2. 中山大学附属第六医院普外科,广东 广州 510655
2. Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, China
急性肠系膜缺血在所有的急腹症中占1%~2%,多发于老年女性。而57%的急性肠系膜缺血患者最终因肠坏死而接受肠切除术[1]。在急诊手术中,由肠系膜缺血导致的肠坏死占了超过3%[2]。由于肠系膜缺血并不常见,它的症状与很多疾病重叠,因此,即使是经验丰富的医师,也难以对此病做出快速的诊断。急性肠坏死的死亡率可达60%~80%。而这个死亡率跟70年前的数据差不多,最重要的原因可能由于现代老年化社会逐渐加重[3]。研究指出,超过24 h对急性肠系膜缺血导致的肠坏死进行干预治疗,患者的死亡率可高达70%。如果确诊小于24 h并及时干预,死亡率可降至14%[4]。
长链非编码RNA(lncRNA)约占人体总RNA的80%以上,它们在细胞中调控着各种生命过程。同时,它们在体内表达失调与多种疾病相关,因此检测它们在体内的表达水平有助于协助诊断多类疾病。血清、唾液、尿液等中的各种分子,如DNA、RNA、代谢物等,可比拟组织病理活检术,对体内的疾病起着协助确诊的作用[5]。肠系膜缺血使缺氧肠细胞逐渐降解坏死。这过程破坏肠道黏膜屏障,使消化酶进入肠道基层,进而使肠道发生自我消化。Zou等[6]研究指出,上皮屏障破坏过程中,长链非编码RNA H19在肠道黏膜细胞中高表达,高表达H19使紧密连接蛋白ZO-1及黏连蛋白E-cadherin表达降低,进而破坏着黏膜屏障的功能。但该研究并未指出H19可否作为标志物协助诊断急性肠坏死。
迄今为止,对急性肠坏死的早诊研究依然缺乏,仍未发现或报道敏感性和特异性较强的检验、检查,多项分析急性肠坏死标志物的研究依然存在许多缺陷,诊断效果难以令人满意[7-9],因此许多患者往往漏诊,导致这类患者的死亡率居高不下[10-11]。基于此,有必要挖掘出敏感性和特异性高的分子标志物协助诊断急性肠坏死,以期减少患者的的死亡率及提高预后。多项研究指出,尿液中的分子物质的表达与体内多种组织的表达有超过75%的一致性。同时,某些分子在尿液与血浆的表达量一致性可达95%以上[12]。基于上述研究,我们猜想,在急性肠系膜缺血中,坏死肠组织释放高表达的H19到血液,血液H19通过肾脏进入尿液使尿液中的H19高表达。因此,尿液中高表达H19可以助诊断肠系膜缺血引起的肠组织坏死。
1 资料和方法 1.1 研究资料所纳入全部病患来源于中山大学附属第六医院,收集时间在2013年~2018年期间。所有患者均签署知情同意书同意贡献标本。肠坏死患者:共纳入因急性肠坏死而接受手术切除小肠的患者51例。该51例患者切除的小肠小于1 m,术后无并发短肠综合征。收集这些患者术后切除的坏死肠组织及配对的坏死组织旁3 cm以上的正常肠组织。同时收集这些患者的血液及尿液。对照者:共纳入与肠坏死在性别、年龄、种族配对的健康对照者51例。健康对照者的纳入标准为胸部平片、腹部超声、生化、肝肾功能化验、血液常规、尿常规、大便常规、AFP、CEA、乙肝表面抗原、丙肝抗体、HIV抗体、梅毒抗体无异常发现。同时纳入10种最常见的急腹症,每种急腹症患者各35例。这10种常见的急腹症为胃肠炎、消化性溃疡、阑尾炎、消化道穿孔、急性胆囊炎、急性胆管炎、泌尿系统结石、胰腺炎、心肌梗死、肠易激综合征。收集这些患者的血液和中段尿液标本。
根据既往文献报道,H19是一个在多种肿瘤中高表达,并起重要调控作用的长链非编码RNA。较多报道与之相关的癌症有:乳腺癌[13]、胃癌[14]、膀胱癌[15]、急性髓性白血病[16]、以及肺癌[17]。同时收集这些患者的血清和唾液样本各10例。
1.2 标本处理 1.2.1 组织标本处理术后切除1 min内立即放入液氮冰冻,然后再放入-80 ℃冰箱保存。51例肠坏死组织均经术后病理确诊。从坏死肠旁3 cm以上取的肠组织也经病理证实无坏死表现,为正常小肠组织。
1.2.2 血液标本处理用2 mL干燥管收集血液。然后放入离心机2000 g 5 min 4 ℃收集血清置入无菌无酶EP管中。最后再放入-80 ℃冰箱保存。
