2. 南方医科大学基础医学院,广东 广州 510515;
3. 南方 医科大学南方医院体检中心,广东 广州 510631
2. Department of Health Management, Nanfang Hospital;
3. Southern Medical University, Guangzhou 510515, China
肝癌排我国肿瘤相关死因第3位,严重影响生命健康[1-2]。尽管随着早期诊断技术和外科治疗技术的提高,肝癌发病率和死亡率都呈下降趋势,但是仍有80%的病例发现于中晚期,无法接受根治性手术,且术后复发转移率居高不下[3-4]。近年来,中医药在肝癌防治领域展现出巨大的潜力。发掘治疗肝癌的有效中药成分并阐明其作用靶点,无疑是个值得鼓励的研究方向。姜酚是生姜中的主要的活性成分,具有抗肿瘤作用,包括6-姜酚、8-姜酚、10-姜酚等[5]。10-姜酚是抗肿瘤的有效成分,关于10-姜酚抑制结肠癌和乳腺癌等其他癌症的增殖作用已有报道[6-11],但尚未见10-姜酚治疗肝癌的研究报道。酪氨酸激酶Src是一个重要的促癌基因,在肝癌的发生和发展中起重要作用;STAT3能被Src调控,介导Src的促肿瘤作用[12],Cyclin D1和c-myc是STAT3的下游靶基因[13-15]。本课题采用虚拟软件筛选出中药有效成分数据库中具有抑制Src作用的潜在成分。并采用细胞实验进一步验证,10-姜酚可能作为一种天然的Src抑制剂,抑制肝癌Src/STAT3通路而抑制肝癌细胞增殖。本文旨在揭示10-姜酚抑制肝癌细胞生长的作用与机制,为中医药防治肝癌提供科学证据。
1 材料和方法 1.1 材料人肝癌细胞株HepG2购自中国科学院上海细胞库;RPMI 1640培养液、双抗、胰蛋白酶、胎牛血清为Hyclone;MTT为Biosharp;Src、p-Src、STAT3、p-STAT3一抗均购自美国CST;引物由南京凯基生物公司合成;qPCR试剂盒为Takara。
1.2 细胞培养与分组HepG2细胞为贴壁细胞,培养于10%胎牛血清、100 U/mL青霉素和100 mg/L链霉素的RPMI 1640培养基中,每2~3 d传代1次。10-姜酚低、中、高浓度(1、3、10 μmol/L)处理肝癌细胞HepG2 24 h,同时设立正常对照组(Con)。
1.3 MTT检测HepG2细胞增殖取对数生长期HepG2细胞,按每孔5×104细胞接种于96孔板,每组设4个复孔,同时设空白对照,分别于10-姜酚低、中、高浓度(1、3、10 μmol/L)处理24 h后加入5 mg/mL的MTT溶液,继续培养3 h,弃去上清液,每孔加入二甲基亚砜150 μL,震荡10 min,使结晶溶解混匀。以空白孔为对照,酶标仪读取各孔吸光度值A490 nm,吸光度值越高,代表细胞增殖能力越强。
