2. 中国人民解放军第 458医院临床营养科,广东 广州 510602;
3. 暨南大学第二临床医学院深圳市人民医院肿瘤科,广东 深圳 518020;
4. 暨南大学第二临床医学院深圳市人民医院临床医学研究中心,广东 深圳 518020
2. Department of Clinical Nutrition, 458 Hospital of PLA, Guangzhou 510602, China;
3. Department of Oncology, Shenzhen People's Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, China;
4. Clinical Medical Research Center, Shenzhen People's Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, China
乳腺癌是女性最常见的恶性肿瘤,同时也是全世界女性因癌症致死的主要原因之一[1]。尽管目前在乳腺癌治疗方法和理念上有显著的进步,但是晚期乳腺癌患者往往因为对化疗药物耐药而导致转移进展和预后不良[2-3]。因此,乳腺癌对各类化疗药物耐药已成为乳腺癌治疗中的主要障碍[4-5]。miRNA在控制细胞分化、凋亡、增殖和致癌等多种生理和病理过程中有重要作用[6-7]。最近的研究也证明miRNA在不同类型癌症中产生化疗耐药的功能[8-15]。而作为紫杉烷类药物之一的多西他赛在乳腺癌化学治疗中非常重要[16],部分乳腺癌患者在多西他赛化疗期间对其具有内在的抵抗或获得性耐药[17],然而有关其在乳腺癌细胞中的耐药机制尚无报道。本研究首次建立了来自人乳腺癌细胞系MCF-7的耐多西他赛乳腺癌细胞系(MCF-7/DOX)探索人乳腺癌细胞对多西他赛耐药的分子机制。
1 材料和方法 1.1 材料HEK-239T细胞和人乳腺癌细胞系MCF-7从ATCC(Rockville, USA)获得;RPMI 1640培养基、Lipofectamine2000试剂、ECL试剂盒(Thermo Fisher Scientific, USA);miR-433模拟物(miR-433 mimics),miR-433抑制剂(miR-433 inhibitor)及相应对照组(mimics-Ctrl and inhibitor-Ctrl)的相对混杂miRNA,以及针对Notch1的siRNA和对照组的杂乱siRNA(siCtrl)(Genepharma, 中国上海);TRIzol试剂、One Step PrimeScriptmiRNAcDNA合成试剂盒、PrimeScript RT试剂盒、SYBR Green PCR试剂(Takara, 中国大连);ABI 7500 FAST Real-Time PCR(Applied Biosystems, USA);酶标仪(BioTek, USA);膜联蛋白V-FITC/PI染色试剂盒、FACS流式细胞仪(BD Biosciences, USA);psiCHECK-2载体(Promega, USA);BCA试剂盒(BioRad, USA);抗Notch1、抗GAPDH(Abcam, USA)。
1.2 方法 1.2.1 细胞培养将MCF-7细胞暴露于逐渐增强浓度的多西他赛中,培养耐多西他赛的乳腺癌细胞系(MCF-7/DOX)。所有细胞培养于RPMI 1640培养基,其中含10%胎牛血清。培养瓶置于37 ℃,5% CO2饱和湿度培养箱培养。
