2. 南昌大学第二附属医院重症医学科,南昌 江西 330006;
3. 南昌大学第二附属医院呼吸科,南昌 江西 330006
2. Department of Intensive Care Medicine, Second Affiliated Hospital of Nanchang University, Nanchang 330006, China;
3. Department of Respiratory Diseases, Second Affiliated Hospital of Nanchang University, Nanchang 330006, China
肺间质纤维化是一类以肺间质中细胞外基质沉积为特征的疾病[1],目前认为主要是肺部慢性非特异性炎症反应导致肺成纤维细胞活化导致细胞外基质沉积,但确切的机制尚不明确[2-3]。因此,进一步明确其发病机制,寻求新的治疗靶点是目前研究的热点。巨噬细胞迁移抑制因子(MIF)是一类重要的促炎因子,可以促进肿瘤坏死因子α(TNF-α)、白介素1(IL-1)、白介素6(IL-6)、白介素8(IL-8)等炎症因子产生,进而在急慢性炎症发挥重要作用[4-5]。现有研究表明,在肺纤维组织中,MIF的表达显著增加,抑制MIF可以改善博莱霉素诱导的小鼠肺纤维化[6-7],但其机制尚不明确。有氧糖酵解是指细胞在有氧环境中仍发生大量的糖酵解来进行糖代谢[8-9]。既往的研究也表明,肺纤维中有氧糖酵解的水平明显升高,抑制有氧糖酵解可以减轻博来霉素诱导的肺纤维化和转化生长因子(TGF-β)诱导的collagen生成[10-12]。那么,MIF是否通过促进肺组织的有氧糖酵解促进肺纤维的发生发展及其机制如何尚不可知。因此,本研究通过体内外实验阐明MIF对肺成纤维细胞有氧糖酵解的影响,并探讨其中的机制,进一步明确MIF在肺纤维的发生发展中的作用和机制,为肺纤维化的防治提供新的思路。
1 材料和方法 1.1 材料2-脱氧葡萄糖(2-deoxy-[3H]-D-glucose,[3H]-2DG)(Amersham);羊抗兔MIF抗体和collagen抗体(Abcam),羊抗鼠β-actin抗体(CST),近红外二抗山羊抗鼠(DyLight 680/DyLight800)(Odyssey);博莱霉素(海正药业);ELISA试剂盒(R & D);活性氧检测试剂盒(碧云天);胎牛血清(Gibco),F-12K培养基(Gibco);兔抗HRP标记二抗(中山金桥);DAB显色液(博士德);重组人MIF(rMIF)(sigma)。人胚肺成纤维(HLF)实验室自留(含10% FBS F-12K,37 ℃、5% CO2孵箱中培养)。
1.2 方法 1.2.1 博莱霉素诱导的肺纤维小鼠模型20只雄性成年(8周大)C57饲养在南昌大学医学院SPF级动物实验室,被随机分为对照组和博莱霉素组。博莱霉素组,气道内注射博莱霉素(50 μL,2.5 mg/kg),对照气道内注射生理盐水(50 μL)[13]。1月后,收集右下肺行病理检测,右肺上叶和中叶蛋白匀浆做western blot检测,左肺行肺泡灌洗。
1.2.2 免疫组化检测MIF表达右肺福尔马林固定16 h后,常规脱水、石蜡包埋、切片。切片脱蜡、水化、抗原修复后,用含5% BSA和2.5‰曲拉通的封闭液常温孵育1 h后,PBS洗3次×5 min。孵一抗MIF(1:200),4 ℃过夜,PBS洗3次×5 min。HRP标记的兔二抗(1:50)常温孵育1 h,PBS洗3次×5 min。DAB显色,苏木素染红,封片拍照。评分:按棕色所占观察面积≤1%,2%~25%,26%~50%,51%~75% and ≥75%分别计0、1、2、3和4分;没有棕色、浅棕色、棕色、深棕色分别计0、1、2、3分,上述两者相乘为最终得分[14]。