1.2.3 尿液标本处理用收集尿常规的管子(总容量约12 mL)收集随机尿液约10 mL,然后放入离心机2000 g 5 min 4 ℃收集上清置入无菌无酶EP管中。最后再放入-80 ℃冰箱保存。上述样本放入-80 ℃冰箱7 d内完成下述后续的试验。
1.3 RNA提取 1.3.1 组织RNA利用TRIzol(货号12183555,Thermo Fisher Scientific,美国)试剂,根据厂商说明操作[18]。
1.3.2 血清及尿液RNA因RNA在血清及尿液含量较少,一般提取RNA的TRIzol法通常不能成功提取。因此利用专门提取体液RNA的mirVana PARIS试剂盒(货号AM1556,Thermo Fisher Scientific, 美国)试剂盒提取。根据厂商说明操作。
1.4 RNA表达水平的检测取大概500 ng RNA样本,加入ReverTra Ace qPCR RT Kit(货号FSQ-101,Toyobo, 日本)做逆转录合成cDNA。反应条件为:37 ℃ 15 min → 98 ℃ 5 min→ 4 ℃ ∞。最后进行荧光定量PCR反应检测RNA的表达水平。采用Maxima SYBR Green qPCR Master Mixes(货号K0252,Thermo Fisher Scientific, 美国)试剂盒进行PCR试验。定量PCR仪使用美国Bio-Rad CFX,40次循环以下程序95 ℃ 5 s→60 ℃ 32 s。最后设溶解曲线反应温度:95 ℃ 15 s→60 ℃ 1 min→95 ℃ 15 s→ 60 ℃ 15 s。每个cDNA样本做3个平行重复,同时预留3个孔做无模板对照。以β-actin作为内部参照物[19]。LncRNA-H19引物序列:上游:5'-TGCTGCACTTTACAACCACTG-3',下游:5'-ATGGTGTCTTTGATGTTGGGC-3'。β-actin引物序列:上游:5'-GTCTTCCCCTCCATCGTG-3',下游:5'-AGGGTGAGGATGCCTCTCTT-3'。H19的表达水平使用2-ΔΔCt表示[20]。
1.5 统计学方法数据采用SPSS13.0软件进行统计分析,计量资料用均数±标准差进行统计描述。使用Mann-Whitney U检验(两组)或Kruskal-Wallis H检验(两组以上)比较H19表达量在组间的差异性。Spearman检验组间的相关性。最后构建受试者工作特征(ROC)曲线分析血清和尿液H19对急性肠坏死的诊断价值。P < 0.05(双侧)为差异有统计学意义。
2 结果 2.1 纳入急性肠坏死患者的病例特点本研究共收集急性肠坏死患者51例。患者的病因及确诊均基于影像学及病理学。其中男性患者16例(31.4%),女性患者35例(68.6%)。发病年龄平均岁数74.4岁,多发于60岁以上的老年女性。26例(51.0%)病因为肠系膜动脉栓塞或栓子形成,15例(29.4%)为肠系膜静脉非血栓性狭窄。7例(13.7%)为肠系膜静脉栓塞,3例(5.9%)为肠系膜动脉的炎症。与世界上发病的流行病学特点相似。
2.2 长链非编码RNA H19在坏死肠组织中的表达相对于坏死旁的正常肠组织,H19在肠坏死组织中的表达平均升高11.2倍,差异具有统计学意义(P < 0.001,图 1)。
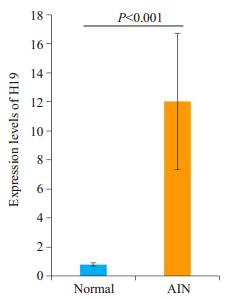
|
图 1 lncRNA-H19在肠坏死组织与旁正常肠组织的表达 Figure 1 Expression of lncRNA H19 in acute necrotic intestinal tissues (AIN) and adjacent normal intestinal tissues. |
对H19在健康对照者分别与其在其他10种常见急腹症及5种癌症(乳腺癌、胃癌、膀胱癌、急性髓性白血病、以及肺癌)患者的血清、尿液中的表达水平进行组间的两两比较及多组间比较,并未发现H19在血清、尿液任何组间出现显著性差异(P > 0.05)。然后将H19在急性肠坏死患者中的血清、尿液分别与健康对照以及10种最常见的急腹症及5种癌症患者的血清、尿液的表达进行两两比较,发现H19在急性肠坏死患者中的血清、尿液的表达量均显著升高(P < 0.001,图 2)。
