糖尿病作为一种常见的慢性代谢性疾病,也是心血管疾病的独立危险因子。2型糖尿病患者易并发缺血性心脏病(IHD),心肌梗死后的病死率更高[1-2]。Lirk等[3]利用Zucker肥胖大鼠实验证实,糖尿病状态下心肌缺血/再灌注(MI/R)后损伤明显增加。目前对糖尿病加重MI/R损伤的作用机制及防治方法尚有争议[4-6]。越来越多的证据表明二磷酸腺苷核糖聚合酶(PARP-1)过度激活在心肌缺血再灌注损伤、循环休克、糖尿病心血管并发症和动脉粥样硬化中扮演了重要的角色[7]。PARP-1作为一个DNA损伤的感应器,由自由基和氧化细胞损伤引起的DNA断裂反应而激活。然而PARP-1的过度激活耗尽其作用底物尼克酰胺腺嘌呤二核苷酸(NAD)导致细胞死亡。有报道PARP-1抑制剂能有效提高线粒体的抗氧化功能,保护心肌细胞,而且PARP-1基因敲除小鼠心肌细胞抗氧化能力明显增强[7-8]。MRL-45696作为PARP-1特异性抑制剂,在糖尿病大鼠脑缺血再灌注损伤中发挥了保护性作用[8]。目前尚未见MRL- 45696对糖尿病MI/R损伤影响的研究报道。本研究旨在探讨PARP-1新型特异性抑制剂MRL-45696对糖尿病大鼠MI/R损伤中的保护作用。为减轻糖尿病患者心肌缺血再灌注损伤提供有效的药物选择。
1 材料和方法 1.1 实验动物、主要试剂及仪器 1.1.1 实验动物健康雄性SD大鼠,体质量150~180 g,由南方医科大学实验动物中心提供,许可证号:SCXK(粤)2016-0041。
1.1.2 主要试剂高脂高糖饲料(D12492)购自广东省医学动物实验中心;链脲佐菌素(STZ,Sigma-Aldrich);MRL-45696(默克);脂质过氧化丙二醛(MDA)检测试剂盒和超氧化物歧化酶(SOD)检测试剂(碧云天);心肌肌钙蛋白(I cTnI)ELISIA试剂盒(ANDYGENE);BCA法蛋白含量检测试剂盒(KeyGEN);TUNEL试剂盒(Roche);cleaved caspase-3、γ-H2AX、PARP-1、PAR抗体(CST);β-actin抗体(Kang Chen Bio-tech)。
1.1.3 仪器血糖仪(罗氏,卓越型);胰岛素放射免疫分析试剂盒(华英生物);垂直电泳仪(Bio-Rad)。
1.2 方法 1.2.1 建立2型糖尿病大鼠模型SD大鼠适应1周后喂养高糖高脂饲料8周,糖尿病组(DM)大鼠单次腹腔注射链脲佐菌素(STZ)30 mg/kg(柠檬酸和柠檬酸钠的缓冲液溶解STZ粉,配置浓度为10 mg/mL,pH值4.2~ 4.5)。对照组(Con)注射相同剂量的生理盐水。STZ注射1周后,全部大鼠断料不断水12 h,鼠尾动脉采血1 mL,以2000 r/min离心20~30 min,取上层血清0.4 mL分别检测血糖值和血清胰岛素值,随机血糖值≥16.7 mmol/L同时表现为对胰岛素抵抗,符合2型糖尿病大鼠模型。
1.2.2 实验分组选取2型糖尿病大鼠40只,随机分为糖尿病组(DM)、假手术组(S)、MRL-45696治疗+假手术组(NO)、缺血再灌注损伤模型组(MI/R)、MRL- 45696治疗+缺血再灌注损伤组(MRL),每组各8只。S组暴露心脏不结扎,仅留线。NO组和MRL组分别参考Eija等[8]对MRL-45696的使用方法,生理盐水将MRL- 45696溶解,配置浓度为1%,持续1周每天灌胃给予MRL-45696(50 mg/kg)。