1.2.3 ELISA检测BALF中MIF水平ELISA检测BALF中MIF参照说明书进行。
1.2.4 Western blot检测MIF和collagen35 mm皿收集细胞,提取蛋白。浓缩胶70 V恒压40 min,离胶恒压100 V,60 min,湿转恒流250 mA,100 min;37 ℃摇床BSA封闭2 h,加入兔抗MIF抗体和collagen抗体(1: 1000)、小鼠抗β-actin抗体(1:1000),4 ℃摇床孵育过夜;TBST洗膜3次× 5 min,1:15 000山羊抗兔或鼠(DyLight 680/800),避光常温摇床孵育1 h,避光TBST洗膜3次×10 min。用Odyssey V3.0近红外扫描仪扫描膜。重复3次。
1.2.5 酶标仪检测ROS水平5×103细胞种于96孔黑底不透明版中,细胞处理完后更换无血清无酚红培养基(含1‰的荧光探针DCFH-DA)37 ℃、5% CO2孵箱中孵育30 min,无血清无酚红培养基洗3次后,酶标仪检测荧光值(488 nm激发波长,525 nm发射波长)。相对活性氧(ROS)水平=(实验组荧光值-空白组荧光值)(/对照组荧光值-空白组荧光值)。
1.2.6 confocal检测ROS水平5×104细胞种于confocal中,细胞处理完后更换无血清无酚红培养基(含1‰的荧光探针DCFH-DA)37 ℃、5% CO2孵箱中孵育30 min,无血清无酚红培养基洗3次后,confocal拍照。
1.2.7 [3H]-2DG法检测葡萄糖摄取根据Fischer等[15]的报道,细胞对葡萄糖的摄取效率通过检测细胞对[3H]-2DG摄取量来反映。细胞饥饿24 h后,更换低糖含37 kBq/mL [3H]-2DGDMEM(1 mg/mL)培养液继续培养24 h。细胞经胰蛋白酶消化后,留少许细胞计数用,其他用细胞离心沉淀后0.5 mol/L NaOH裂解15 min,再用等体积0.5 mol/L盐酸中和。用液体闪烁计数仪(HIDEX300SL, Finland)检测细胞裂解液的每分钟衰变数(DPM)值。[3H]-2DG摄取量(kBq/h/105)=(DPM实验组-DPM空白对照组)(/DPM对照组-DPM空白对照组)。
1.2.8 微孔法检测乳酸脱氢酶(LDH)活性LDH活性检测根据产品说明书(Sigma-Aldrich, MAK066)进行。收集各组细胞1×106,加入100 μL细胞裂解液孵育冰上10 min后,13 000 g离心10 min去除杂质,收集上清。乳酸溶液、1×的INT溶液、酶溶液等体积混合为工作液。50 μL工作液等体积混合后加入96孔板中,室温避光孵育30 min,酶标仪测量样品吸光度A490 nm。相对LDH活性=(样品孔吸光度-背景空白对照孔吸光度)、(对照组管吸光度-标准空白管吸光度)。
1.2.9 微孔法检测Lactate水平Lactate水平检测根据产品说明书(Sigma-Aldrich, MAK064)进行。各组细胞按1×105/孔种于6孔版中,12 h候更换1 mL/孔无血清培养基培养24 h。收集细胞培养基,13 000 g离心10 min去除杂质。将20 μL样品、26 μL乳酸盐测定缓冲液、2 μL乳酸酶混合物和2 μL乳酸盐探针混合,在室温下孵育30 min。酶标仪570 nm测量样品吸光度。
1.3 统计分析实验数据,应用SPSS13.0统计学软件分析。计量资料采用均数±标准差表进行描述。用One-way ANOVA方差分析法,首先经Levene方法检测方差齐性,明确方差齐性且整体比较组间差异有统计学意义,然后采用LSD法多重比较。P < 0.05为差异有统计学意义。