1.2.2 细胞转染转染细胞分6组:分别转染miR-433 inhibitor(化学修饰的专门针对细胞中特异的靶miRNA的抑制剂,它可以特异的靶向和敲除单个的miRNA分子)和Notch1 siRNA及其相应对照组mimics-Ctrl、siCtrl的MCF-7/DOX细胞组。根据生产说明用Lipofectamine2000试剂进行体外转染24 h。
1.2.3 实时定量PCR(qRT-PCR)用TRIzol试剂提取乳腺癌细胞系中的miRNA和mRNA,分别用One Step PrimeScriptmiRNAcDNA合成试剂盒、PrimeScript RT试剂盒将miRNA、mRNA逆转录成cDNA,然后用SYBR Green PCR试剂和ABI 7500 FAST Real-Time PCR仪进行实时荧光定量PCR。采用2-ΔΔCt方法评估miR-433和Notch1 mRNA(两者分别用U6、GAPDH作内参校正后)的表达水平。miR-433引物:正向,5'-TGCGGTACGGTGAGCTGTC-3',反向,5'-CCAGTGCAGGGTCCGAGGT-3';Notch1引物:正向,5'-GTGGATGACCTAGGCAAGTCG-3'和反向5'-GTCTCCTCCTTGTTGTTCTGC-3'。
1.2.4 细胞活力实验细胞转染24 h后,将其接种到96孔培养板。用不同浓度的多西他赛(MCF-7细胞:多西他赛10~80 ng/L;MCF/DOX细胞:多西他赛100~ 800 ng/L)培养细胞48 h,然后每孔加入100 μL无血清培养液和10 μL CCK-8溶液,37 ℃潮湿环境中继续培养1 h,酶标仪(BioTek)在450 nm的波长处测量吸光度。
1.2.5 细胞凋亡实验miRNA或siRNA转染细胞24 h后(同前),胰蛋白酶处理细胞。用膜联蛋白V-FITC/PI染色试剂盒进行细胞凋亡:冷PBS洗涤细胞,然后将细胞重悬于结合缓冲液(100 mmol/L NaCl, 25 mmol/L CaCl2, 100 mmol/L HEPES, pH 7.4)中,室温暗处膜联蛋白V-FITC/PI染色15 min,最后FACS流式细胞仪评估细胞凋亡率。
1.2.6 荧光素酶报告基因测定法以人基因组DNA为模板PCR扩增人Notch1的野生型或突变的3'UTR,PCR产物插入psiCHECK-2载体。HEK-293T细胞与Notch1 3'UTR报道分子和miR-433 mimics,miR-433 inhibitor或它们各自的对照(mimics-Ctrl, inhibitorCtrl)共转染48 h后,双荧光素酶(Promega)测定萤火虫和海肾萤光素酶活性。
1.2.7 蛋白印迹法含蛋白酶抑制剂的RIPA裂解缓冲液提取癌细胞的蛋白质,BCA试剂盒检测分离得到的蛋白质浓度。SDS-聚丙烯酰胺凝胶电泳分离蛋白质后将其转移至硝酸纤维素滤膜,5%脱脂奶粉封闭1 h,分别加入抗体:抗Notch1、抗GAPDH,4 ℃孵育过夜。洗涤滤膜后用结合过氧化物酶的二抗室温孵育1 h。最后用ECL试剂盒显影条带。
1.2.8 统计学分析所有的体外测定1式3份进行,数据以均数±标准差表示。采用GraphPad Prism版本6.0软件对数据进行独立样本检验分析各组间的统计学意义。P < 0.05为差异有统计学意义。