1.4 Western blot检测Src、p-Src、STAT3、p-STAT3取处理后的HepG2细胞,冰PBS冲洗2遍,每孔加入150 μL的裂解抽提液比例,混合并匀浆。取上清2~4 μL,进行蛋白定量。取蛋白样品,加入上样缓冲液混匀,煮沸10 min后,上样,蛋白样品经8%~15%的聚丙烯酰胺凝胶电泳分离后,运用湿转法将凝胶上的蛋白条带转移至聚偏二氟乙烯(PVDF)膜。用5%的牛血清白蛋白(BSA)封闭PVDF膜,置于脱色摇床上,室温封闭2 h。加入相应的一抗,4 ℃孵育过夜。TBST冲洗,二抗敷育2 h,TBST冲洗,常规显色曝光。
1.5 qPCR检测CyclinD1和cmyc用Trizol裂解液裂解取处理后的HepG2细胞,按常规方法提取纯化总RNA,溶于适量DEPC水后分装,取适量测其纯度和总量,其余于-80 ℃冰箱保存。按试剂说明书配RT反应液,RNA反转录为cDNA,将反转录好的cDNA于-80 ℃冰箱保存。CyclinD1引物:上游5'- ACCAGCTCCTGTGCTGCGAA-3',下游5'-CAGGA CCTCCTTCTGCACAC-3'。Cmyc引物:上游5'-TAC AACACCCGAGCAAGGAC-3',下游5'-AGCTAACG TTGAGGGGCATC-3'。按照说明书配制PCR反应液,PCR反应液混匀后在荧光PCR仪上扩增目的基因:95 ℃预变性30 s,然后执行(95 ℃变性5 s,60 ℃退火25 s,72 ℃延伸30 s)45个循环。
1.6 数据统计处理研究数据用SPSS 16.0统计软件进行统计学处理,正态分布的数据以均数±标准差表示,并用单因素方差分析比较各组间差异。P < 0.05为差异有统计学意义。
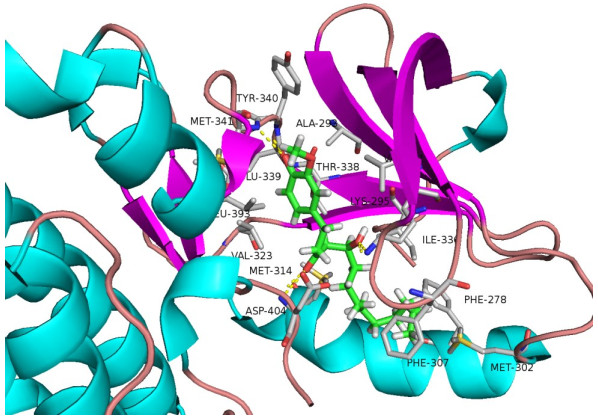
2 结果 2.1 10-姜酚是潜在的Src-抑制剂使用SYBYL-X2.1软件对10-姜酚与Src之间的相互结合进行模拟。分子对接主要考察最佳复合物中受体和配体的结合亲和力,配体和受体之间的相互作用细节和参与作用的重要氨基酸残基。10-姜酚与Src的结合评分为10.21。10-姜酚可以与Src的氨基酸残基:TRY-340、MET-341、MET-314、ASP404、ILE-336形成氢键连接(图 1)。
|
图 1 10-姜酚与Src相互作用的分子模拟 Figure 1 Molecular model of the interaction between 10-gingerol and Src. |
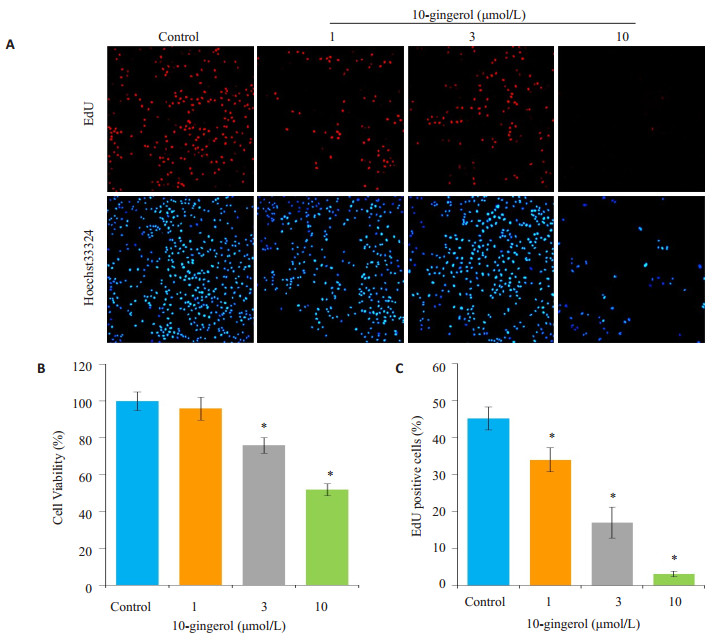
选择10-姜酚低、中、高浓度(1、3、10 μmol/L)处理肝癌细胞HepG2 24 h,同时设立正常对照组,比较各不同浓度10-姜酚对HepG2细胞活力的影响。3~10 μmol/L的10-姜酚能降低HepG2的细胞活性,与正常组比较差异有统计学意义(P < 0.001)。EdU染色结果显示,1、3、10 μmol/L的10-姜酚均能显著降低HepG2细胞的EdU染色阳性细胞数,与正常组比较差异有统计学意义(P < 0.001,图 2)。
|