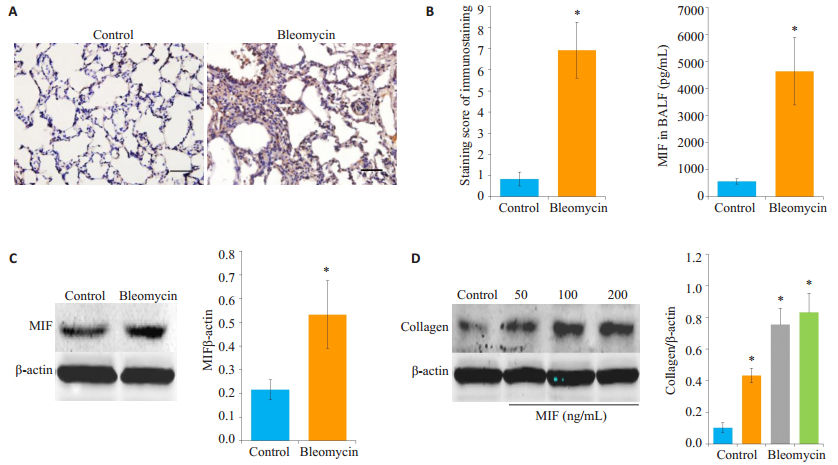
2 结果 2.1 MIF可促进肺成纤维细胞collagen蛋白生成首先在博莱霉素诱导的肺纤维小鼠肺组织中MIF蛋白的表达,免疫组化的结果显示博莱霉素诱导的肺纤维小鼠组肺组织MIF表达较对照组显著增加(图 1A);在肺泡灌洗液中,博莱霉素组MIF的水平较对照组显著增多(图 1B);Western blot检测结果也显示MIF蛋白的水平较对照组显著增多(图 1C)。进一步用不同浓度重组的rMIF刺激HLF细胞24 h后,Western blot检测发现collagen蛋白表达也显著增加(图 1D)。
|
图 1 MIF对肺成纤维细胞collagen蛋白生成的影响 Figure 1 Effect of macrophage migration inhibitory factor (MIF) on collagen production in lung fibroblasts. A: Expression of MIF in mouse lungs with fibrosis detected by immunohistochemistry (Original magnification: ×200); B: Expression of MIF in bronchoalveolar lavage fluid detected by ELISA; C: Western blottingfor detecting MIF expression in mouse lungs with fibrosis; D: Collagen production in HLFs detected by Western blotting following rMIF stimulation for 24 h. *P < 0.05 vs control. |
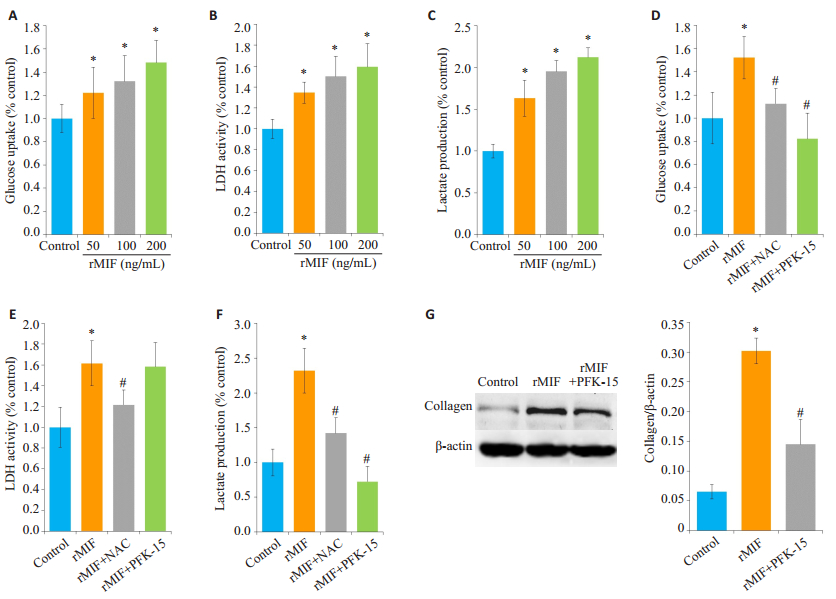
在不同溶度rMIF刺激下,HLF细胞葡萄糖摄取(图 2A)、LDH活性(图 2B)和乳酸生成(图 2C)都随rMIF浓度升高而增加。提前用糖酵解抑制剂PFK-15抑制葡萄糖摄取(图 2D)和乳酸生成(图 3F)后,可抑制rMIF诱导的collagen表达增加(图 2G)。
|
图 2 有氧糖酵解在rMIF促进collagen生成中的作用 Figure 2 Effect of aerobic glycolysis on rMIF-promoted collagen production. A: Glucose uptake measured by [3H]-2DG method in HLFs stimulated with different concentrations of rMIF for 24 h; B: LDH activity was measured by micropore method; C: Lactate production detected using microsphere method. HLFs were pretreated with NAC (1 mmol/L) and PFK-15 (10 μmol/L) for 1 h, followed by rMIF (200 ng/mL) stimulation for 24 h. Glucose uptake (D) and LDH activity (E) were detected by [3H]-2DG method and microwell assay, respectively; lactate production (F) and collagen expression (G) were detected with microwell assay and Western blotting, respectively. *P < 0.05 vs control; #P < 0.05 vs rMIF group. |