2 结果 2.1 miR-433在耐多西他赛的MCF-7细胞中表达下调qRT-PCR分别测定MCF-7细胞和耐多西他赛MCF-7/DOX细胞,以及miR-433 inhibitor或对照组(inhibitor-Ctrl)转染MCF-7细胞,miR-433 mimics或对照组(mimics-Ctrl)转染MCF-7/DOX细胞中miR-433的相对表达水平。实验结果显示:相比MCF-7细胞,MCF-7/DOX细胞中miR-433表达显着下调(图 1A)。转染miR-433 inhibitor可以降低MCF-7细胞中miR-433的表达(图 1B)。与对照组(mimics-Ctrl)相比,MCF-7/DOX中miR-433 mimics的转染使miR-433的水平增强30倍以上(图 1C)。
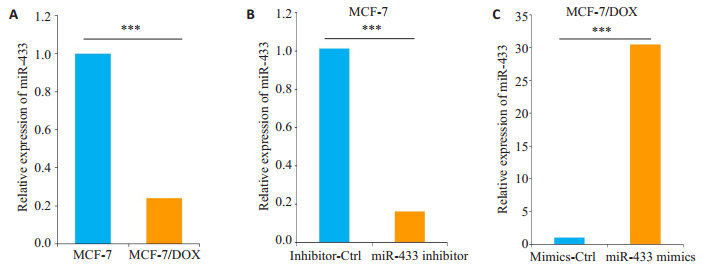
|
图 1 MCF-7和MCF-7/DOX细胞中miR-433的表达水平分析 Figure 1 Analysis of miR-433 expression levels in MCF-7 and MCF-7/DOX cells (Mean±SD, n=3). ***P < 0.001. A: Blank; B: MCF-7; C: MCF-7/DOX. |
我们用不同浓度的多西他赛溶液处理不同miRNA转染的MCF-7细胞和MCF-7/DOX后,MTT法检测CCK-8以测定细胞活力。如图 2A所示,多西他赛以剂量依赖性的方式降低MCF-7细胞的活力,用不同浓度的多西他赛溶液(10~80 ng)处理时,miR-433 inhibitor转染的MCF-7细胞比对照组(inhibitor-Ctrl)具有更高的细胞活力。然而,相比对照组转染的MCF-7/DOX细胞,需要更高的多西他赛(100~800 ng/L)浓度才能降低转染miR-433 mimics的MCF-7/DOX细胞的活力,提示miR-433表达水平升高可能逆转了MCF-7/DOX细胞对多西他赛的耐药性(图 2B)。进一步的分析发现,转染了miR-433 inhibitor的MCF-7细胞的多西他赛IC50值均高于其对照组(inhibitor-Ctrl)(图 2C),而转染miR-433 mimics的MCF-7/DOX细胞的IC50值则低于其对照组(mimics-Ctrl)(图 2D)。流式细胞术测定细胞凋亡率以研究miR-433对MCF-7/DOX细胞凋亡是否有影响。在MCF-7细胞中,miR-433 inhibitor转染后显着降低细胞凋亡率(图 2E),转染miR-433 mimics的MCF-7/DOX细胞则具有更高的细胞凋亡率(图 2F)。
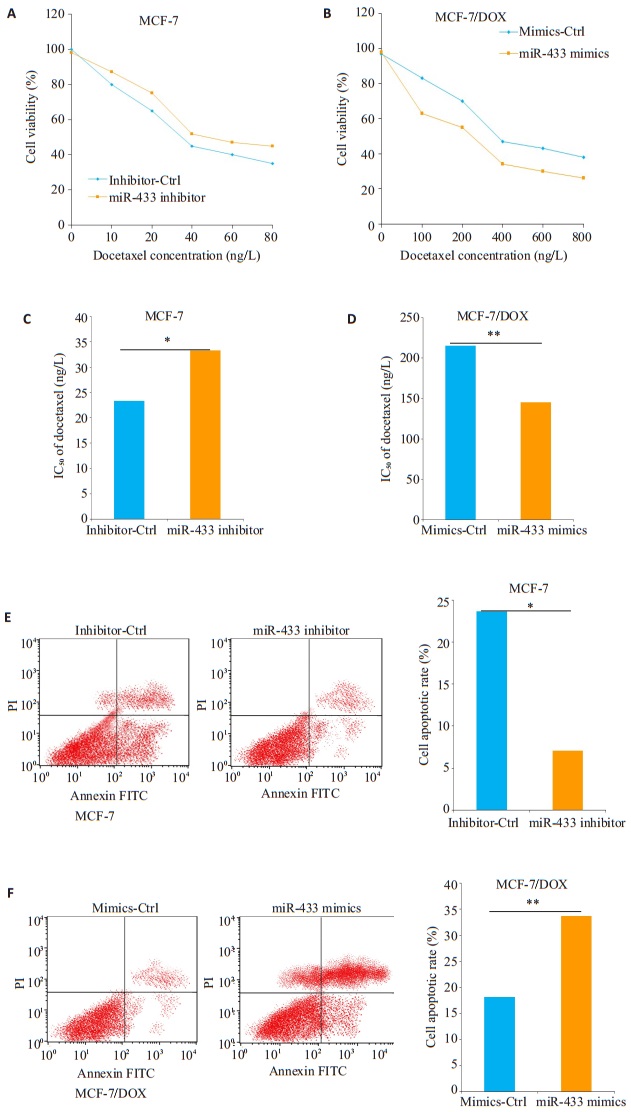
|
图 2 miR-433对MCF-7和MCF-7/DOX细胞对多西他赛化学敏感性的影响 Figure 2 Effect of miR-433 down-regulation and over-expression on chemosensitivity of MCF-7 and MCF- 7/DOX cells to docetaxel. Data are presented as Mean±SD from 3 independent experiments. *P < 0.05; **P < 0.01.A-B: Cell viavility assay; C-D: IC50 of docetaxel; E-F: Cell apoptosis rate. |
