2. 中国科学院大学,北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
原子力显微镜(AFM)可在近生理条件下进行高分辨成像,且样品制备简单,不需要特殊标记等特点[1-3],已经在生物学领域获得了广泛的应用,如单个生物大分子成像、分子间相互作用力的测量、细胞膜表面细微结构与功能关系的研究等[4]。但是,大多数生物分子的移动、构型和构象的变化、以及相互作用等多发生在极短时间内,普通AFM很难捕捉它们的动态过程[5-7]。快速AFM(HS-AFM)可以在100 ms甚至更短的时间内扫描一幅图像,大大提高了成像速度[8],使原来无法检测到的中间过程,在HS-AFM上得到了实现[9-13]。这种兼顾空间和时间分辨率的检测技术,对深入理解生命的奥秘具有重要作用。本文将简要论述HS-AFM在细胞生物学研究中取得的突破性进展,包括DNA构象的调控、DNA与蛋白质的作用、膜蛋白结构域的变化和细胞内吞机制等方面,并讨论HS-AFM的发展趋势和应用前景。
1 HS-AFM在细胞生物学中的应用 1.1 DNA分子构象、DNA分子马达的研究DNA是生物体最重要的生命分子之一,承载着生物体的遗传信息。同时,DNA本身也是一种卓越的纳米材料,利用DNA的特殊性质,如杂交的特异性和编程性,可构建多维度DNA纳米结构和纳米器件。而HSAFM为DNA及其DNA自组装纳米结构的动态过程研究提供了精准的检测手段[14]。
光照和离子浓度可影响DNA构型的转变和DNA构象的稳定。HS-AFM研究观察到了光调控下的DNA “纳米剪刀”的打开和闭合[14]、寡核苷酸的杂交和降解[15]以及DNA在DNA折纸纳米结构界面上的运动[16]。通过HS-AFM,也观察到了镁离子调控的DNA分子B型与Z型之间的相互转换[17-18],钾离子调控下的DNA四联体的形成[19],酶作用下的分子马达运动(步幅7 nm)(图 1)[20]。这些研究取得了传统手段无法获取的单分子动态信息,加深了人们对DNA及其DNA自组装纳米结构的理解。
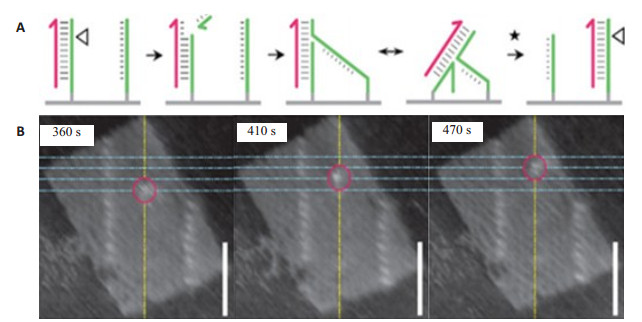
|
图 1 DNA分子马达沿着DNA折纸上的设定轨道运动[20] Figure 1 DNA motor movement along the track of DNA origami[20]. A: DNA molecular motor movement diagram on DNA origami interface: when the immobilized strand is combined with the kinematic strand, the nicking enzyme cleaves the immobilized strand (green strand) to create a nick (triangular position), which facilitates the transfer of the molecular motor to the adjacent intact anchored strand. This chain of processes is repeated and thus the motor continuously moves along the track of stators until reaching the track end; B: Successive AFM images showing discrete steps of a single DNA motor. The highest point on each profile along the yellow line is interpreted as the motor position and marked by the red circle. The motor steps between four stators (blue lines). Imaging rate: 0.1 frame/s; Scale bar: 50 nm |