|
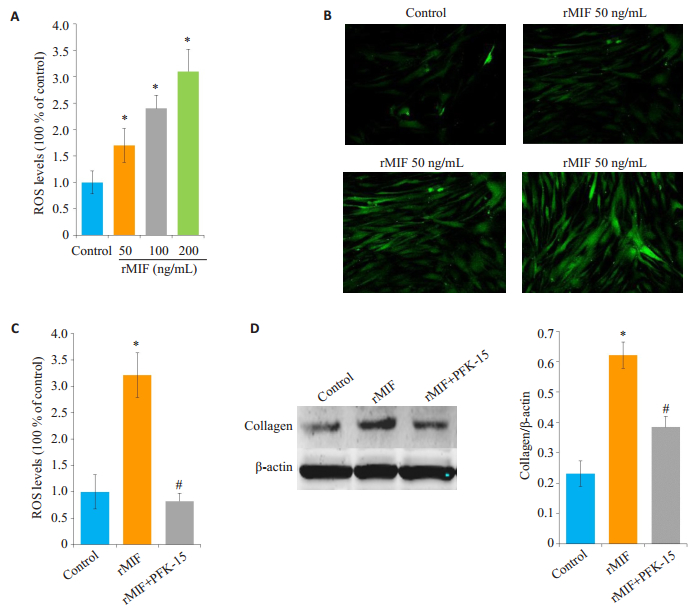
图 3 ROS在rMIF促进collagen生成中的作用 Figure 3 Effect of ROS on rMIF-promoted collagen production. HLF cells were stimulated with different concentrations of rMIF for 24 h and after staining with fluorescent probe DCFH-DA, ROS levels were measured with a microplate reader (A) and confocal microscopy (B) (×600). After pretreatment with NAC (1 mmol/L) for 1 h, HLFs were stimulated with rMIF (200 ng/mL) for 24 h; after staining with DCFH-DA fluorescent probe, ROS level was detected by microplate reader (C), and Western blotting was used to detect collagen expression (D). *P < 0.05 vs control; #P < 0.05 vs rMIF group. |
用不同溶度的rMIF刺激HLF细胞24 h,检测ROS水平,结果显示ROS的水平醉着刺激浓度的升高而增加(图 3A、B)。而用NAC抑制ROS后(图 3C),collagen的表达也被抑制(图 3D)。
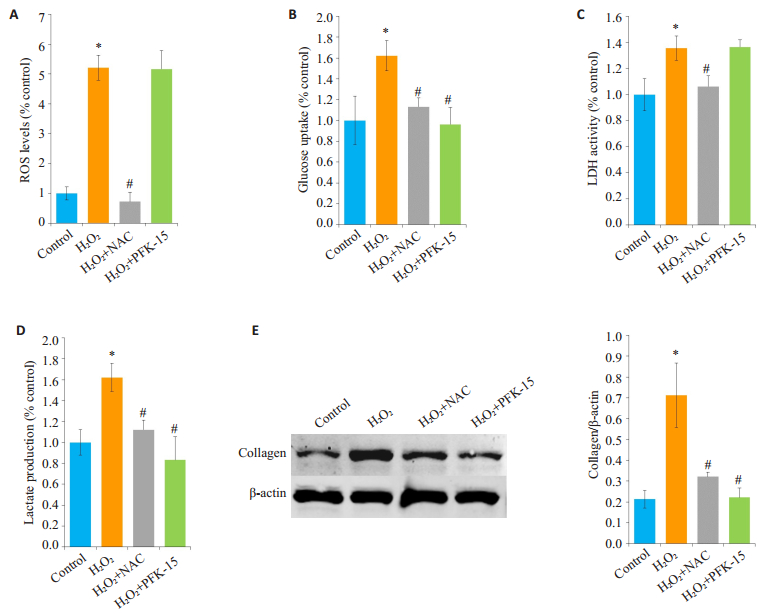
2.4 活性氧通过诱导有氧糖酵解促进collagen生成结果显示,H2O2可增加HLF细胞葡萄糖摄取(图 4B)、LDH活性(图 4C)和lactate产生(图 4D)。而使用PFK-15对H2O2诱导的活性氧生成没有影响(图 4A),但可以抑制H2O2诱导葡萄糖摄取(图 4B)、LDH活性(图 4C)、lactate产生(图 4D)和collagen生成(图 4E)。
|
图 4 活性氧通过诱导有氧糖酵解促进collagen生成 Figure 4 Active oxygen promotes collagen production by inducing aerobic glycolysis. HLFs were pretreated with NAC (1 mmol/L) and PFK-15 (10 μmol/L) for 1 h before stimulation with H2O2 (500 μmol/L) for 24 h. A: HLFs were stained with fluorescent probe DCFH-DA, and ROS level was detected by a microplate