我们用TargetScan进行生物信息学分析miR-433潜在的下游靶点,初步选择Notch1作进一步实验。如图 3A所示,构建含有Notch1的野生型或突变型3'UTR的萤光素酶报告基因。HEK-293T细胞共转染实验显示miR-433表达水平与野生型Notch1 3'UTR报告基因的相对荧光素酶活性呈负相关(P < 0.05,图 3B),但miR- 433过表达或下调均不能抑制突变型Notch13'UTR报告基因的荧光素酶活性(P < 0.05,图 3C)。另外,MCF-7/ DOX细胞中Notch1的mRNA和蛋白表达水平显着高于MCF-7细胞(P < 0.05,图 4A)。我们用miR-433 inhibitor或对照组(inhibitor-Ctrl)转染MCF-7细胞,进一步检测miR-433对Notch1表达的影响。结果显示:在miR-433表达显著下调的MCF-7细胞中,Notch1的mRNA和蛋白质表达水平明显增强(P < 0.05,图 4B)。miR-433mimics转染的MCF-7/DOX细胞与对照组(mimics-Ctrl)相比,Notch1的mRNA和蛋白表达显着降低(P < 0.05,图 4C)。
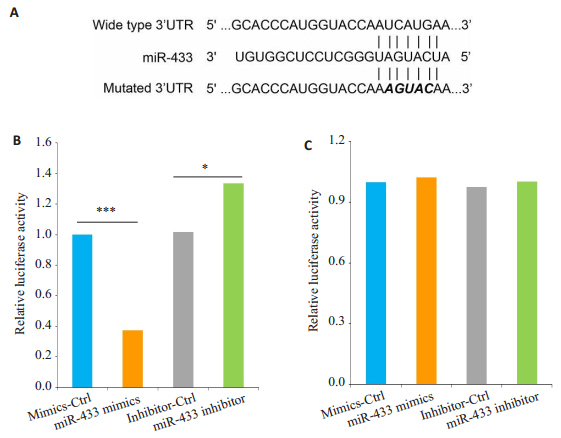
|
图 3 荧光素酶报告基因测定 Figure 3 Notch1 is a direct target of miR-433. Data are presented as Mean ± SD from 3 independent experiments. *P < 0.05; ***P < 0.001. A: Luciferase reporter gene Notch 1; B:Wide of Notch 1 3'UTR; C: Mutated of Notch 1 3'UTR. |
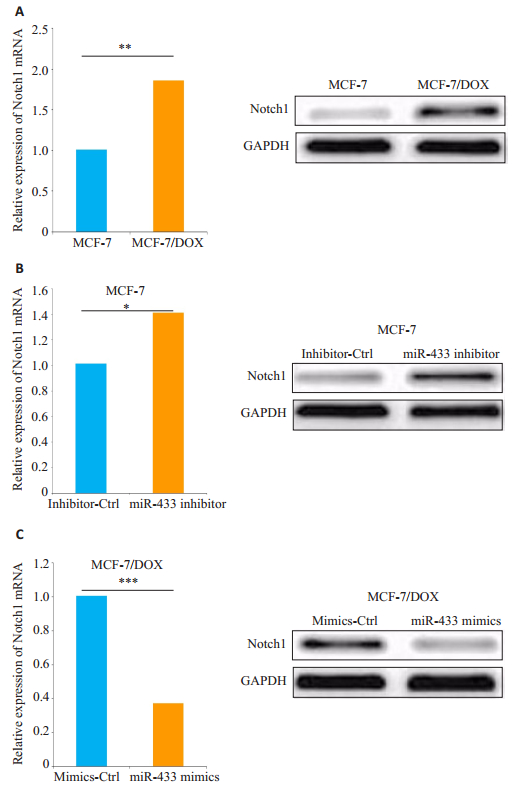
|
图 4 MCF-7和MCF-7/DOX细胞中Notch1表达水平的分析 Figure 4 Analysis of Notch1 expression levels in MCF-7 and MCF-7/DOX cells with miR-433 down-regulation and over-expression. Data are presented as Mean±SD from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. |
为了研究Notch1在MCF-7/DOX细胞对多西他赛药物敏感性中的抑制作用,用Notch1siRNA(siNotch1)转染MCF-7/DOX细胞,显着降低Notch1的mRNA和蛋白的表达水平(P < 0.05,图 5A)。与对照组(siCtrl)相比,siNotch1转染的MCF-7/DOX细胞对多西他赛的耐药性得到逆转(P < 0.05,图 5B),转染siNotch1的MCF- 7/DOX细胞对多西他赛的IC50低于对照组(siCtrl)(P < 0.05,图 5C)。
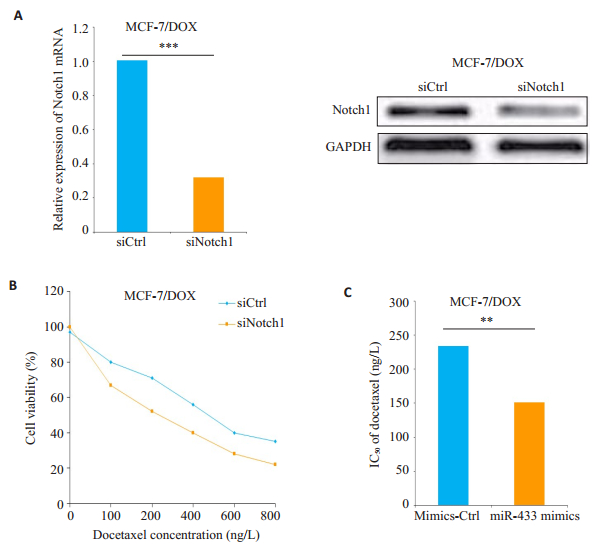
|
图 5 敲除Notch1增强MCF-7/DOX细胞对多西他赛的化学敏感性 Figure 5 Knock-down of Notch1 increases chemosensitivity of MCF-7/DOX cells to docetaxel. Data are presented as Mean±SD from 3 independent experiments. **P < 0.01; ***P < 0.001. |