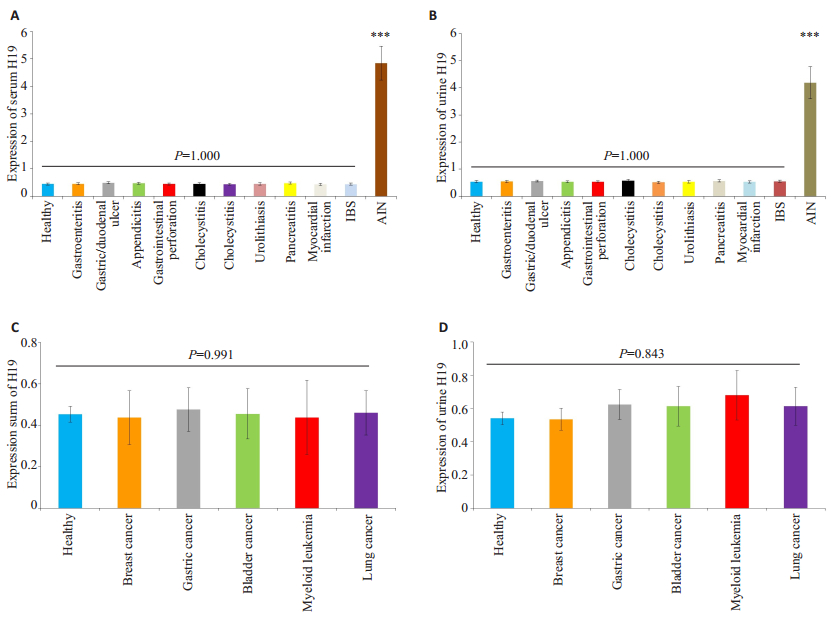
|
图 2 H19在包括急性肠坏死等急腹症患者中的血清与尿液表达水平 Figure 2 Serum and urine levels of H19 in patients with acute intestinal necrosis (AIN) and other acute abdominal conditions and different cancers. A: Serum H19 was significantly elevated in patients with acute intestinal necrosis compared with healthy controls and patients with other acute abdominal conditions; B: Urine H19 was significantly elevated in patients with acute intestinal necrosis compared with healthy controls and patients with other acute abdominal conditions; C: Serum H19 level was similar between healthy controls and patients with different cancers; D: Urine H19 level was similar between healthy controls and patients with different cancers; ***P < 0.001 healthy controls vs acute intestinal necrosis. IBS: Irritable bowel syndrome. |
将H19在坏死肠组织、患者血清、及尿液中的表达量进行两两相关性的Spearman统计学分析,发现H19在坏死肠组织与血清、肠组织与尿液、血清与尿液中的表达均呈两两显著正相关(P < 0.001),组织与血清H19表达的相关性为0.974,血清与尿液H19表达的相关性为0.967,组织与尿液H19表达的相关性为0.917(图 3)。
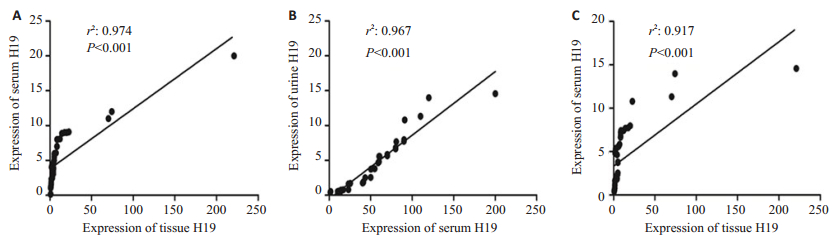
|
图 3 H19在急性肠坏死患者坏死肠组织、血清及尿液表达的相关性 Figure 3 Correlations of H19 levels in necrotic intestinal tissues, serum, and urine in patients with acute intestinal necrosis. A: Correlation of H19 levels between necrotic intestinal tissue and serum samples; B: Correlation of H19 levels between serum and urine samples; C: Correlation of H19 levels between necrotic intestinal tissue and urine samples. The correlation coefficients (r2) are shown. |
曲线下面积(AUC)为构建ROC曲线后判断生物标志物对疾病诊断价值的评判指标。一般AUC越大,诊断价值越高。通过构建ROC曲线发现,血清H19对急性肠坏死诊断的AUC值为0.951,敏感性为94%,特异性为100%。而尿液H19对急性肠坏死的诊断的AUC值为0.915,诊断敏感性为79.6%,特异性为100%(图 4)。
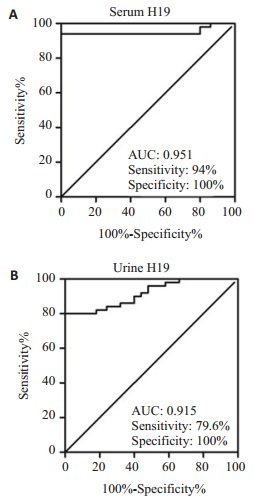
|