reader; B: Glucose uptake measured by [3H]-2DG method; C: LDH activity measured by micropore method; D: Microsphere method was used to detect lactate production; E: Western blotting for detecting collagen expression. *P < 0.05 vs control. #P < 0.05 vs H2O2 group. |
本研究通过体内外实验首次发现,博莱霉素诱导的肺纤维化小鼠组织和肺泡灌洗液中MIF表达增加;并且使用rMIF可以诱导HLF细胞ROS、有氧糖酵解和collagen表达增加。进一步发现抑制ROS和有氧糖酵解可以减少rMIF诱导的collagen表达增加。提示,rMIF通过ROS诱导有氧糖酵解促进collagen生成。
MIF因可以促进巨噬细胞迁移而得名。最新的研究发现,MIF在各种组织和细胞中都表达,参与急性肺损伤[16]、哮喘[17]、肺结核[18]、肺癌[19]等肺部疾病的发病过程。同时,通过分析纤维化病人和博莱霉素诱导的肺纤维鼠模型肺组织中MIF表达水平,发现相对于健康对照组,MIF表达都显著升高;并且抑制MIF可以减轻博莱霉素诱导的小鼠肺纤维化[6-7]。我们的结果也显示,在博莱霉素诱导的肺纤维化小鼠组织和肺泡灌洗液中MIF表达增加;并且用不同浓度rMIF人胚肺成纤维细胞(HLF),发现collagen表达水平也显著增加。由此提示,MIF可促进成纤维细胞合成细胞外基质参与肺纤维的发生发展。
通过快速的有氧糖酵解,细胞不仅获得ATP,还可以补充大量DNA合成所需要的原料[20-22]。研究发现在纤维化组织中氧糖酵解的水平明显升高,使用有氧糖酵解的抑制剂可以减轻博莱霉素诱导的肺纤维化和TGF-β诱导的肺成纤维细胞collagen生成[10-12]。我们的研究发现,在rMIF的HLF细胞中,有氧糖酵解显著增加;而使用PFK-15抑制有氧糖酵解后,rMIF的肺成纤维细胞collagen生成被明显抑制。提示,MIF可能是通过促进肺成纤维细胞有氧糖酵解来促进细胞外基质的生成的。
活性氧(ROS)是细胞代谢的产物,在正常的细胞生理活动中有重要作用,但其也参与了很多疾病的发生发展[23-24]。有研究表明,肺纤维中ROS水平显著增加,而抑制ROS可以减轻肺纤维化[25-26]。而MIF可以促进ROS的生成[27-28],我们的研究也发现,rMIF处理HLF细胞后,ROS的水平显著升高。因此,我们进一步使用NAC抑制ROS水平,发现可以显著抑制rMIF诱导的collagen生成。说明,MIF诱导ROS升高,是其促进collagen生成的重要机制。
ROS可以促进细胞的有氧糖酵解[29],通过诱导HIF-1α生成,增加糖酵解相关蛋白表达[30]。因此,我们进一步使用H2O2模拟ROS,发现细胞有氧糖酵解明显增加;抑制ROS可以减少有氧糖酵解和collagen生成,而抑制用H2O2诱导的有氧糖酵解也减少了collagen生成。由此说明,rMIF通过ROS上调细胞有氧糖酵解,促进college生成。
综上所述,肺纤维中高表达的rMIF可以促进ROS的生成,进而诱导细胞发生有氧糖酵解,增加collagen的生成,导致纤维的发生发展。
| [1] |
Carvajalino S, Reigada C, Johnson M J, et al. Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review[J].