多西他赛是治疗乳腺癌的常用化学药物。其主要的药理机制是通过与β-微管蛋白结合抑制微管解聚,使肿瘤细胞停滞于细胞周期G2/M期而逐步凋亡[18-19]。但是,乳腺癌患者对多西他赛产生获得性耐药已经成为治疗乳腺癌的严重障碍。研究表明miRNA在不同癌症化疗耐药的机制中发挥着非常重要的作用,但在乳腺癌中的研究鲜有报导。本研究首次阐明了miR-433在耐多西他赛乳腺癌细胞(MCF-7/DOX)中的潜在作用。
miR-433在胃癌,膀胱癌,卵巢癌,肝癌和口腔鳞癌等多种癌症中的作用已被破译。研究发现:miR-433能抑制肿瘤细胞的增殖、侵袭和迁移[20-21],但miR-433在肿瘤化疗耐药中的机制和作用尚不清楚。在卵巢癌中,miR-433过表达能够诱导细胞衰老并促进对紫杉醇的耐药。相反,miR-433也能通过调节胸腺嘧啶合成酶mRNA和蛋白的表达,增强Hela细胞对5-氟尿嘧啶的敏感性。我们的研究结果表明:miR-433的过度表达能够增强MCF/DOX细胞对多西他赛的敏感性,而miR-433的下调能导致MCF-7细胞的化疗耐药。这表明miR-433能够降低乳腺癌细胞对多西他赛的化疗耐药性。
miR-433在上述不同肿瘤耐药中的作用差异可能是癌症类型依赖性的或化学治疗剂依赖性的,需要进一步的研究去证实。而我们的体外机制研究进一步证实miR-433能够诱导乳腺癌细胞凋亡,这与既往关于miR-433诱导视网膜母细胞瘤和人类牙髓细胞的细胞凋亡的研究结果相一致[22-23]。因此,不同肿瘤的研究结果提示:miR-433可能通过诱导细胞凋亡来增强乳腺癌细胞对多西他赛的敏感性。Notch信号通路是大多数多细胞生物中高度保守的信号通路,Notch1在调控细胞周期、细胞转化和祖细胞/干细胞维持中起重要作用[24-25]。Notch1也是miR-433预测的靶点之一。Notch1在化疗耐药中的作用已经在几种类型的癌症中进行了相关研究:Notch1过度表达可以促进5-氟尿嘧啶在结直肠癌细胞中的化疗疗效[26];而敲除Notch1能够增强多西他赛诱导前列腺癌细胞有丝分裂停滞和凋亡的作用[27];抑制Notch1还可以激活凋亡途径,使小细胞肺癌细胞对阿霉素的化疗耐受能力下降[28-31]。本研究发现:miR-433表达水平与野生型Notch1 3'UTR报告基因的相对荧光素酶活性呈负相关。另外,MCF-7/DOX细胞中Notch1的mRNA和蛋白表达水平显着高于MCF-7细胞。在miR-433表达显著下调的MCF-7细胞中,Notch1的mRNA和蛋白质表达水平明显增强;敲除Notch1基因能够诱导细胞凋亡来增强乳腺癌细胞对多西他赛的敏感性,从而逆转乳腺癌细胞对多西他赛的化疗耐药性。然而,miR-433也靶向作用于TargetScan预测的其他基因,需要通过进一步的研究证实其对其他潜在靶点的作用。
综上所述,本研究表明:Notch1是miR-433的潜在靶标,在乳腺癌细胞增殖、分化、凋亡和肿瘤进展中发挥重要作用,Notch1的异常表达与乳腺癌进展相关,miR-433可通过抑制Notch1的表达来逆转乳腺癌对多西他赛的耐药性,继续发挥药物的有效性。这对Notch1作为乳腺癌患者总体生存率的预测指标提供了重要证据。
| [1] |
Gelband H, Sankaranarayanan R, Gauvreau CL, et al. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: key messages from disease control priorities, 3rd edition[J].