生物体中的多种DNA修饰酶,需要与DNA分子上的两个不相邻的位点结合才能行使功能,这些酶与DNA分子的结合时间短、速度快,难以直接观测。利用HS-AFM,测得了EcoRⅡ酶沿着DNA链寻找特异识别位点的扩散常数(1.8×10-5 μm2/s)[21],发现了EcoP15I限制酶Ⅲ采用将两个间隔的识别位点直接连接起来的办法,增加了酶的移位速度[22]。此外,利用HS-AFM还记录到EcoRⅠ甲基转移酶对拉紧态DNA链和松弛态DNA链结合效率的差异(87%和13%)[23],这种细微状态的差别说明DNA状态可影响DNA甲基转移酶的作用效果。HS-AFM研究也发现,DNA与核蛋白结合时发生了构象变化[24]。不仅如此,在研究CRISPR-Cas9指导的基因编程中还发现了BCL2-IGH的易位现象[25]。记录到了Cas9-RNA酶切割DNA的动态过程(图 2),获得了酶与DNA结合时间(0.4~29.2 s),其切割释放出的DNA片段长度(2.7 nm)[26]。以上研究结果均以分子图像可视化的形式展示了DNA与蛋白相互作用的动态过程,为研究酶与DNA间的作用机制提供了直接证据,与传统方法获得的结果相比是重大的突破,给生物学家带来了前所未有的认识。
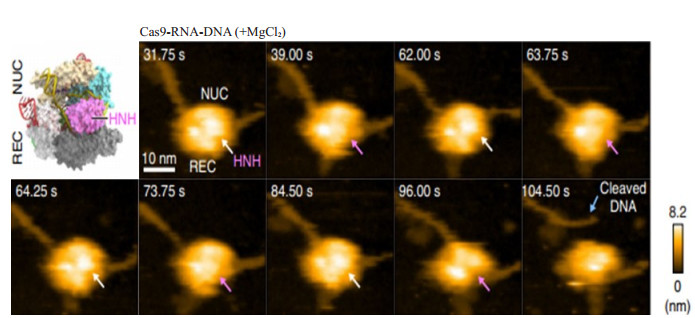
|
图 2 HS-AFM观察Cas9-RNA对DNA的切割过程[26] Figure 2 HS-AFM observations of DNA cleavage by Cas9-RNA[26]. Sequential HS-AFM images of Cas9-RNADNA in the presence of MgCl2. The HNH domains in the inactive (high-height) and active (low-height) states are indicated by white and magenta arrows, respectively. The scale bar is 10 nm |
蛋白质是细胞生物学的重要核心内容,也是生命的最基本物质之一,对生物细胞的功能维持起到举足轻重的作用。利用HS-AFM既能观察蛋白质的超微结构和动态变化,也能监测蛋白质的聚集和降解过程[27],为研究蛋白质结构与功能的相关性提供丰富的信息。
嗜盐菌细胞膜上的视紫红质(bR),呈现为有序的2D阵列分布[28]。HS-AFM研究发现,相邻单体间可形成类似三叶草的结构(图 3)[9],且单体bR-bR间有一定作用力(0.9 kcal/mol)[29]。光照可使bR发生构象变化,其中心按顺时针方向运动(位移0.7~0.8 nm,角度7~8°)。细胞膜谷氨酸转运蛋白对神经传导功能的恢复至关重要,HS-AFM研究发现该转运蛋白类似物插入胞膜中使局部膜高度上升约1.8 nm,直接证实了转运蛋白与胞膜间的相互作用[30]。
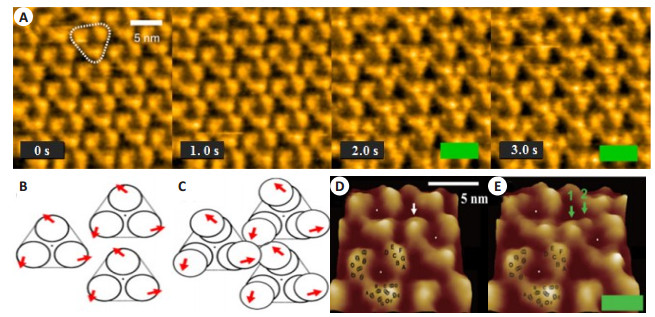
|
图 3 光诱发的视紫红质结构的变化[9] Figure 3 Structural change in bR induced by light absorption[9]. A: Successive high-speed AFM images of the cytoplasmic surface of D96N bR under dark or illuminated conditions. Frame rate, 1 frame/s; pixel size, 200 pixels × 200 pixels. A bR trimer is highlighted by the white triangle. The green bars indicate illumination by 532 nm green light of 0.5 μW; B: Schematic of arrangement of bR molecules and bR trimers under dark. The arrows indicate the directions in which respective bR molecules displace upon light illumination; C: Schematic of