图 2 10-姜酚抑制HepG2细胞增殖 Figure 2 Inhibitory effect of 10-gingerol on the proliferation of HepG 2 cells (*P < 0.05 vs control). A: EdU staining of the cells (×10); B: Quantification of cell viability; C: Quantification of the EdU-positive cells. |
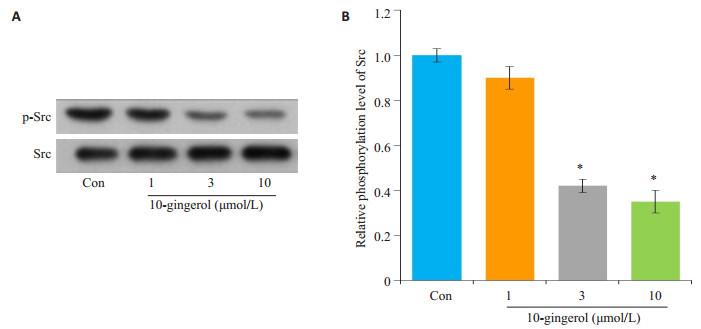
采用Western blot检测不同剂量10-姜酚对肝癌细胞磷酸化Src(p-Src)及总Src的表达水平的影响。不同剂量10-姜酚能抑制p-Src蛋白水平升高,与对照组比较差异有统计学意义(P < 0.01,图 3)。
|
图 3 10-姜酚抑制肝癌细胞Src活性 Figure 3 10-gingerol decreases Src activity in hepatocellular carcinoma cells (*P < 0.05 vs Con). A: Western blotting for the expression of p-Src and Src; B: Quantification of the results. |
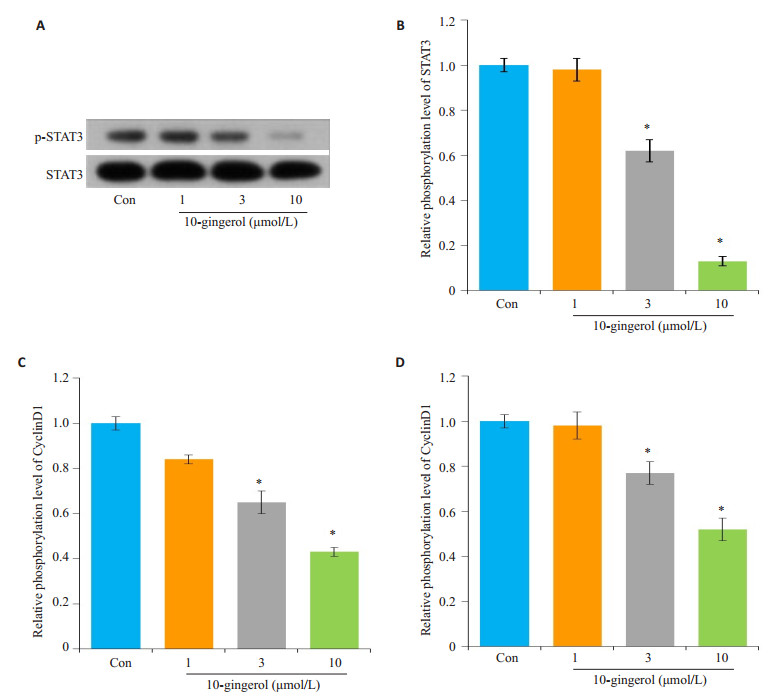
采用Western blot检测不同剂量10-姜酚对肝癌细胞HepG2中磷酸化STAT3(p-STAT3)的表达水平。不同剂量10-姜酚能抑制p-STAT3蛋白水平,与对照组比较差异有统计学意义(P < 0.01,图 4A、B)。进一步采用qPCR技术检测STAT3靶基因CyclinD1和cmyc的表达,10-姜酚能剂量依赖性的降低CyclinD1和cmyc的表达,与对照组比较差异有统计学意义(P < 0.01,图 4C、D)。
|
图 4 10-姜酚抑制STAT3信号通路激活 Figure 4 10-gingerol inhibits activation of STAT3 signaling pathway (*P < 0.05 vs control). A: Western blotting for expression of p-STAT3 and STAT3; B: Quantification of p-STAT3; C-D: Relative expression of CyclinD1 and cmyc mRNA. |
本文采用计算机辅助虚拟筛选技术筛选了来自ZINC数据库中的4200个中药有效成分,结果显示10-姜酚能与Src发生分子对接,是潜在的Src抑制剂。采用肝癌HepG2细胞探索了10-姜酚治疗肝癌的疗效,结果显示10-姜酚能剂量依赖性的抑制肝癌的增殖。采用Western blot技术验证10-姜酚对Src的抑制作用,结果显示10-姜酚能剂量依赖性的抑制肝癌HepG2细胞中磷酸化Src/STAT3的表达水平。由此可见10-姜酚可能是一种天然的Src抑制剂。并进一步采用qPCR技术探索10-姜酚对STAT3靶基因c-myc、Cyclin D1的影响,结果显示:10-姜酚能显著抑制STAT3靶基因c-myc、Cyclin D1的表达。