1.2.3 建立2型糖尿病心肌缺血再灌注损伤大鼠模型采用冠状动脉左前降支结扎30 min再灌注120 min的方法制作心肌缺血再灌注损伤模型。盐酸戊巴比妥钠麻醉后大鼠被固定于操作台,胸部剃毛、消毒,气管切开,机械辅助通气,胸骨左缘切开,暴露心脏,在左冠状动脉前降支根部环绕穿线、结扎,30 min后剪开结扎线,恢复血流使冠脉再灌注120 min。以心电图Ⅱ导联ST段明显抬高、心肌颜色变暗为结扎成功标志。
1.2.4 心肌梗死面积的测量用伊文蓝-TTC法检测心肌梗死范围[9]。
1.2.5 血浆心肌肌钙蛋白Ⅰ(cTnI)、血清肌酸激酶(CK)、乳酸脱氢酶(LDH)活性、丙二醛(MDA)、超氧化物歧化酶活性(SOD)、烟酰胺腺嘌呤二核苷酸(NAD)水平(以NAD+/NADH比例代表)的检测按照试剂盒说明书指示操作。
1.2.6 缺血区心肌细胞凋亡检测按照TUNEL试剂盒说明书检测心肌TUNEL阳性细胞比例。
1.2.7 Western blot常规行SDS-PAGE电泳,将蛋白转移至PVDF膜。加入一抗4 ℃孵育过夜,洗膜后加入相应二抗,37 ℃孵育1 h,用ECL法行化学发光。
1.3 统计学分析采用SPSS 20.0统计软件分析,计量资料以均数±标准差表示,多组间比较采用单因素方差分析(one-way ANOVA),两两之间比较采用LSD法,P < 0.05为差异有统计学意义。
2 结果 2.1 一般情况高糖高脂喂食8周的SD大鼠在STZ腹腔注射1周后,检测的血糖、血清胰岛素值符合2型糖尿病大鼠模型,与文献报道的结果相符[10](表 1)。
| 表 1 大鼠的体质量、血糖及血清胰岛素数值 Table 1 Body weight, blood glucose and plasma insulin levels of the rats (Mean±SD, n=40) |
MRL组心肌梗死面积显著小于MI/R组(P < 0.05),与DM组相比,S组、NO组心肌梗死面积值相近,差异无统计学意义(P>0.05)。与MI/R组相比,MRL组大鼠cTnI、CK、LDH、MDA值及细胞凋亡比例显著降低(P < 0.05),而SOD活性和NAD值水平明显升高(P < 0.05,图 1~2,表 2)。
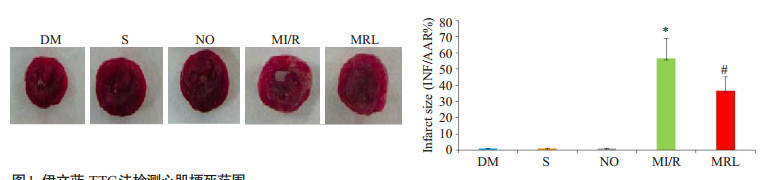
|
图 1 伊文蓝-TTC法检测心肌梗死范围 Figure 1 Myocardial infarct size measured using Evans blue. The data are presented as the percentage of the infarct area in the ischemic area (Mean ± SD, n=8). DM: diabetes mellitus group. S: DM group with exposure but not ligation of the anterior descending