arrangement of bR molecules and bR trimers under illumination. The three bR molecules in a trefoil form a transient assembly. The arrows indicate the directions in which respective bR molecules displaced upon light illumination; D, E: Surface maps of the magnified images in the dark (D) and under illumination (E). The position of each trimer center is denoted by the white dots. A monomer in the dark is indicated by a single white arrow. Under illumination, the topography of the monomer splits into major and minor protrusions, as indicated by green arrows 1 and 2, respectively. The positions of 7 transmembrane α helices A-G are schematically indicated on the left bottom trimer images |
细胞膜上离子通道具有选择性和开关性[31],受到多种因素的影响。HS-AFM研究显示,ATP的结合可以影响P2X4受体亚基的重组和离子通道的状态,对离子通道造成影响[32-33]。HS-AFM观察到了P2X4受体形貌的变化,激活前呈圆形,激活后,中心形成孔洞,半径增加,膜高度增加(0.3 nm)[34],这些结构的细微变化,加深了人们对P2X作用效果的认识。运用HS-AFM也发现了多种水通道蛋白的布朗运动[11-12],这些现象对于揭示水通道蛋白的生物功能起到重要作用。
HS-AFM在其它蛋白质结构和功能的研究中也起到独特的作用。利用HS-AFM观察到链霉亲和素晶格表面出现了多种形貌的凹陷,以及不同凹陷的快速扩散,记录到的最快速度可达48.8 nm2/s[35]。此外,通过HS-AFM的研究,首次捕捉到了亚秒时间尺度下肌球蛋白Ⅴ沿着微丝运动的过程(图 4),发现肌球蛋白Ⅴ的运动是不连续的,每步约36 nm。也有研究者证实了微管和微管原细丝在驱动蛋白上的滑行,发现微管移动速度(100 nm/s)比原细丝快(40 nm/s)[36]。以上HS-AFM的快速高分辨成像应用于蛋白质分子及其反应过程的表征,为蛋白质结构和功能相关性研究提供了一种独特的研究手段。
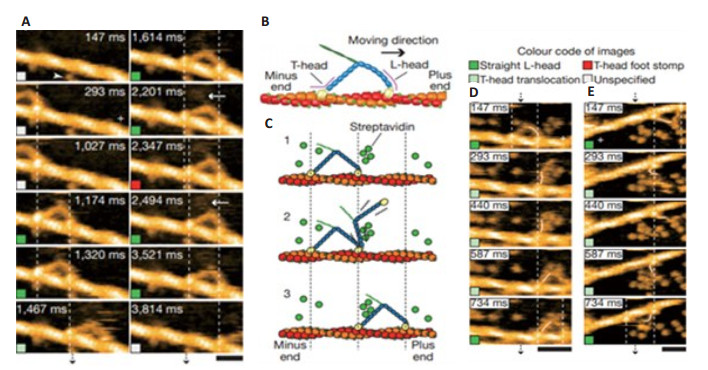
|
图 4 直接观察M5-HMM的步行[13] Figure 4 Directly visualized walking M5-HMM[13]. A: Successive AFM images showing processive movement of M5- HMM in 1 mM ATP. Arrowhead, streptavidin molecule; arrows, coiled-coil tail of M5-HMM tilted towards the minus end of actin. Scan area, 130×65 nm2; scale bar, 30 nm; B: Schematic of two-headed bound M5-HMM; C: Schematic explaining the images in D and E. D, E: Successive AFM images showing hand-over-hand movement in 1 mM ATP (D: scan area, 150×75 nm2; scale bar, 50 nm) and in 2 mM ATP (E: scan area, 130 × 65 nm2; scale bar, 30 nm). The swinging lever is highlighted with a thin white line. Vertical dashed lines in A, D and E show the centres of mass of the motor domains, and a plus sign is used to indicate the plus end of actin. All images were taken at 146.7 ms per frame |