图 4 判断血清、尿液H19对急性肠坏死诊断的ROC曲线 Figure 4 ROC curves of serum (A) and urine (B) H19 for diagnosis of acute intestinal necrosis. |
本研究根据既往的基础实验研究结果,受损的肠组织可出现高表达H19[6]。我们的研究也发现了急性肠坏死的组织相对于正常肠组织也显著高表达。同时,据我们所知,本研究首次报道了相对于健康对照者及常见的急腹症患者,H19在急性肠坏死患者的血清及尿液中也显著高表达,对急性肠坏死表现出一定的鉴别诊断价值。
急性肠坏死的病因大致可分为4类:40%~50%为肠系膜动脉栓塞或栓子形成;20%~35%为肠系膜静脉非血栓性狭窄;5%~15%位肠系膜静脉栓塞;5%是由于动脉的炎症或夹层。无论何种原因导致的肠坏死,死亡率均非常高,在24 h以内进行干预可将死亡率降低至60%以上,同时短肠综合征等严重并发症也可大大降低。但现时,由于肠坏死的缺乏特异的症状及实验室检查,漏诊率非常高。因此急性肠坏死的致死率一直居高不下。虽然CT及血管造影敏感性和特异性非常高,但当急诊医生怀疑此病时患者往往到了非常严重的状况[21-22]。因此,急需挖掘一种敏感性和特异性都非常高的检验协助诊断。我们收集的肠坏死患者坏死的肠组织小于1 m,且术后无重大并发症,因此尚属于早期肠坏死患者。本研究发现H19在坏死肠组织、血清、尿液中都显著升高。而相比其他10种常见的急腹症未见升高。因此H19可能是协诊早期肠坏死的理想分子标志物。但要进一步转化于临床应用尚需大样本的验证。但本研究结果为此理想提供了重要的思路。
由于尿液的产生与排泄需要经过肾脏、膀胱、前列腺等尿路系统,因此许多研究表明尿液中的DNA、RNA、蛋白等分子物质可作为生物标志物协助诊断膀胱癌[23]、间质性膀胱炎[24]、前列腺癌[25]等泌尿系统疾病。同时,当体内的器官组织坏死时,组织细胞内的分子物质可释放入血液循环,血液循环中的分子物质都会流经肾脏,除了大分子蛋白,经过滤过后进入尿液。因此除了部分蛋白分子,细胞及血液中的大部分分子物质都可见于尿液[16]。因此,尿液中的分子物质并非全部来源于尿道系统的分泌与排泄,也可能来源全身的各个器官组织的分泌与释放。已发现尿液中的多种分子,如RNA、DNA、蛋白等也可作为生物标志物对胃癌[26]、肺癌[27]、骨关节炎[28]、系统性红斑狼疮[29]、卵巢癌[30]、乳腺癌[31]等多种非尿道疾病具有一定的诊断价值。同时,尿液中的代谢物也可预测直肠癌患者对化疗的耐受性和反应性[32]。本研究发现H19在肠坏死组织、患者血清、尿液中的表达水平两两显著正相关。初步提示尿液H19的高表达可能来源于体内坏死的肠组织,有望成为肠坏死的“液体活检术”之一,成为协诊早期急性肠坏死的无创标志物之一。同时本研究进一步提示体内坏死的分子物质释放入血液循环后,经肾脏滤过后排泄入尿液。但也有研究发现,癌组织与血清中的RNA表达并非完全一致,只有51.6%的组织RNA同时存在于血清中[33]。原因可能由于组织细胞中存在某种调控机制,选择性释放RNA至血液中。因此在本研究中,虽然其它报道H19在乳腺癌、胃癌、膀胱癌、急性髓性白血病、以及肺癌肿瘤细胞中高表达,但肿瘤细胞并无选择释放H19至血液中。所以这些癌症患者的H19并未在血清和尿液中表达升高。由于尿液收集较取血更为无创、简便,患者受检的依从性更好。同时由于部分急腹症患者疼痛较为剧烈,抽血可加重患者的抵抗情绪,而尿液收集可无需专业人员操作,甚至可以从导尿患者尿袋中收集,同时本研究发现血清与尿液H19对肠坏死的诊断价值相差不大。鉴于取样的简便性而言,尿液的标志物更值得推广研究。
综上所述,本研究发现了尿液中的H19在急性肠坏死中与其它常见的急腹症具有良好的鉴别诊断价值。深入研究尿液中的生物标志物,相信有更多精彩的发现,更能简便、精确地协诊疾病。
| [1] |
Lapsekili E, Menteş Ö, Balkan M, et al. Role of alkaline phosphatase intestine-isomerase in acute mesenteric ischemia diagnosis[J].
