类风湿关节炎(RA)是以滑膜细胞进行性增生和慢性炎症为主要特征的慢性系统性自身免疫性疾病[1-2]。其中增生的滑膜细胞与新生血管、炎症细胞等组成的血管翳是RA特征性病理改变,也是侵袭关节软骨导致骨组织破坏的主要原因[3-4]。有效控制血管的新生可以阻止血管翳的形成改成减轻类风湿关节炎症状,已经成为治疗RA新药研发的热点[5-6]。
糖酵解是糖代谢中的一种,有研究表明,在RA的发生发展过程中发挥重要作用,多种糖酵解的关键酶在RA滑膜中表达增高[7-9]。同时由于糖酵解过程中代谢产物乳酸和丙酮酸增加,易造成滑膜组织低氧环境,诱导细胞上缺氧诱导因子-1(HIF-1)表达增加,并促使TNF-α等多种炎症因子表达增加,导致滑膜增生和血管新生[10-11]。
2-脱氧葡萄糖(2-DG)是一种葡萄糖类似物,竞争性抑制已糖激酶与葡萄糖转运蛋白的反应,限制了糖酵解后继反应的发生,减少了ATP的生成,促使多种肿瘤细胞的凋亡[12-14]。近年研究也发现,2-DG可有效缓解实验关节炎小鼠的关节肿胀[15]。这种作用是否通过抑制血管功能,阻断血管新生尚不清楚。因此本实验体内观察2-DG是否可以减少AA大鼠滑膜组织血管翳形成,并通过观察2-DG体外对于人脐静脉血管内皮细胞(HUVEC)增殖、迁移和体外成管的影响,探讨2-DG抑制血管新生治疗类风湿关节炎的作用和机制。
1 材料和方法 1.1 主要试剂RPMI 1640培养基、胰蛋白酶、胎牛血清购于Gibco;2-DG 100 g和10 mg,分别购自上海麦克林和Sigma;完全弗氏佐剂购自Chondrex Inc(USA);CCK-8试剂盒,购自中国四季青公司;8 μm Transwell小室,购自美国Corning;Matrigel基质胶,购自日本BD Biosciences;Compound C购自Selleck;p-AMPK购自CST;TNF-α,Bcl-2,β-actin抗体均购自Proteintech。
1.2 大鼠滑膜病理组织检查将10 mg/mL CFA充分混匀,于每只大鼠右后足跖皮内注射CFA 0.1 mL制备AA模型。正常组用生理盐水同法注射。将造模成功的大鼠分层随机分为模型组与2-DG(200 mg/kg)组,10只/组。2-DG(200 mg/kg)组:灌胃给药,1次/d,用药15 d,第33天处死大鼠,取大鼠滑膜10%甲醛固定、不同浓度梯度乙醇逐级脱水,石蜡包埋、切片、HE染色,光学显微镜观察滑膜病理组织学变化。
血管翳评分:0=无改变;1=两个部位出现血管翳;2=四个部位出现血管翳;3=四个以上部位出现血管翳或二个部位出现大范围的血管翳。
1.3 细胞株培养人脐静脉内皮细胞(HUVEC)由上海中国科学院细胞库购买。细胞培养于含10%胎牛血清,100 U/mL青霉素、100 g/mL链霉素的高糖培养基RPIM 1640中,在37 ℃、5% CO2饱和湿度的细胞培养箱中培养。
1.4 CCK-8法检测细胞增殖培养瓶中已经长满的HUVEC经消化、计数后,用5% FBS培养液稀释成1×105/mL的细胞悬液,按每孔100 μL加入96孔板,于培养箱中培养细胞贴壁后,按分组情况每孔加用1640培养液配制的10 μL 2-DG或TNF-α(2.5 nmol/L)的刺激剂使2-DG终浓度分别为(0.5、5、50 μmol/L、0.5、5 mmol/L),最终体积为100 μL在培养箱中培养24 h,培养结束前每孔加入10 μL CCK-8试剂并震荡混匀后继续培养1~3 h肉眼观察细胞孔中颜色由黄色变成深橙色,于酶标仪中读取吸光度值A450。