细胞膜是细胞与外界环境隔开的一道屏障,也是细胞与周围环境发生交流和联系的必经之路,对物质的转运、代谢调控、分子免疫和药物作用等都具有重要的作用。HS-AFM为研究细胞膜表面形貌结构提供了纳米级分辨率的表征手段,成为荧光显微技术的有力补充。
HS-AFM研究发现,多种抗菌和杀菌物质的作用机理是先作用于细胞膜,进而对多种细菌、真菌、病毒及癌细胞产生极强的杀伤力[37]。如抗菌肽(CM15)能够使大肠杆菌的表面在短时间内由光滑变得粗糙[38],而溶菌酶使细菌表面的粗糙度不断增加,同时细胞体积逐渐膨胀,最后细菌被裂解死亡[39-40]。此外,还观察到磁螺菌表面覆盖有大小相似、排列规则的颗粒状物,中心内部的小孔与边框交织成网状结构[41]。通过对比不同细菌表面结构的差异,发现了大肠杆菌和类球红细菌表面的孔洞大小不同[42]。这些结构差异提示可能与微生物的特异抗性有关。
细胞边缘的肌动蛋白微丝,可以调控细胞的行为,如细胞迁移和内吞等。HS-AFM对HeLa细胞边缘肌动蛋白的研究发现,细胞边缘不断延伸,且速度快(54 nm/s)[43],而微丝破坏后,细胞边缘的活动明显受限[44]。另外,HS-AFM研究发现,哺乳动物细胞膜表面的许多凹陷能在特殊位置上不断重复开和闭,而dynasore会引起COS-7细胞表面的凹陷消失,是可逆的,去除药物后,凹陷得到恢复[45]。HS-AFM研究表明细胞表面凹陷与Rab5 GTP酶活性相关[46],在过表达Rab5的COS-7细胞的表面凹陷明显增多,内吞活性增强,且凹陷的周期也明显缩短,表明了细胞表面的凹陷与内吞密切关系。同时,在HeLa和海马神经细胞中均发现了内吞活性,且凹陷重复出现在特殊部位,说明内吞发生在细胞表面的特定位置,为哺乳细胞作用机制研究提供了证据[45](图 5)。
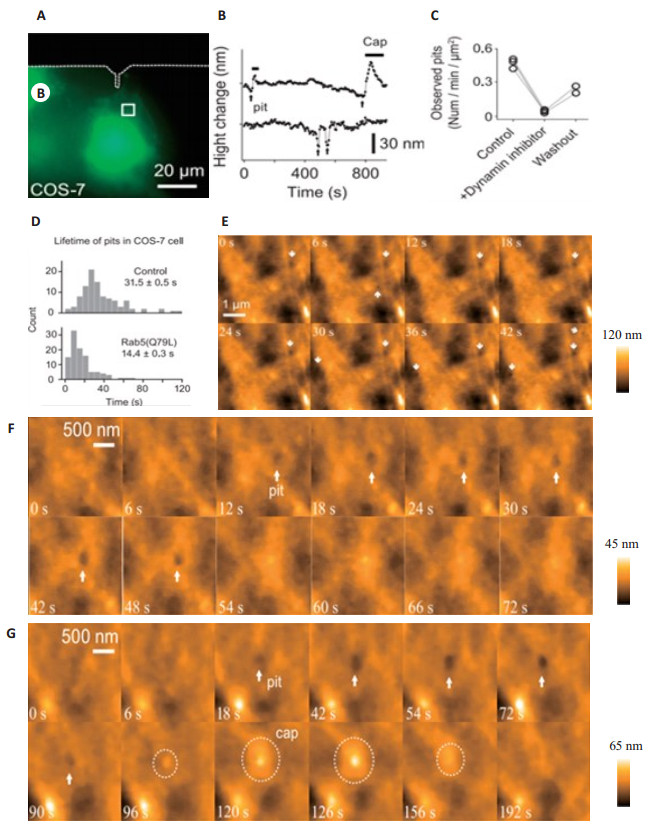
|
图 5 COS-7细胞质膜上凹陷形成和内吞过程[45] Figure 5 Pits formation and endocytosis process on the plasma membrane of the living COS-7 cells[45]. A: Fluorescence images of a COS-7 cells transfected with mEGFP. The area indicated with the white square was subjected to HS-AFM imaging; B: Time courses of the depth of pits with and without closure caps. Arrows indicate pit formation; C: The number of observed pits per min per μm2 area before, after and washout of dynasore application; D: The histogram of the lifetime of pits for COS-7 cells transfected with mEGFP (upper) and mEGFP-Rab5 (bottom); E: A sequence of HS-AFM topographical images of a COS-7 cell transfected with constitutively active Rab5 mutant (mEGFPRab5). The white arrows indicate pits formations; F: The images of endocytosis process on the plasma membrane; G: The images of the closure of the pit with a cap process. Dotted circles indicate the formation of the closure cap. All images were taken at 6 s per frame |