Lancet, 2016, 387(10033): 2133-44.
DOI: 10.1016/S0140-6736(15)00755-2. |
| [2] |
Leone J, Leone BA, Leone JP. Adjuvant systemic therapy in older women with breast cancer[J].
Breast Cancer (Dove Med Press), 2016, 8: 141-7.
|
| [3] |
Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, et al. Management and Outcomes in Metaplastic Breast Cancer[J].
Clin Breast Cancer, 2016, 6(6): 437-43.
|
| [4] |
Pan ST, Li ZL, He ZX, et al. Molecular mechanisms for tumour resistance to chemotherapy[J].
Clin Exp Pharmacol Physiol, 2016, 43(8): 723-37.
DOI: 10.1111/cep.2016.43.issue-8. |
| [5] |
Wang J, Yang M, Li Y, et al. The Role of MicroRNAs in the Chemoresistance of Breast Cancer[J].
Drug Dev Res, 2015, 76(7): 368-74.
DOI: 10.1002/ddr.v76.7. |
| [6] |
Chang K, Elledge SJ, Hannon GJ. Lessons from Nature: microRNAbased shRNAlibraries[J].
Nat Methods, 2006, 3(9): 707-14.
DOI: 10.1038/nmeth923. |
| [7] |
Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way[J].
Cancer Res, 2010, 70(16): 6401-6.
DOI: 10.1158/0008-5472.CAN-10-1346. |
| [8] |
Hu Y, Qiu Y, Yague E, et al. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer[J].
Cell Death Dis, 2016, 7(6): e2291.
DOI: 10.1038/cddis.2016.194. |
| [9] |
Gu X, Xue JQ, Han SJ, et al. Circulating microRNA-451 as a predictor of resistance to neoadjuvant chemotherapy in breast cancer[J].
Cancer Biomark, 2016, 16(3): 395-403.
DOI: 10.3233/CBM-160578. |
| [10] |
Liu X, Xie T, Mao X, et al. MicroRNA-149 Increases the sensitivity of colorectal cancer cells to 5-fluorouracil by targeting forkhead box transcription factor FOXM1[J].
Cell Physiol Biochem, 2016, 39(2): 617-29.
DOI: 10.1159/000445653. |
| [11] |
Zhao H, Yu X, Ding Y, et al. MiR-770-5p inhibits cisplatinchemoresistance in human ovarian cancer by targeting ERCC2[J].
Oncotarget, 2016, 7(33): 53254-68.
|
| [12] |
Li X, Yang L, Shuai T, et al. MiR-433 inhibits retinoblastoma malignancy by suppressing Notch1 and PAX6 expression[J].
Biomed Pharmacother, 2016, 82: 247-55.
DOI: 10.1016/j.biopha.2016.05.003. |
| [13] |
Wang XC, Ma Y, Meng PS, et al. miR-433 inhibits oral squamous cell carcinoma (OSCC) cell growth and metastasis by targeting HDAC6[J].
Oral Oncol, 2015, 51(7): 674-82.
DOI: 10.1016/j.oraloncology.2015.04.010. |
| [14] |
Xu X, Zhu Y, Liang Z, et al. c-Met and CREB1 are involved in miR- 433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3beta/Snail signaling[J].