1.5 HUVEC迁移HUVEC平铺至24孔板中时,按分组情况每孔加用DMEM培养液配制的100μL 2-DG或TNF-α(2.5 nmol/L),使2-DG终浓度分别为(0.5、5、50 μmol/L,0.5、5 mmol/L),处理24 h的HUVEC消化后用含10%FBS的DMEM培养液制备成细胞悬液(1 × 105/mL),取100 μL加入Transwell小室的上室,下室加入600 μL含20% FBS的1640培养液。培养箱中培养过夜,棉签擦去内面未迁移的细胞,下室已迁移的细胞用20%甲醇配制的结晶紫室温下染色10 min。显微成像系统(正置)镜检迁移细胞数,4倍镜视野统计细胞数目。
1.6 基质胶体外成管Matrigel基质胶溶解后取60 μL包被于96孔板中,室温平衡30 min,37 ℃凝胶60 min。加入药物处理24 h的HUVEC,胰酶消化,用2% FBS的DMEM培养液制备细胞悬液。取细胞数为约20 000/50 μL铺在含基质胶的96孔板中,培养箱中培养6 h,显微成像系统(倒置)镜检显微镜下拍片。计数方法参考文献[16]。
1.7 Western blot实验将细胞接种于60 mmol/L平皿中,每个平皿接种50×104细胞。药物刺激24 h后收集细胞,每组加入适量的RIPA蛋白裂解液,冰上裂解30 min,4 ℃低温离心机中离心,提取细胞总蛋白。使用BCA法进行蛋白定量,然后取50 mg蛋白进行12% SDS-PAGE电泳,转膜至PVDF膜;室温封闭2~3 h,一抗4 ℃孵育过夜,TPBS洗3次,相应二抗室温孵育2 h,TPBS清洗3遍。化学发光增强试剂盒显影,Bio-Rad凝胶成像仪对膜进行曝光获取图像。
1.8 AMPK通路抑制剂Compound C处理HUVEC培养瓶中已经长满的HUVEC经消化、计数后,用5% FBS培养液重悬到适当的密度,分别平铺于96孔和24孔板。细胞贴壁后,首先使用AMPK抑制剂Compund C(5 µmol/L)作用1 h,然后将处理后的HUVEC给予2-DG(5 mmol/L),作用24 h后分别进行增殖、迁移、体外成管和Western blot实验。
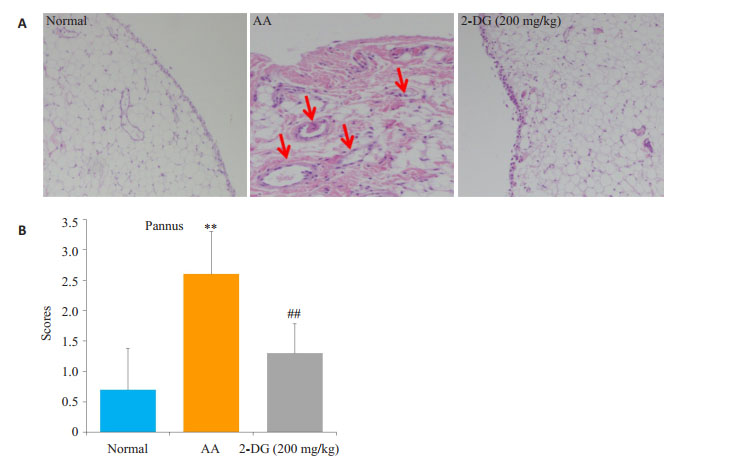
2 结果 2.1 2-DG减少AA大鼠滑膜血管翳生成第33天将大鼠处死,取足踝关节滑膜,进行HE染色,观察组织病理变化。从滑膜血管翳形成进行评分。与正常组大鼠相比,AA大鼠滑膜可见明显的滑膜组织增生,血管翳出现(图 1A)。滑膜血管翳评分,与正常对照组比较,AA组滑膜显著增生,2-DG(200 mg/kg)组滑膜血管翳明显被抑制。血管翳评分,与正常对照组比较,AA组血管翳形成明显(P < 0.01),2-DG(200 mg/kg)可显著抑制AA组血管翳的形成(P < 0.01,图 1B)。
|
图 1 2-DG对AA大鼠滑膜血管翳形成的影响 Figure 1 The effect of 2-DG on synovial pannus in AA rats. A: Photomicrograph (original magnification, × 200, H & E stain) of synovium; B: Synovial pannus were evaluated. **P < 0.01 vs Normal, ##P < 0.01 vs AA. (x±s, n=10). |
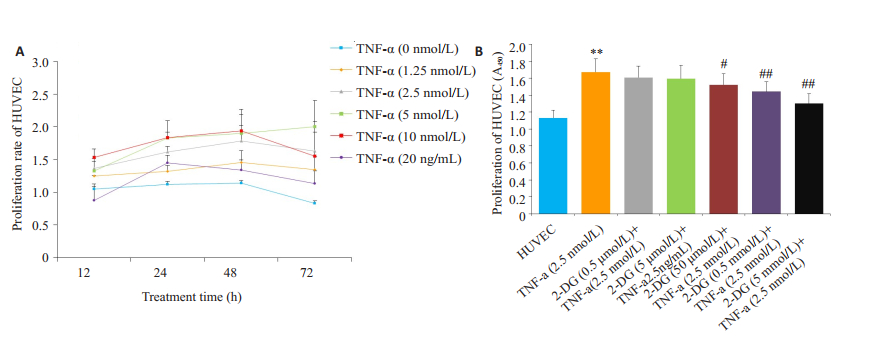