利用HS-AFM对活细胞的研究,在近生理状态下观察细胞的运动、结构组成及对药物的反应,有利于更好地揭示生物体的生命活动。因为有些生命现象,如神经胶质细胞的丝状伪足的动态过程、树突膜的波纹、膜表面的小凸出等现象只有在活细胞状态下才能被发现[47],这些生命现象也可对药物的疗效提供一个直观证据,有望用于药物的生物安全监测[48]。
2 前景与展望HS-AFM可达到原子级的分辨率和毫秒级成像,理论上,任何在毫秒内发生的生物过程,都能进行结构和动态的研究。如上所述,科学家们已利用HS-AFM观察到了许多传统检测技术和方法无法看到的“分子事件”的动态过程,为生命过程的研究提供了独特的、不可多得的手段。然而,HS-AFM也有其自身的局限性,如:多用于研究较纯的生物样品或相对独立的生物分子的动态。另外,提高探针的共振频率,扫描速度可以达到100帧/s,但扫描范围通常只有20 nm×20 nm,这些还远不能满足多数生物分子的成像需求[40, 49]。HS-AFM与其它仪器联用,能够克服自身的一些局限,扩大它的使用范围。如HS-AFM与X-射线晶体分析仪或表面增强拉曼光谱的联用,已成功对2-半胱氨酸-抗氧化酶蛋白的结构进行了解析[50],并证明了脂筏在鼠诺如病毒的感染中起到了重要作用[51]。因此,采用HS-AFM与其它仪器联用的方式,研究多种生物反应过程和生命现象,解决传统生物学方法难以揭示的现象和规律,是目前HSAFM的发展的主要趋势。
总之,随着AFM技术的发展,HS-AFM已具备了观察生化反应过程及生物分子构象变化的能力,在生物学领域中展现出了非凡的优势。随着仪器技术本身和相关联用技术的完善和改进,符合生命科学研究需要的HS-AFM将在生物学领域中具备广阔的应用前景,有望成为生物学领域的重要研究工具之一。
| [1] |
Marti O, Drake B, Hansma PK. Atomic force microscopy of liquidcovered surfaces: atomic resolution images[J].
Appl Phys Lett, 1998, 51(7): 484-6.
|
| [2] |
Drake B, Prater CB, Weisenhorn AL, et al. Imaging crystals, polymers, and processes in water with the atomic force microscope[J].
Science, 1989, 243(4898): 1586-9.
DOI: 10.1126/science.2928794. |
| [3] |
Müller DJ, Dufrêne YF. Atomic force microscopy: a nanoscopic window on the cell surface[J].
Trends Cell Biol, 2011, 21(8): 461-9.
DOI: 10.1016/j.tcb.2011.04.008. |
| [4] |
Ando T. High-speed atomic force microscopy coming of age[J].
Nanotechnology, 2012, 23(6): 062001.
DOI: 10.1088/0957-4484/23/6/062001. |
| [5] |
Pyne A, Marks W M, Picco L, et al. High-speed atomic force microscopy of dental enamel dissolution in citric acid[J].
Arch Histol Cytol, 2009, 72(4/5): 209-15.