Ulus TravmaAcil Cerrahi Derg, 2016, 22(2): 115-20.
|
| [2] |
Yang K, Wang W, Zhang WH, et al. The combination of D-Dimer and peritoneal irritation signs as a potential indicator to exclude the diagnosis of intestinal necrosis[J].
Medicine (Baltimore), 2015, 94(40): e1564.
DOI: 10.1097/MD.0000000000001564. |
| [3] |
Carver TW, Vora RS, Taneja A. Mesenteric ischemia[J].
Crit Care Clin, 2016, 32(2): 155-71.
DOI: 10.1016/j.ccc.2015.11.001. |
| [4] |
Gonenc M, Dural CA, Kocatas A, et al. The impact of early diagnostic laparoscopy on the prognosis of patients with suspected acute mesenteric ischemia[J].
Eur J Trauma Emerg Surg, 2013, 39(2): 185-9.
DOI: 10.1007/s00068-013-0253-y. |
| [5] |
Shi T, Gao G, Cao Y. Long noncoding RNAs as novel biomarkers have a promising future in cancer diagnostics[J].
Dis Markers, 2016, 25(10): 9085195.
|
| [6] |
Zou T, Jaladanki SK, Liu L, et al. H19 long noncoding RNA regulates intestinal epithelial barrier function via MicroRNA 675 by interacting with RNA-Binding protein HuR[J].
Mol Cell Biol, 2016, 36(9): 1332-41.
DOI: 10.1128/MCB.01030-15. |
| [7] |
Chiu YH, Huang MK, How CK, et al. D-Dimer in patients with suspected acute mesenteric ischemia[J].
Am J Emerg Med, 2009, 27(8): 975-9.
DOI: 10.1016/j.ajem.2009.06.006. |
| [8] |
Cossé C, Zogheib E, Dupont H, et al. New biomarkers for outcomes of acute mesenteric ischemia[J].
Intensive Care Med, 2015, 41(7): 1376-7.
DOI: 10.1007/s00134-015-3852-8. |
| [9] |
Nuzzo A, Ronot M, Maggiori L, et al. Early acute mesenteric ischemia: many rivers to cross[J].
Ann Surg, 2017, 29(8): 36.
|
| [10] |
Shirasu T, Hosaka A, Okamoto H, et al. Bowel necrosis following endovascular revascularization for chronic mesenteric ischemia: a case report and review of the literature[J].
BMC Gastroenterol, 2013, 19(13): 118.
|
| [11] |
Leone M, Bechis C, Baumstarck K, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases[J].
Intensive Care Med, 2015, 41(4): 667-76.
DOI: 10.1007/s00134-015-3690-8. |
| [12] |
Salvi S, Martignano F, Molinari C, et al. The potential use of urine cell free DNA as a marker for Cancer[J].
Expert Rev Mol Diagn, 2016, 16(12): 1283-90.
DOI: 10.1080/14737159.2016.1254551. |
| [13] |
Yu G, Zhang W, Zhu L, et al. Upregulated long non- coding RNAs demonstrate promising efficacy for breast cancer detection: a metaanalysis[J].
Onco Targets Ther, 2018, 16(11): 1491-9.
|
| [14] |
Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer[J].