相关研究表明姜酚具有很多有益的功能,包括抗氧化和抗炎、抗癌、抑制环氧化酶COX-2表达、抗真菌等[16-19]。本研究发现,10-姜酚能剂量依赖性的抑制肝癌的增殖。可见,10-姜酚是姜酚抗肿瘤的有效成分。关于10-姜酚的抑制细胞增殖的研究早有报道,姜酚能抑制细胞周期蛋白D1(Cyclin D1)的表达,抑制细胞增殖,诱导细胞凋亡和细胞周期停止[20];姜酚还能抑制血管内皮生长因子(VEGF)和碱性成纤维细胞生长因子(bFGF)诱导的内皮细胞增殖作用,从而抑制血管增生[21]。关于10-姜酚的抑制其他癌症细胞增殖的研究也有报道,关于乳腺癌的研究显示[5-9],姜酚剂量依赖性的降低基质金属蛋白酶-2(MMP-2)和MMP-9的表达,而抑制细胞转移和活性;或者通过下调细胞周期蛋白依赖性激酶和细胞周期蛋白而抑制细胞增殖。关于结肠癌的研究显示[10-11],10-姜酚通过升高caspase-3和caspase-9的表达,促进细胞凋亡。
酪氨酸激酶Src(Src)广泛存在于组织细胞中,参与细胞的代谢过程[22-23]。异常激活的Src蛋白与人类的许多肿瘤如结肠癌、乳腺癌、前列腺癌、肺癌及胃癌等的发生有着联系,并且其活性的高低与肿瘤的发展密切相关[24-27]。研究发现,Src在肝癌中的表达及活性明显增加。其机制可能是,Src增加雄激素受体(AR)氨基末端转录活性域(NTD)而影响肝癌细胞的侵袭转移;或者抑制TGF-β介导的细胞凋亡[28-30]。可见,Src在肝癌中发挥重要作用是治疗肝癌的有效靶点。
信号转导和转录活化因子(STATs)家族是一种存在于胞浆并在激活后能够转入核内与DNA结合的蛋白家族。其中STAT3与肿瘤的关系最为密切。在调节肿瘤细胞增殖、侵袭、迁移等功能过程中,Src的磷酸化信号交互能作用于STAT3信号通路,肝癌细胞中Src和STAT3同时升高[12];采用RNA干扰技术或者Src抑制剂作用于肝癌细胞,STAT3磷酸化受到抑制。可见,Src/ STAT3信号通路在肝癌的生长中发挥了重要的作用[31-33]。综上所述,10-姜酚通过Src/STAT3信号通路抑制肝癌生长,是治疗肝癌的有效药物,是姜酚中发挥抗肿瘤作用的有效成分,为中医药防治肝癌提供科学证据。
| [1] |
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J].
CA Cancer J Clin, 2015, 65(2): 87-108.
DOI: 10.3322/caac.21262. |
| [2] |
Cheng S, Yang J, Shen F, et al. Multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus - eastern hepatobiliary surgical hospital consensus statement[J].