BMC Pulm Med, 2018, 18(1): 78.
DOI: 10.1186/s12890-018-0651-3. |
| [2] |
Kreuter M, Spagnolo P, Wuyts W, et al. Antacid therapy and disease progression in patients with idiopathic pulmonary fibrosis who received pirfenidone[J].
Respiration, 2017, 93(6): 415-23.
DOI: 10.1159/000468546. |
| [3] |
Kropski JA, Reiss S, Markin C, et al. Rare genetic variants in PARN are associated with pulmonary fibrosis in families[J].
Am J Respir Crit Care Med, 2017, 196(11): 1481-4.
DOI: 10.1164/rccm.201703-0635LE. |
| [4] |
Donnelly SC, Haslett C, Reid PT, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome[J].
Nat Med, 1997, 3(3): 320-3.
DOI: 10.1038/nm0397-320. |
| [5] |
Husebo GR, Bakke PS, Gronseth R, et al. Macrophage migration inhibitory factor, a role in COPD[J].
Am J Physiol Lung Cell Mol Physiol, 2016, 311(1): L1-7.
DOI: 10.1152/ajplung.00461.2015. |
| [6] |
Olivieri C, Bargagli E, Inghilleri S, et al. Macrophage migration inhibitory factor in lung tissue of idiopathic pulmonary fibrosis patients[J].
Exp Lung Res, 2016, 42(5): 263-6.
DOI: 10.1080/01902148.2016.1199744. |
| [7] |
Tanino Y, Makita H, Miyamoto K, et al. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice[J].
Am J Physiol Lung Cell Mol Physiol, 2002, 283(1): L156-62.
DOI: 10.1152/ajplung.00155.2001. |
| [8] |
Shang Y, He J, Wang Y, et al. CHIP/Stub1 regulates the warburg effect by promoting degradation of PKM2 in ovarian carcinoma[J].
Oncogene, 2017, 36(29): 4191-200.
DOI: 10.1038/onc.2017.31. |
| [9] |
Jiang SH, Li J, Dong FY, et al. Increased serotonin signaling contributes to the warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice[J].
Gastroenterology, 2017, 153(1): 277-91.
DOI: 10.1053/j.gastro.2017.03.008. |
| [10] |
Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta[J].
Am J Respir Crit Care Med, 2012, 186(8): 740-51.
DOI: 10.1164/rccm.201201-0084OC. |
| [11] |
Xie N, Tan Z, Banerjee S, et al. Glycolytic Reprogramming in myofibroblast differentiation and lung fibrosis[J].
Am J Respir Crit Care Med, 2015, 192(12): 1462-74.
DOI: 10.1164/rccm.201504-0780OC. |
| [12] |
Chen XS, Li LY, Guan YD, et al. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the warburg effect[J].
Acta Pharmacol Sin, 2016, 37(8): 1013-9.
DOI: 10.1038/aps.2016.47. |
| [13] |
李伟峰, 胡玉洁, 袁伟锋, 等. 气管内滴入与雾化博莱霉素致小鼠肺纤维化模型的比较研究[J].
南方医科大学学报, 2012(2): 221-5.
|
| [14] |
Yu C, Tang W, Wang Y, et al. Downregulation of ACE2/Ang-(1-7)/ Mas axis promotes breast cancer metastasis by enhancing storeoperated calcium entry[J].