由于大鼠关节炎血管翳的血管难以取出,根据文献报道以HUVEC作为替代来研究药物对血管新生的影响[17-18]。为确定TNF-α刺激HUVEC的最佳条件,用TNF-α(1.25、2.5、5、10、20 nmol/L)体外作用人HUVEC不同时间(12、24、36、48 h)诱导HUVEC增殖,CCK-8法检测细胞增殖水平。结果显示,TNF-α(2.5 nmol/L)作用HUVEC 24 h可作为最适实验条件用于后面实验(P < 0.01,图 2A)。2-DG(0.5、5、50 μmol/L,0.5、5 mmol/L)作用于HUVEC,结果显示2-DG(0.5、5 mmol/L)明显抑制TNF-α(2.5 nmol/L)刺激的HUVEC的增殖活性(P < 0.01,图 2B)。
|
图 2 2-DG对HUVEC增殖的影响。 Figure 2 Effects of 2-DG on the proliferation of HUVEC. A: Proliferation of HUVEC treated in defferent concentration of TNF-α at various time points. *P < 0.05, **P < 0.01 vs HUVEC group; B: Effects of 2- DG on the proliferation of HUVEC. **P < 0.01 vs HUVEC group; #P < 0.05, ##P < 0.01 vs TNF-α group. (x±s, n=3). |
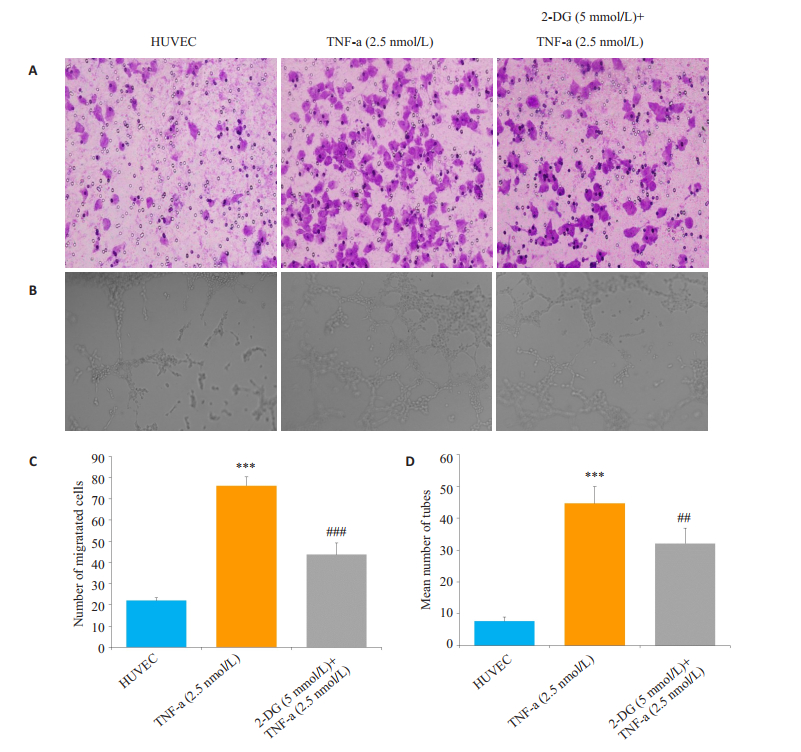
TNF-α(2.5 nmol/L)明显增加HUVEC的迁移和体外成管能力,而2-DG(5 mmol/L)明显抑制TNF-α(2.5 nmol/L)刺激的HUVEC的迁移和体外成管能力(P < 0.001,图 3A、C)。
|
图 3 2-DG对HUVEC迁移和体外成管的影响 Figure 3 Effect of 2-DG on migration and tube formation of HUVEC. A: Migration of the HUVEC were measured by transwell migration assay(original magnification, scale×100); B: Photomicrograph of tube formation assay (original magnification, × 100); C: Chemotaxis was quantified by counting the migrated cells. 2- DG (5 mmol/L) inhibited HUVEC migration; D: Number of tube formation. ***P < 0.001 vs HUVEC group; ##P < 0.001, ###P < 0.001 vs AA group (x±s, n=3). |
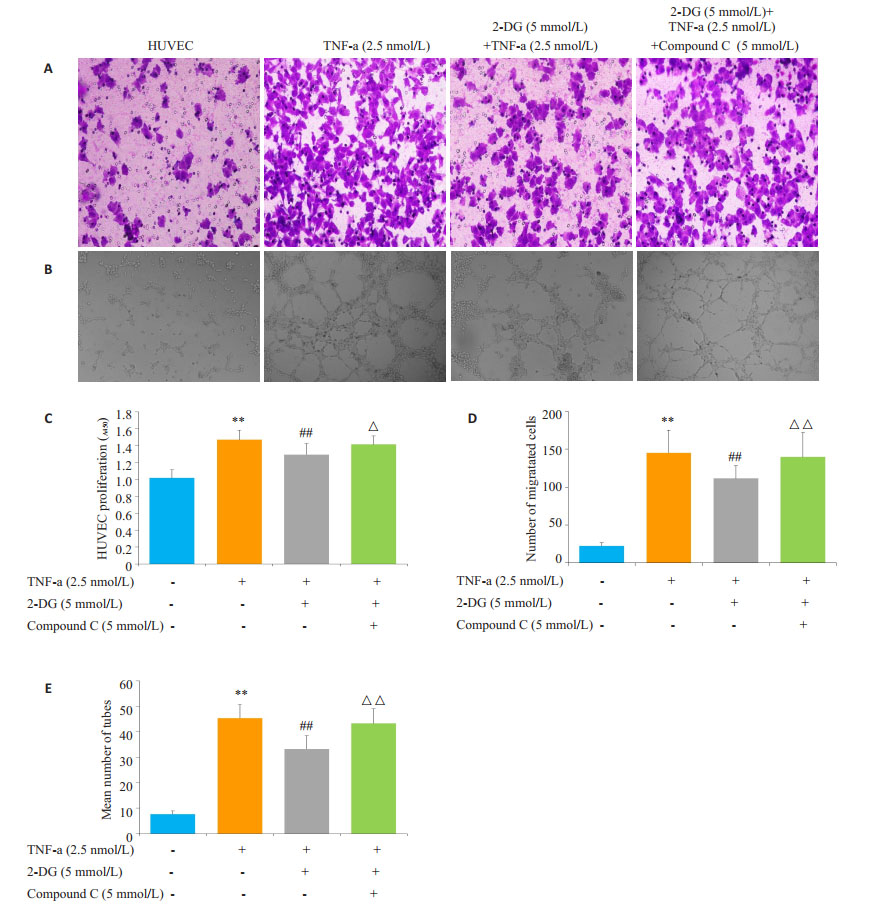
实验加入AMPK通路抑制剂Compound C(5 μmol/L),结果显示2-DG(5 mmol/L)抑制TNF-α(2.5 nmol/L)刺激的HUVEC的增殖、迁移和体外成管的作用被Compound C阻断(P < 0.01,图 4A、C、D)。
|