DOI: 10.1679/aohc.72.209. |
| [6] |
Chiaruttini N, Redondo-Morata L, Colom A, et al. Relaxation of loaded ESCRT-Ⅲ spiral springs drives membrane deformation[J].
Cell, 2015, 163(4): 866-79.
DOI: 10.1016/j.cell.2015.10.017. |
| [7] |
Sakiyama Y, Mazur A, Kapinos LE, et al. Spatiotemporal dynamics of the nuclear pore complex transport barrier resolved by highspeed atomic force microscopy[J].
Nat Nanotechnol, 2016, 11(8): 719-23.
DOI: 10.1038/nnano.2016.62. |
| [8] |
Ando T, Uchihashi T, Kodera N. High-speed atomic force microscopy[J].
J Appl Phys, 2012, 51: 08KA2.
|
| [9] |
Shibata M, Uchihashi T, Yamashita H, et al. Structural changes in bacteriorhodopsin in response to alternate illumination observed by high-speed atomic force microscopy[J].
Angew Chem Int Ed Engl, 2011, 50(19): 4410-3.
DOI: 10.1002/anie.201007544. |
| [10] |
Uchihashi T, Iino R, Ando T, et al. High-speed atomic force microscopy reveals rotary catalysis of rotorless F1-ATPase[J].
Science, 2011, 333(643): 755-8.
|
| [11] |
Colom A, Casuso I, Rico F, et al. A hybrid high-speed atomic forceoptical microscope for visualizing single membrane proteins on eukaryotic cells[J].
Nat Commun, 2013, 4: 2155.
DOI: 10.1038/ncomms3155. |
| [12] |
Gonen T, Cheng Y, Sliz P, et al. Lipid-protein interactions in doublelayered two-dimensional AQP0 crystals[J].
Nature, 2005, 438(768): 633-8.
|
| [13] |
Kodera N, Yamamoto D, Ishikawa R, et al. Video imaging of walking myosin V by high-speed atomic force microscopy[J].
Nature, 2010, 468(7320): 72-6.
DOI: 10.1038/nature09450. |
| [14] |
Willner EM, Kamada Y, Suzuki Y, et al. Single-Molecule observation of the photoregulated conformational dynamics of DNA origami nanoscissors[J].
Angew Chem Int Ed Engl, 2017, 56(48): 15324-8.
DOI: 10.1002/anie.201708722. |
| [15] |
Endo M, Yang Y, Suzuki Y, et al. Single-molecule visualization of the hybridization and dissociation of photoresponsive oligonucleotides and their reversible switching behavior in a DNA nanostructure[J].
Angew Chem Int Ed Engl, 2012, 51(42): 10518-22.
DOI: 10.1002/anie.201205247. |
| [16] |
Yang Y, Goetzfried MA, Hidaka K, et al. Direct visualization of walking motions of photocontrolled nanomachine on the DNA nanostructure[J].
Nano Lett, 2015, 15(10): 6672-6.
DOI: 10.1021/acs.nanolett.5b02502. |
| [17] |
Endo M, Sugiyama H. Single-molecule imaging of dynamic motions of biomolecules in DNA origami nanostructures using high-speed atomic force microscopy[J].
Acc Chem Res, 2014, 47(6): 1645-53.
DOI: 10.1021/ar400299m. |
| [18] |
Rajendran A, Endo M, Hidaka K, et al. Direct and real-time observation of rotary movement of a DNA nanomechanical device[J].
J Am Chem Soc, 2013, 135(3): 1117-23.
DOI: 10.1021/ja310454k. |
| [19] |
Sannohe Y, Endo M, Katsuda Y, et al. Visualization of dynamic conformational switching of the G-quadruplex in a DNA nanostructure[J].
J Am Chem Soc, 2010, 132(46): 16311-3.
DOI: 10.1021/ja1058907. |
| [20] |
Wickham SF, Endo M, Katsuda Y, et al. Direct observation of stepwise movement of a synthetic molecular transporter[J].
Nat Nanotechnol, 2011, 6(3): 166-9.
