of adenosine Monophosphate-Activated protein kinase α1 in vascular smooth muscle cells promotes Diet-Induced atherosclerotic calcification in vivo[J].
Circ Res, 2016, 119(3): 422-33.
DOI: 10.1161/CIRCRESAHA.116.308301. |
| [4] |
Durham AL, Speer MY, Scatena M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness[J].
Cardiovasc Res, 2018, 114(4): 590-600.
DOI: 10.1093/cvr/cvy010. |
| [5] |
Yamada S, Taniguchi M, Tokumoto M, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease[J].
J Bone Miner Res, 2012, 27(2): 474-85.
DOI: 10.1002/jbmr.539. |
| [6] |
Mody N, Parhami F, Sarafian TA, et al. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells[J].
Free Radic Biol Med, 2001, 31(4): 509-19.
DOI: 10.1016/S0891-5849(01)00610-4. |
| [7] |
Sudo R, Sato F, Azechi T, et al. 7-Ketocholesterol-induced lysosomal dysfunction exacerbates vascular smooth muscle cell calcification via oxidative stress[J].
Genes Cells, 2015, 20(12): 982-91.
DOI: 10.1111/gtc.12301. |
| [8] |
Yan J, Stringer SE, Hamilton A, et al. Decorin GAG synthesis and TGF-β signaling mediate Ox-LDL-induced mineralization of human vascular smooth muscle cells[J].
Arterioscler Thromb Vasc Biol, 2011, 31(3): 608-15.
DOI: 10.1161/ATVBAHA.110.220749. |
| [9] |
Nègre-Salvayre A, Augé N, Camaré C, et al. Dual signaling evoked by oxidized LDLs in vascular cells[J].
Free Radic Biol Med, 2017, 106: 118-33.
DOI: 10.1016/j.freeradbiomed.2017.02.006. |
| [10] |
Liao L, Zhou Q, Song Y, et al. Ceramide mediates Ox-LDLinduced human vascular smooth muscle cell calcification via p38 mitogen-activated protein kinase signaling[J].
PLoS One, 2013, 8(12): e82379.
DOI: 10.1371/journal.pone.0082379. |
| [11] |
Tang Y, Xu Q, Peng H, et al. The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification[J].
Atherosclerosis, 2015, 243(2): 357-63.
DOI: 10.1016/j.atherosclerosis.2015.08.047. |
| [12] |
Goettsch C, Rauner M, Hamann C, et al. Nuclear factor of activated T cells mediates oxidised LDL-induced calcification of vascular smooth muscle cells[J].
Diabetologia, 2011, 54(10): 2690-701.
DOI: 10.1007/s00125-011-2219-0. |
| [13] |
Higashimori M, Tatro JB, Moore KJ, et al. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice[J].
Arterioscler Thromb Vasc Biol, 2011, 31(1): 50-7.
DOI: 10.1161/ATVBAHA.110.210971. |
| [14] |
Edfeldt K, Swedenborg J, Hansson GK, et al. Expression of tolllike receptors in human atherosclerotic lesions: a possible pathway for plaque activation[J].
Circulation, 2002, 105(10): 1158-61.
|
| [15] |
Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL[J].
Circulation, 2001, 104(25): 3103-8.
DOI: 10.1161/hc5001.100631. |
| [16] |
Yang K, Zhang XJ, Cao LJ, et al. Toll-like receptor 4 mediates inflammatory cytokine secretion in smooth muscle cells induced by oxidized low-density lipoprotein[J].
PLoS One, 2014, 9(4): e95935.
DOI: 10.1371/journal.pone.0095935. |
| [17] |
Song Y, Hou M, Li Z, et al. TLR4/NF-κB/ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells[J].
Eur J Pharmacol, 2017, 794: 45-51.
DOI: 10.1016/j.ejphar.2016.11.029. |
| [18] |
Abarikwu SO, Pant AB, Farombi EO. Quercetin decreases steroidogenic enzyme activity, NF-κB expression, and oxidative stress in cultured Leydig cells exposed to atrazine[J].
Mol Cell Biochem, 2013, 373(1/2): 19-28.
|
| [19] |
颜建云, 周芹, 于汇民, 等. 高糖激活WNT信号通路促进血管平滑肌细胞钙化[J].
南方医科大学学报, 2015, 35(1): 29-33.
DOI: 10.3969/j.issn.1673-4254.2015.01.06. |
| [20] |
Tyson KL, Reynolds JL, Mcnair R, et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification[J].
Arterioscler Thromb Vasc Biol, 2003, 23(3): 489-94.
DOI: 10.1161/01.ATV.0000059406.92165.31. |
| [21] |
Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers[J].
Circ Res, 2001, 89(12): 1147-54.
DOI: 10.1161/hh2401.101070. |
| [22] |
Lin ME, Chen TM, Wallingford MC, et al. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation[J].
Cardiovasc Res, 2016, 112(2): 606-16.
DOI: 10.1093/cvr/cvw205. |
| [23] |
Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation - A possible explanation for the paradox of arterial calcification in osteoporotic patients[J].
Arterioscler Thromb Vasc Biol, 1997, 17(4): 680-7.
DOI: 10.1161/01.ATV.17.4.680. |
| [24] |
Aghagolzadeh P, Radpour R, Bachtler M, et al. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/ NRF2/NQO1 activation[J].
Atherosclerosis, 2017, 265: 78-86.
DOI: 10.1016/j.atherosclerosis.2017.08.012. |
| [25] |
Yin YW, Liao SQ, Zhang MJ, et al. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression[J].
Cell Death Dis, 2014, 5: e1574.
DOI: 10.1038/cddis.2014.535. |
| [26] |
Suganya N, Dornadula S, Chatterjee S, et al. Quercetin improves endothelial function in diabetic rats through inhibition of endoplasmic reticulum stress-mediated oxidative stress[J].
Eur J Pharmacol, 2018, 819: 80-8.
DOI: 10.1016/j.ejphar.2017.11.034. |
| [27] |
Luangaram S, Kukongviriyapan U, Pakdeechote P, et al. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats[J].
Food Chem Toxicol, 2007, 45(3): 448-55.
DOI: 10.1016/j.fct.2006.09.008. |
| [28] |
Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling[J].
J Biol Chem, 2008, 283(22): 15319-27.
DOI: 10.1074/jbc.M800021200. |
 2018, Vol. 38
2018, Vol. 38