图 4 2-DG通过AMPK通路抑制HUVEC的增殖、迁移和体外成管 Figure 4 2-DG inhibited proliferation, migration and tube formation of HUVEC via AMPK pathway. A: Migration of the HUVEC were measured by transwell migration assay (Original magnification, scale × 100); B: Photomicrograph of tube formation assay (original magnification, ×100); C: 2-DG inhibited proliferation of HUVEC via AMPK pathway; D: Chemotaxis was quantified by counting the migrated cells; E: Number of tube formation. **P < 0.01 vs HUVEC group, ##P < 0.01 vs TNF-α group, △△P < 0.01 vs 2-DG+TNF-α group. (x±s, n=3). |
Western blot方法检测不同用药组的HUVEC的P-AMPK和Bcl-2的蛋白表达,结果显示TNF-α组的HUVEC的Bcl-2表达明显增加,2-DG(5 mmol/L)可以增加P-AMPK蛋白表达,而减少Bcl-2表达。加入Compound C(5 µmol/L)可以减少HUVEC的P-AMPK的表达,同时Bcl-2表达增加(P < 0.01,图 5A~C)。
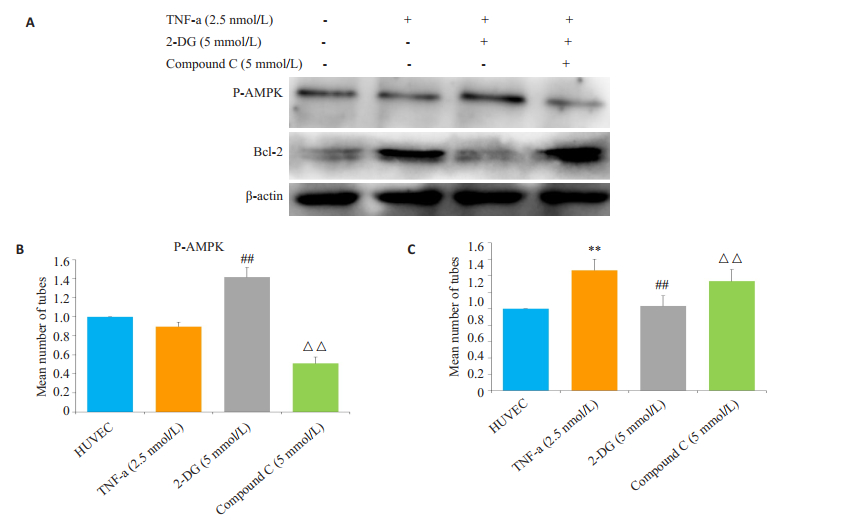
|
图 5 2-DG和Compound C对HUVEC的P-AMPK和Bcl-2蛋白表达的影响。 Figure 5 Effects of 2-DG and Compound C on the expression of P-AMPK and Bcl-2 in HUVEC. A: Expression of P-AMPK and Bcl-2 in HUVEC by western blot; B and C: Bar graphs show quantitative evaluation of expression of P-AMPK and Bcl-2 by densitometry. **P < 0.01 vs HUVEC group, ##P < 0.01, ###P < 0.001 vs TNF-α group, △△P < 0.01 vs 2-DG+TNF-α group. (x±s, n=3). |
RA是一类复杂的、多系统性的自身免疫性疾病,整个病程伴随着持续增生的滑膜细胞、炎细胞浸润、血管新生,并组成RA特征性的病理表现血管翳的形成。它是引起软骨破坏和关节功能丧失的主要原因[19-20]。RA病程与新生血管密切相关,阻断血管新生可以明显抑制滑膜炎症和血管翳的形成。RA病人关节腔分泌许多促血管的物质,比如TNF-α,可激活内皮细胞,促进迁移,使新生微血管形成[21-22]。有效控制血管的活化将成为控制类风湿关节炎的新靶点。