DOI: 10.1038/nnano.2010.284. |
| [21] |
Gilmore JL, Suzuki Y, Tamulaitis G, et al. Single-molecule dynamics of the DNA-EcoRⅡ protein complexes revealed with high-speed atomic force microscopy[J].
Biochemistry, 2009, 48(44): 10492-8.
DOI: 10.1021/bi9010368. |
| [22] |
Crampton N, Yokokawa M, Dryden DT, et al. Fast-scan atomic force microscopy reveals that the type Ⅲ restriction enzyme EcoP15I is capable of DNA translocation and looping[J].
Proc Natl Acad Sci USA, 2007, 104(31): 12755-60.
DOI: 10.1073/pnas.0700483104. |
| [23] |
Endo M, Katsuda Y, Hidaka K, et al. Regulation of DNA methylation using different tensions of double strands constructed in a defined DNA nanostructure[J].
J Am Chem Soc, 2010, 132(5): 1592-7.
DOI: 10.1021/ja907649w. |
| [24] |
Lee AJ, Endo M, Hobbs JK, et al. Direct Single-Molecule observation of mode and geometry of RecA-Mediated homology search[J].
ACS Nano, 2018, 12(1): 272-8.
DOI: 10.1021/acsnano.7b06208. |
| [25] |
Mikheikin A, Olsen A, Leslie K, et al. DNA nanomapping using CRISPR-Cas9 as a programmable nanoparticle[J].
Nat Commun, 2017, 8(1): 1665.
|
| [26] |
Shibata M, Nishimasu H, Kodera N, et al. Real-space and real-time dynamics of CRISPR-Cas9 visualized by high-speed atomic force microscopy[J].
Nat Commun, 2017, 8(1): 1430.
DOI: 10.1038/s41467-017-01466-8. |
| [27] |
Eghiaian F, Rico F, Colom A, et al. High-speed atomic force microscopy: imaging and force spectroscopy[J].
FEBS Lett, 2014, 588(19): 3631-8.
DOI: 10.1016/j.febslet.2014.06.028. |
| [28] |
Yamashita H, Voïtchovsky K, Uchihashi T, et al. Dynamics of bacteriorhodopsin 2D crystal observed by high-speed atomic force microscopy[J].
J Struct Biol, 2009, 167(2): 153-8.
DOI: 10.1016/j.jsb.2009.04.011. |
| [29] |
Henderson R, Unwin PN. Three-dimensional model of purple membrane obtained by electron microscopy[J].
Nature, 1975, 257(5521): 28-32.
DOI: 10.1038/257028a0. |
| [30] |
Ruan Y, Miyagi A, Wang X, et al. Direct visualization of glutamate transporter elevator mechanism by high-speed AFM[J].
Proc Natl Acad Sci USA, 2017, 114(7): 1584-8.
DOI: 10.1073/pnas.1616413114. |
| [31] |
Allsopp RC, Lalo U, Evans RJ. Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP: chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors[J].
J Biol Chem, 2010, 285(43): 32770-7.
DOI: 10.1074/jbc.M110.148940. |
| [32] |
Kawate T, Michel JC, Birdsong WT, et al. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state[J].
Nature, 2009, 460(7255): 592-8.
DOI: 10.1038/nature08198. |
| [33] |
Khakh BS, Bao XR, Labarca C, et al. Neuronal P2X transmittergated cation channels change their ion selectivity in seconds[J].
Nat Neurosci, 1999, 2(4): 322-30.
DOI: 10.1038/7233. |
| [34] |
Shinozaki Y, Sumitomo K, Tsuda M, et al. Direct observation of ATP-induced conformational changes in single P2X(4) receptors[J].
PLoS Biol, 2009, 7(5): e1000103.
DOI: 10.1371/journal.pbio.1000103. |
| [35] |
Yamamoto D, Uchihashi T, Kodera N, et al. Anisotropic diffusion of point defects in a two-dimensional crystal of streptavidin observed by high-speed atomic force microscopy[J].
Nanotechnology, 2008, 19(38): 384009.