Cell Death Dis, 2016, 7: e2088.
DOI: 10.1038/cddis.2015.274. |
| [15] |
Weiner-Gorzel K, Dempsey E, Milewska M, et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells[J].
Cancer Med, 2015, 4(5): 745-58.
DOI: 10.1002/cam4.409. |
| [16] |
Biganzoli L, Aapro M, Loibl S, et al. Taxanes in the treatment of breast cancer: Have we better defined their role in older patients? A position paper from a SIOG Task Force[J].
Cancer Treat Rev, 2016, 43: 19-26.
DOI: 10.1016/j.ctrv.2015.11.009. |
| [17] |
McGrogan BT, Gilmartin B, Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer[J].
Biochim Biophys Acta, 2008, 1785(2): 96-132.
|
| [18] |
Hernandez-Vargas H, Palacios J, Moreno-Bueno G. Telling cells how to die: docetaxel therapy in cancer cell lines[J].
Cell Cycle, 2007, 6(7): 780-3.
DOI: 10.4161/cc.6.7.4050. |
| [19] |
Hernandez-Vargas H, Palacios J, Moreno-Bueno G. Molecular profiling of docetaxel cytotoxicity in breast cancer cells: uncoupling of aberrant mitosis and apoptosis[J].
Oncogene, 2007, 26(20): 2902-13.
DOI: 10.1038/sj.onc.1210102. |
| [20] |
Luo H, Zhang H, Zhang Z, et al. Down-regulated miR-9 and miR-433 in human gastric carcinoma[J].
J Exp Clin Cancer Res, 2009, 28: 82.
DOI: 10.1186/1756-9966-28-82. |
| [21] |
Tan Y, Ge G, Pan T, et al. A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis B virus[J].
PLoS One, 2014, 9(9): e107986.
DOI: 10.1371/journal.pone.0107986. |
| [22] |
Gotanda K, Hirota T, Matsumoto N, et al. MicroRNA-433 negatively regulates the expression of thymidylate synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa cells[J].
BMC Cancer, 2013, 13: 369.
DOI: 10.1186/1471-2407-13-369. |
| [23] |
Wang K, Li L, Wu J, et al. The different expression profiles of microRNAs in elderly and young human dental pulp and the role of miR-433 in human dental pulp cells[J].
MechAgeing Dev, 2015, 146-148: 1-11.
|
| [24] |
Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival[J].
Carcinogenesis, 2013, 34(7): 1420-30.
DOI: 10.1093/carcin/bgt127. |
| [25] |
Kangsamaksin T, Tattersall IW, Kitajewski J. Notch functions in developmental and tumour angiogenesis by diverse mechanisms[J].
Biochem Soc Trans, 2014, 42(6): 1563-8.
DOI: 10.1042/BST20140233. |
| [26] |
Meng RD, Shelton CC, Li YM, et al. gamma- Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity[J].
Cancer Res, 2009, 69(2): 573-82.
DOI: 10.1158/0008-5472.CAN-08-2088. |
| [27] |
Hassan WA, Yoshida R, Kudoh S, et al. Notch1 controls cell chemoresistance in small cell lung carcinoma cells[J].
Thorac Cancer, 2016, 7(1): 123-8.
DOI: 10.1111/1759-7714.12297. |
| [28] |
Liu Y, Su C, Shan Y, Yang S, et al. Targeting Notch1 inhibits invasion and angiogenesis of human breast cancer cells via inhibition Nuclear Factor-kappaB signaling[J].
Am J Transl Res, 2016, 8(6): 2681-92.
|
| [29] |
Reedijk M, Odorcic S, Chang L, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival[J].
Cancer Res, 2005, 65(18): 8530-7.
DOI: 10.1158/0008-5472.CAN-05-1069. |
| [30] |
Xu J, Song F, Jin T, et al. Prognostic values of Notch receptors in breast cancer[J].
Tumour Biol, 2016, 37(2): 1871-7.
DOI: 10.1007/s13277-015-3961-6. |
| [31] |
Yuan X, Zhang M, Wu H, et al. Expression of Notch1 correlates with breast cancer progression and prognosis[J].
PLoS One, 2015, 10(6): e0131689.
DOI: 10.1371/journal.pone.0131689. |
 2018, Vol. 38
2018, Vol. 38