Oncotarget, 2016, 7(26): 40816-29.
|
| [3] |
Khemlina G, Ikeda S, Kurzrock R. The biology of hepatocellular carcinoma: implications for genomic and immune therapies[J].
Mol Cancer, 2017, 16(1): 149.
DOI: 10.1186/s12943-017-0712-x. |
| [4] |
Montalbano M, Georgiadis J, Masterson AL, et al. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma (Review)[J].
Oncol Rep, 2017, 37(3): 1291-300.
DOI: 10.3892/or.2017.5387. |
| [5] |
Zhang F, Thakur K, Hu F, et al. 10-Gingerol, a phytochemical derivative from "tongling white ginger", inhibits cervical cancer: insights into the molecular mechanism and inhibitory targets[J].
J Agric Food Chem, 2017, 65(10): 2089-99.
DOI: 10.1021/acs.jafc.7b00095. |
| [6] |
Martin ACBM, Fuzer AM, Becceneri AB, et al. 10-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo[J].
Oncotarget, 2017, 8(42): 72260-71.
|
| [7] |
Bernard MM, Mcconnery JR, Hoskin DW. 10-Gingerol, a major phenolic constituent of ginger root, induces cell cycle arrest and apoptosis in triple-negative breast cancer cells[J].
Exp Mol Pathol, 2017, 102(2): 370-6.
DOI: 10.1016/j.yexmp.2017.03.006. |
| [8] |
Fuzer AM, Lee SY, Mott JD, et al. 10-Gingerol reverts malignant phenotype of breast cancer cells in 3D culture[J].
J Cell Biochem, 2017, 118(9): 2693-9.
DOI: 10.1002/jcb.v118.9. |
| [9] |
Joo JH, Hong SS, Cho YR, et al. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity[J].
Oncol Rep, 2016, 35(2): 779-84.
DOI: 10.3892/or.2015.4405. |
| [10] |
Ryu MJ, Chung HS. 10-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells[J].
In Vitro Cell Dev Biol Anim, 2015, 51(1): 92-101.
DOI: 10.1007/s11626-014-9806-6. |
| [11] |
Chen CY, Li YW, Kuo SY. Effect of 10-gingerol on[ca2+]i and cell death in human colorectal cancer cells[J].
Molecules, 2009, 14(3): 959-69.
DOI: 10.3390/molecules14030959. |
| [12] |
Liu H, Xu J, Zhou L, et al. Hepatitis B virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway[J].
Cancer Res, 2011, 71(24): 7547-57.
DOI: 10.1158/0008-5472.CAN-11-2260. |
| [13] |
Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review[J].
Int J Cancer, 2016, 138(11): 2570-8.
DOI: 10.1002/ijc.v138.11. |
| [14] |
Wang Y, Fu D, Chen Y, et al. G3BP1 promotes tumor progression and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal cell carcinomas[J].
Cell Death Dis, 2018, 9(5): 501.
DOI: 10.1038/s41419-018-0504-2. |
| [15] |
Wu K, Xu K, Liu K, et al. Long noncoding RNA BC200 regulates cell growth and invasion in colon cancer[J].
Int J Biochem Cell Biol, 2018, 99(5): 219-25.
|
| [16] |
Ho SC, Chang YH. Comparison of inhibitory capacities of 6-, 8- and 10-Gingerols/shogaols on the canonical NLRP3 Inflammasome-Mediated IL-1β secretion[J].
Molecules, 2018, 23(2): 156.
DOI: 10.3390/molecules23020156. |
| [17] |
Li Z, Wang Y, Gao M, et al. Nine new gingerols from the rhizoma of zingiber officinale and their cytotoxic activities[J].
Molecules, 2018, 23(2): 28.
DOI: 10.3390/molecules23020028. |
| [18] |
Yu HL, Mao SH, Zhao TF, et al. Antagonistic effect of gingerols against TNF-α release, ROS overproduction and RIP3 expression increase induced by lectin from pinellia ternata[J].