正常关节组织细胞是通过葡萄糖的有氧氧化来获取能量和满足生长代谢生理活动,而研究表明RA组织在缺氧的环境下从氧化磷酸化向糖酵解途径转换以维持其能量供应[23-24]。有效抑制糖酵解可缓解滑膜细胞增生和调节免疫细胞的功能,但是否可以调节血管内皮细胞活化抑制血管新生尚不清楚[25]。本研究通过AA大鼠模型的制备,观察滑膜病理组织血管翳变化,同时给予糖酵解抑制剂2-DG(200 mg/kg)治疗发现,AA大鼠滑膜组织血管翳形成明显减少。血管新生与内皮细胞的激活,迁移和存活密切相关,考虑血管翳内的内皮细胞很难分离,所以选取人的HUVEC作为替代,观察2-DG对HUVEC的增殖、迁移以及体外成管的作用。由于TNF-α与RA的关节组织炎症微环境密切相关,体外用TNF-α刺激HUVEC,模拟RA关节腔环境。实验结果显示选用TNF-α(2.5 nmol/L)作用HUVEC 24 h可作为最适浓度和时间用于后续实验。研究结果显示2-DG(0.5、5 mmol/L)可明显抑制TNF-α(2.5 nmol/L)刺激HUVEC的增殖、迁移和体外成管。
AMPK是调控细胞内代谢和能量平衡的重要“能量传感器”,对ATP/AMP比值非常敏感[26]。AMPK激活对其下游细胞脂质和糖代谢可产生直接或间接地影响,同时在调节细胞生长、迁移和免疫反应等方面发挥关键作用[27-28]。2-DG是否由于影响ATP的变化激活AMPK对HUVEC的活化产生作用?我们使用AMPK通路抑制剂Compound C作用于HUVEC,研究结果显示,Compound C可以明显阻断2-DG对HUVEC的增殖、迁移和体外成管的抑制作用。同时Western blot结果显示,TNF-α刺激的HUVEC的抗凋亡蛋白Bcl-2表达明显增高,而2-DG(5 mmol/L)可以增加P-AMPK蛋白表达,Bcl-2表达降低。提示2-DG可能激活AMPK通路减少抗凋亡蛋白Bcl-2的表达,从而抑制血管内皮细胞活化。
综上所述,糖酵解抑制剂2-DG可能通过抑制内皮细胞的增殖、迁移和血管新生阻断内皮活化有效控制RA血管翳形成,这种作用与其阻断AMPK通路,减少抗凋亡蛋白Bcl-2的表达有密切关系,但具体机制有待进一步研究。本研究为能量代谢参与RA关节炎症病理过程以及糖酵解抑制剂可能作用的靶点治疗类风湿关节炎提供重要依据。
| [1] |
Bustamante MF, Garcia-Carbonell R, Whisenant KD, et al. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis[J].
Arthritis Res Ther, 2017, 19(1): 110.
DOI: 10.1186/s13075-017-1303-3. |
| [2] |
Firestein GS, Mcinnes IB. Immol/Lunopathogenesis of rheumatoid arthritis[J].
Immol/Lunity, 2017, 46(2): 183-96.
|
| [3] |
Sawaguchi Y, Hirata K, Suzuki R, et al. Suppression of murine collagen-induced arthritis by vaccination of synovial vascular endothelial cells[J].
Life Sci, 2013, 92(23): 1125-30.
DOI: 10.1016/j.lfs.2013.04.012. |
| [4] |
Macdonald IJ, Liu SC, Su CM, et al. Implications of angiogenesis involvement in arthritis[J].
Int J Mol Sci, 2018, 19(7): 25.
|
| [5] |
Xia ZB, Meng FR, Fang YX, et al. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis[J].