Oncotarget, 2014, 5(8): 2318-29.
|
| [15] |
Zhu Z, Xu L, Wan Y, et al. Inhibition of E-cadherin expression by lncRNA H19 to facilitate bladder cancer metastasis[J].
Cancer Biomark, 2018, 19(12): 109.
|
| [16] |
Zhang TJ, Zhou JD, Zhang W, et al. H19 overexpression promotes leukemogenesis and predicts unfavorable prognosis in acute myeloid leukemia[J].
Clin Epigenetics, 2018, 10(10): 47.
|
| [17] |
Cui J, Mo J, Luo M, et al. c-Myc-activated long non-coding RNA H19 downregulates miR-107 and promotes cell cycle progression of nonsmall cell lung cancer[J].
Int J Clin Exp Pathol, 2015, 8(10): 12400-9.
|
| [18] |
Liu AM, Yao TJ, Wang W, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study[J].
BMJ Open, 2012, 2(2): e000825.
DOI: 10.1136/bmjopen-2012-000825. |
| [19] |
Xie Z, Chen X, Li J, et al. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer[J].
Oncotarget, 2016, 7(18): 25408-19.
|
| [20] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method[J].
Methods, 2001, 25(4): 402-8.
DOI: 10.1006/meth.2001.1262. |
| [21] |
Clair DG, Beach JM. Mesenteric ischemia[J].
N Engl J Med, 2016, 374(10): 959-68.
DOI: 10.1056/NEJMra1503884. |
| [22] |
Singh M, Long B, Koyfman A. Mesenteric ischemia: a deadly miss[J].
Emerg Med Clin NorthAm, 2017, 35(4): 879-88.
DOI: 10.1016/j.emc.2017.07.005. |
| [23] |
Casadio V, Calistri D, Tebaldi M, et al. Urine cell-free DNA integrity as a marker for early bladder cancer diagnosis: preliminary data[J].
Urol Oncol, 2013, 31(8): 1744-50.
DOI: 10.1016/j.urolonc.2012.07.013. |
| [24] |
Niimi A, Igawa Y, Aizawa N, et al. Diagnostic value of urinary CXCL10 as a biomarker for predicting Hunner type interstitial cystitis[J].
Neurourol Urodyn, 2018, 37(3): 1113-9.
|
| [25] |
Fredsøe J, Rasmussen AKI, Thomsen AR, et al. Diagnostic and prognostic MicroRNA biomarkers for prostate cancer in cell-free urine[J].
Eur Urol Focus, 2017, 17(9): S2405.
|
| [26] |
Kao HW, Pan CY, Lai CH, et al. Urine miR-21-5p as a potential noninvasive biomarker for gastric cancer[J].
Oncotarget, 2017, 8(34): 56389-97.
|
| [27] |
Reckamp KL, Melnikova VO, Karlovich C, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma[J].
J Thorac Oncol, 2016, 11(10): 1690-700.
DOI: 10.1016/j.jtho.2016.05.035. |
| [28] |
Ok SM, Lee SM, Park HR, et al. Concentrations of CTX Ⅰ, CTX Ⅱ, DPD, and PYD in the urine as a biomarker for the diagnosis of temporomandibular joint osteoarthritis: a preliminary study[J].
Cranio, 2017, 45(7): 1-7.
|
| [29] |
Tang D, Chen Y, He H, et al. Integrated analysis of mRNA, microRNA and protein in systemic lupus erythematosus-specific induced pluripotent stem cells from urine[J].
BMC Genomics, 2016, 17(2): 488.
|
| [30] |
Zhou J, Gong G, Tan H, et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma[J].
Oncol Rep, 2015, 33(6): 2915-23.
DOI: 10.3892/or.2015.3937. |
| [31] |
Erbes T, Hirschfeld M, Rücker G, et al. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker[J].
BMC Cancer, 2015, 15(7): 193.
|
| [32] |
Dykstra MA, Switzer N, Eisner R, et al. Urine metabolomics as a predictor of patient tolerance and response to adjuvant chemotherapy in colorectal cancer[J].
Mol Clin Oncol, 2017, 7(5): 767-70.
DOI: 10.3892/mco.2017.1407. |
| [33] |
Gao K, Zhou H, Zhang L, et al. Systemic disease-induced salivary biomarker profiles in mouse models of melanoma and non-small cell lung cancer[J].
PLoS One, 2009, 4(6): e5875.
DOI: 10.1371/journal.pone.0005875. |
 2018, Vol. 38
2018, Vol. 38