Zhong Guo Zhong Yao Za Zhi, 2015, 40(18): 3630-5.
|
| [19] |
Jin YP, Wu H, Yu HL, et al. Antagonism mechanism of gingerols against inflammatory effect of toxic raphides from Pinella pedatisecta[J].
Zhong Guo Zhong Yao Za Zhi,, 2016, 41(6): 1087-92.
|
| [20] |
Lee HS, Seo EY, Kang NE, et al. 6-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells[J].
J Nutr Biochem, 2008, 19(5): 313-9.
DOI: 10.1016/j.jnutbio.2007.05.008. |
| [21] |
Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells[J].
Mol Carcinog, 2008, 47(3): 197-208.
DOI: 10.1002/(ISSN)1098-2744. |
| [22] |
Milanowski L, Rasul F, Gajda SN, et al. Genetic variability of SRC family kinases and its association with platelet hyperreactivity and clinical outcomes: a systematic review[J].
Curr Pharm Des, 2018, 24(5): 628-40.
DOI: 10.2174/1381612824666171213105002. |
| [23] |
Selker HP, Buse JB, Califf RM, et al. CTSA consortium consensus scientific review committee (SRC) working group report on the SRC processes[J].
Clin Transl Sci, 2015, 8(6): 623-31.
DOI: 10.1111/cts.12306. |
| [24] |
Tan J, Liu Y, Maimaiti Y, et al. Combination of SIRT1 and Src overexpression suggests poor prognosis in luminal breast cancer[J].
Onco Targets Ther, 2018, 11(2): 2051-61.
|
| [25] |
Yuan M, Meng W, Liao W, et al. Andrographolide antagonizes TNF-α Induced IL-8 via inhibition of NADPH oxidase/ROS/NF-κB and Src/MAPKs/AP-1 axis in human colorectal cancer HCT116 cells[J].
J Agric Food Chem, 2018, 66(20): 5139-48.
DOI: 10.1021/acs.jafc.8b00810. |
| [26] |
Maruoka T, Kitanaka A, Kubota Y, et al. Lemongrass essential oil and citral inhibit Src/Stat3 activity and suppress the proliferation/survival of small-cell lung cancer cells, alone or in combination with chemotherapeutic agents[J].
Int J Oncol, 2018, 25(3): 11.
|
| [27] |
Beadnell TC, Nassar KW, Rose MM, et al. Src-mediated regulation of the PI3K pathway in advanced papillary and anaplastic thyroid cancer[J].
Oncogenesis, 2018, 7(2): 23.
DOI: 10.1038/s41389-017-0015-5. |
| [28] |
Masaki T, Tokuda M, Shiratori Y, et al. A possible novel src-related tyrosine kinase in cancer cells of LEC rats that develop hepatocellular carcinoma[J].
J Hepatol, 2000, 32(1): 92-9.
DOI: 10.1016/S0168-8278(00)80194-4. |
| [29] |
Suresh S, Durakoglugil D, Zhou X, et al. SRC-2-mediated coactivation of anti-tumorigenic target genes suppresses MYCinduced liver cancer[J].
PLoS Genet, 2017, 13(3): e1006650.
DOI: 10.1371/journal.pgen.1006650. |
| [30] |
O'donnell KA, Keng VW, York B, et al. A Sleeping Beauty mutagenesis screen reveals a tumor suppressor role for Ncoa2/Src-2 in liver cancer[J].
Proc Natl Acad Sci USA, 2012, 109(21): E1377-86.
DOI: 10.1073/pnas.1115433109. |
| [31] |
Basu A, Meyer K, Lai KK, et al. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes[J].
Virology, 2006, 349(2): 347-58.
DOI: 10.1016/j.virol.2006.02.023. |
| [32] |
He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation[J].
Cancer Cell, 2010, 17(3): 286-97.
DOI: 10.1016/j.ccr.2009.12.048. |
| [33] |
Gundala SR, Mukkavilli R, Yang C, et al. Enterohepatic recirculation of bioactive ginger phytochemicals is associated with enhanced tumor growth-inhibitory activity of ginger extract[J].
Carcinogenesis, 2014, 35(6): 1320-9.
DOI: 10.1093/carcin/bgu011. |
 2018, Vol. 38
2018, Vol. 38