Medicine (Baltimore), 2018, 97(23): e10920.
DOI: 10.1097/MD.0000000000010920. |
| [6] |
Kim SJ, Chen Z, Chamberlain ND, et al. Angiogenesis in rheumatoid arthritis is fostered directly by toll-like receptor 5 ligation and indirectly through interleukin-17 induction[J].
Arthritis Rheum, 2013, 65(8): 2024-36.
DOI: 10.1002/art.37992. |
| [7] |
Onuora S. Rheumatoid arthritis: Could glucose metabolism be a sweet target for RA therapy?[J].
Nat Rev Rheumatol, 2016, 12(3): 131.
DOI: 10.1038/nrrheum.2016.20. |
| [8] |
Gaber T, Dziurla R, Tripmacher R, et al. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do![J].
Ann Rheum Dis, 2005, 64(7): 971-80.
DOI: 10.1136/ard.2004.031641. |
| [9] |
Takahashi S, Saegusa J, Sendo S, et al. Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis[J].
Arthritis Res Ther, 2017, 19(1): 76.
DOI: 10.1186/s13075-017-1283-3. |
| [10] |
Xing J, Lu J. HIF-1α activation attenuates IL-6 and TNF-α pathways in hippocampus of rats following transient global ischemia[J].
Cell Physiol Biochem, 2016, 39(2): 511-20.
DOI: 10.1159/000445643. |
| [11] |
Lee JW, Lee J, Um SH, et al. Synovial cell death is regulated by TNF-α-induced expression of B-cell activating factor through an ERK-dependent increase in hypoxia-inducible factor-1α[J].
Cell Death Dis, 2017, 8(4): e2727.
DOI: 10.1038/cddis.2017.26. |
| [12] |
Park GB, Chung YH, Kim D. 2-Deoxy-D-glucose suppresses the migration and reverses the drug resistance of colon Cancer cells through Adam expression regulation[J].