DOI: 10.1088/0957-4484/19/38/384009. |
| [36] |
Keya JJ, Inoue D, Suzuki Y, et al. High-Resolution imaging of a single gliding protofilament of tubulins by HS-AFM[J].
Sci Rep, 2017, 7(1): 6166.
DOI: 10.1038/s41598-017-06249-1. |
| [37] |
Meincken M, Holroyd DL, Rautenbach M. Atomic force microscopy study of the effect of antimicrobial peptides on the cell envelope of Escherichia coli[J].
Antimicrob Agents Chemother, 2005, 49(10): 4085-92.
DOI: 10.1128/AAC.49.10.4085-4092.2005. |
| [38] |
Fantner GE, Barbero RJ, Gray DS, et al. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using highspeed atomic force microscopy[J].
Nat Nanotechnol, 2010, 5(4): 280-5.
DOI: 10.1038/nnano.2010.29. |
| [39] |
Ando T, Uchihashi T, Scheuring S. Filming biomolecular processes by high-speed atomic force microscopy[J].
Chem Rev, 2014, 114(6): 3120-88.
DOI: 10.1021/cr4003837. |
| [40] |
Ghuysen JM. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism[J].
Bacteriol Rev, 1968, 32(4 pt 2): 425-64.
|
| [41] |
Yamashita H, Taoka A, Uchihashi T, et al. Single-molecule imaging on living bacterial cell surface by high-speed AFM[J].
J Mol Biol, 2012, 422(2): 300-9.
DOI: 10.1016/j.jmb.2012.05.018. |
| [42] |
Oestreicher Z, Taoka A, Fukumori Y. A comparison of the surface nanostructure from two different types of gram-negative cells: Escherichia coli and Rhodobacter sphaeroides[J].
Micron, 2015, 72: 8-14.
DOI: 10.1016/j.micron.2015.02.001. |
| [43] |
Suzuki Y, Sakai N, Yoshida A, et al. High-speed atomic force microscopy combined with inverted optical microscopy for studying cellular events[J].
Sci Rep, 2013, 3(3): 2131.
|
| [44] |
Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion[J].
Cell, 1996, 84(3): 371-9.
DOI: 10.1016/S0092-8674(00)81281-7. |
| [45] |
Shibata M, Watanabe H, Uchihashi T, et al. High-speed atomic force microscopy imaging of live mammalian cells[J].
Biophys Physicobiol, 2017, 14: 127-35.
DOI: 10.2142/biophysico.14.0_127. |
| [46] |
Watanabe H, Uchihashi T, Kobashi T, et al. Wide-area scanner for high-speed atomic force microscopy[J].
Rev Sci Instrum, 2013, 84(5): 053702.
DOI: 10.1063/1.4803449. |
| [47] |
Kodera N, Sakashita M, Ando T. Dynamic proportional-integraldifferential controller for high-speed atomic force microscopy[J].
Rev Sci Instrum, 2006, 77(8): 083704.
DOI: 10.1063/1.2336113. |
| [48] |
Mikheikin A, Olsen A, Picco L, et al. High-Speed atomic force microscopy revealing contamination in DNA purification systems[J].
Anal Chem, 2016, 88(5): 2527-32.
DOI: 10.1021/acs.analchem.5b04023. |
| [49] |
Casuso I, Kodera N, Le Grimellec C, et al. Contact-mode highresolution high-speed atomic force microscopy movies of the purple membrane[J].
Biophys J, 2009, 97(5): 1354-61.
DOI: 10.1016/j.bpj.2009.06.019. |
| [50] |
Charoenwattanasatien R, Tanaka H, Zinzius K, et al. X-ray crystallographic and high-speed AFM studies of peroxiredoxin 1 from Chlamydomonas reinhardtii[J].
Acta crystallogr F Struct Biol Commun, 2018, 74(Pt 2): 86-91.
|
| [51] |
Aybeke EN, Belliot G, Lemaire-Ewing S, et al. HS-AFM and SERS analysis of murine norovirus infection: involvement of the lipid rafts[J].
Small, 2017, 13(1): 1600918.
DOI: 10.1002/smll.v13.1. |
 2018, Vol. 38
2018, Vol. 38