Cancer Lett, 2016, 376(2): 268-77.
DOI: 10.1016/j.canlet.2016.04.006. |
| [15] |
Fischer Y, Thomas J, Sevilla L, et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations[J].
J Biol Chem, 1997, 272(11): 7085-92.
DOI: 10.1074/jbc.272.11.7085. |
| [16] |
Nunez C, Rueda B, Martinez A, et al. Involvement of macrophage migration inhibitory factor gene in celiac disease susceptibility[J].
Genes Immun, 2007, 8(2): 168-70.
DOI: 10.1038/sj.gene.6364365. |
| [17] |
El-Adly TZ, Kamal S, Selim H, et al. Association of macrophage migration inhibitory factor promoter polymorphism-173G/C with susceptibility to childhood asthma[J].
Cent Eur J Immunol, 2016, 41(3): 268-72.
|
| [18] |
Das R, Koo MS, Kim BH, et al. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis[J].
Proc Natl Acad Sci USA, 2013, 110(32): E2997-3006.
DOI: 10.1073/pnas.1301128110. |
| [19] |
Li J, Zhang J, Xie F, et al. Macrophage migration inhibitory factor promotes warburg effect via activation of the NFkappaB/HIF1alpha pathway in lung cancer[J].
Int J Mol Med, 2018, 41(2): 1062-8.
|
| [20] |
Liu N, Parry S, Xiao Y, et al. Molecular targets of the Warburg effect and inflammatory cytokines in the pathogenesis of pulmonary artery hypertension[J].
Clin ChimActa, 2017, 466: 98-104.
DOI: 10.1016/j.cca.2017.01.015. |
| [21] |
Gwangwa MV, Joubert AM, Visagie MH. Crosstalk between the Warburg effect, redox regulation and autophagy induction in tumourigenesis[J].
Cell Mol Biol Lett, 2018, 23: 20.
DOI: 10.1186/s11658-018-0088-y. |
| [22] |
徐高峰, 白晓斌, 王茂德, 等. 脑胶质瘤细胞糖酵解表型特征及对细胞增殖与凋亡的影响[J].
南方医科大学学报, 2013, 33(3): 406-11.
|
| [23] |
Perrone S, Bracciali C, Di Virgilio N, et al. Oxygen use in neonatal care: a two-edged sword[J].
Front Pediatr, 2016, 4: 143.
|
| [24] |
Narasimhan M, Rajasekaran N S. Exercise, Nrf2 and antioxidant signaling in cardiac aging[J].
Front Physiol, 2016, 7: 241.
|
| [25] |
Sato N, Takasaka N, Yoshida M, et al. Metformin attenuates lung fibrosis development via NOX4 suppression[J].
Respir Res, 2016, 17(1): 107.
DOI: 10.1186/s12931-016-0420-x. |
| [26] |
Yu WN, Sun LF, Yang H. Inhibitory effects of astragaloside Ⅳ on bleomycin-induced pulmonary fibrosis in rats via attenuation of oxidative stress and inflammation[J].
Inflammation, 2016, 39(5): 1835-41.
DOI: 10.1007/s10753-016-0420-5. |
| [27] |
Zhang Y, Gu R, Jia J, et al. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity suppresses microgliamediated inflammatory responses[J].
Clin Exp Pharmacol Physiol, 2016, 43(11): 1134-44.
DOI: 10.1111/cep.2016.43.issue-11. |
| [28] |
Lv W, Chen N, Lin Y, et al. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/ NF kappa B axis[J].
Cancer Lett, 2016, 375(2): 245-55.
DOI: 10.1016/j.canlet.2016.02.005. |
| [29] |
Molavian HR, Kohandel M, Sivaloganathan S. High concentrations of H2O2 make aerobic glycolysis energetically more favorable for cellular respiration[J].
Front Physiol, 2016, 7: 362.
|
| [30] |
Lim S, Liu H, Madeira DSL, et al. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha[J].
Cancer Res, 2016, 76(8): 2231-42.
DOI: 10.1158/0008-5472.CAN-15-1538. |
 2018, Vol. 38
2018, Vol. 38