Anticancer Drugs, 2017, 28(4): 410-20.
DOI: 10.1097/CAD.0000000000000472. |
| [13] |
Pyaskovskaya ON, Kolesnik DL, Fedorchuk AG, et al. 2-Deoxy-Dglucose enhances dichloroacetate antitumor action against Lewis lung carcinoma[J].
Exp Oncol, 2016, 38(3): 176-80.
|
| [14] |
Sobhakumari A, Orcutt KP, Love-Homan L, et al. 2-Deoxy-dglucose suppresses the in vivo antitumor efficacy of erlotinib in head and neck squamous cell carcinoma cells[J].
Oncol Res, 2016, 24(1): 55-64.
DOI: 10.3727/096504016X14586627440192. |
| [15] |
Garcia-Carbonell R, Divakaruni AS, Lodi A, et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes[J].
Arthritis Rheum, 2016, 68(7): 1614-26.
DOI: 10.1002/art.v68.7. |
| [16] |
Pickens SR, Chamberlain ND, Volin MV, et al. Role of the CCL21 and CCR7 pathways in rheumatoid arthritis angiogenesis[J].
Arthritis Rheum, 2012, 64(8): 2471-81.
DOI: 10.1002/art.34452. |
| [17] |
Amin MA, Mansfield PJ, Pakozdi A, et al. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways[J].
Arthritis Rheum, 2007, 56(6): 1787-97.
DOI: 10.1002/(ISSN)1529-0131. |
| [18] |
Pickens SR, Volin MV, Mandelin AM, et al. IL-17 contributes to angiogenesis in rheumatoid arthritis[J].
J Immol/Lunol, 2010, 184(6): 3233-41.
|
| [19] |
Zhang T, Li H, Shi J, et al. p53 predominantly regulates IL-6 production and suppresses synovial inflammol/Lation in fibroblastlike synoviocytes and adjuvant-induced arthritis[J].
Arthritis Res Ther, 2016, 18(1): 271.
DOI: 10.1186/s13075-016-1161-4. |
| [20] |
Boechat AL, de Oliveira CP, Tarragô AM, et al. Methotrexateloaded lipid-core nanocapsules are highly effective in the control of inflammol/Lation in synovial cells and a chronic arthritis model[J].
Int J Nanomedicine, 2015, 10(3): 6603-14.
|
| [21] |
Hirohata S, Abe A, Murasawa A, et al. Differential effects of IL-6 blockade tocilizumab and TNF inhibitors on angiogenesis in synovial tissues from patients with rheumatoid arthritis[J].
Mod Rheumatol, 2017, 27(5): 766-72.
DOI: 10.1080/14397595.2016.1259717. |
| [22] |
Biniecka M, Connolly M, Gao W, et al. Redox-mediated angiogenesis in the hypoxic joint of inflammol/Latory arthritis[J].
Arthritis Rheum, 2014, 66(12): 3300-10.
DOI: 10.1002/art.v66.12. |
| [23] |
Murata K, Fang C, Terao C, et al. Hypoxia-Sensitive COMMOL/ LD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis[J].
Immol/Lunity, 2017, 47(1): 66-79.
|
| [24] |
Fearon U, Canavan M, Biniecka M, et al. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis[J].
Nat Rev Rheumatol, 2016, 12(7): 385-97.
DOI: 10.1038/nrrheum.2016.69. |
| [25] |
Yang Z, Matteson EL, Goronzy JJ, et al. T-cell metabolism in autoimmol/Lune disease[J].
Arthritis Res Ther, 2015, 35(7): 29.
|
| [26] |
Li FJ, Xu ZS, Soo AD, et al. ATP-driven and AMPK-independent autophagy in an early branching eukaryotic parasite[J].
Autophagy, 2017, 13(4): 715-29.
DOI: 10.1080/15548627.2017.1280218. |
| [27] |
Jang EJ, Kim SC, Lee JH, et al. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress[J].
BMC Complement Altern Med, 2018, 18(1): 97.
DOI: 10.1186/s12906-018-2164-2. |
| [28] |
Law BYK, Gordillo-Martínez F, Qu YQ, et al. Thalidezine, a novel AMPK activator, eliminates apoptosis-resistant Cancer cells through energy-mediated autophagic cell death[J].
Oncotarget, 2017, 8(18): 30077-91.
|
 2018, Vol. 38
2018, Vol. 38