branch of the coronary artery. NO: Sham operation group with MRL-45696 treatment. MI/R: DM group with myocardium ischemia-reperfusion injury. MRL: DM group with myocardium ischemia-reperfusion injury pretreated with MRL- 45696. *P < 0.05 vs DM, S and NO; #P < 0.05 vs MI/R. |
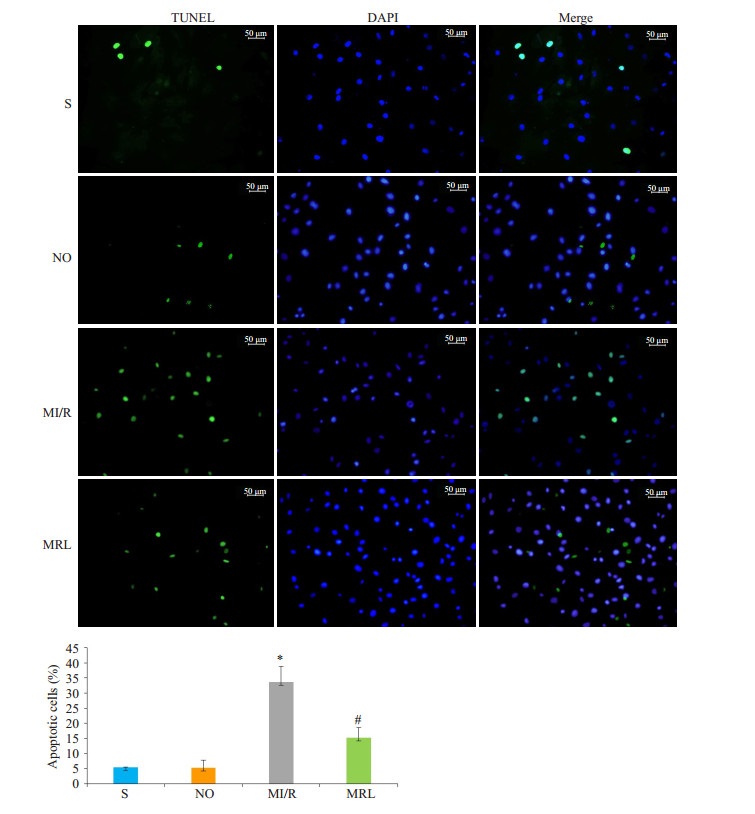
|
图 2 TUNEL法检测大鼠心肌细胞凋亡比例 Figure 2 Myocardial cell apoptotic rates in rats detected by TUNEL assay. *P < 0.05 vs S and NO; #P < 0.05 vs MI/R. |
| 表 2 糖尿病大鼠的cTnI、CK、LDH、MDA、SOD活性及NAD值 Table 2 Levels or activities of cTnI, CK, LDH, MDA, SOD and NAD in diabetic rats (Mean±SD, n=8) |
与MI/R组相比,MRL组大鼠心肌细胞中γ-H2AX、cleaved caspase-3、PARP-1、PAR表达水平明显降低,差异有统计学意义(P < 0.05,图 3、4)。
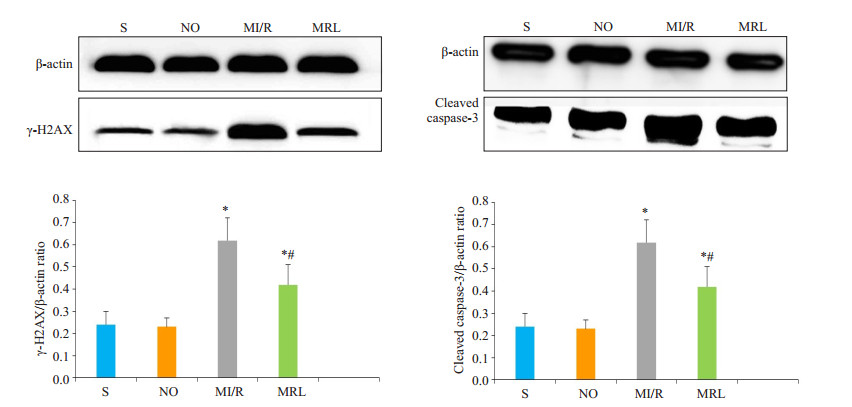
|
图 3 Western Blot法检测大鼠心肌细胞γ-H2AX、cleaved caspase-3蛋白表达 Figure 3 Western blotting for detecting γ-H2AX and cleaved caspase-3 protein expression in the cardiac myocytes in diabetic rats.*P < 0.05 vs S and NO; #P < 0.05 vs MI/R. |
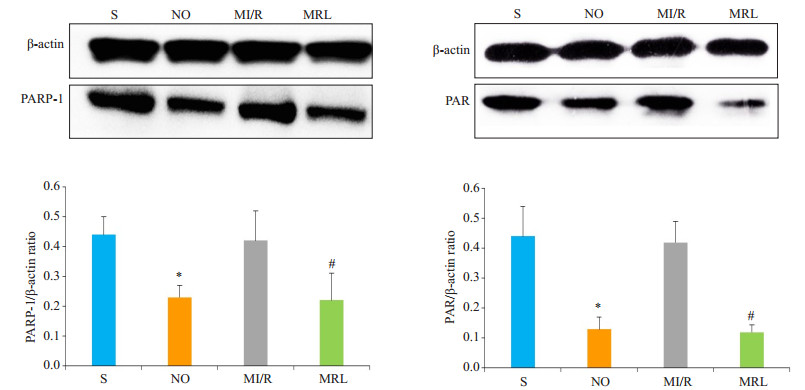
|
图 4 Western blot法检测大鼠心肌细胞PARP-1、PAR蛋白表达 Figure 4 Western blotting for detecting PARP-1 and PAR protein expressions in the cardiac myocytes in diabetic rats. *P < 0.05 vs S; #P < 0.05 vs MI/R. |
糖尿病引起的以缺血性心脏病为代表的心血管并发症是导致糖尿病患者死亡的主要因素[11]。为了更好的模拟临床2型糖尿病模型,本研究以高糖高脂饲料喂食结合小剂量STZ腹腔注射促进胰岛β细胞凋亡,结果表现为血糖值升高,血清胰岛素水平不低,出现典型的胰岛素抵抗等2型糖尿病特征。
MI/R损伤是围术期面临的一种常见病理生理变化,如休克和心搏骤停后的心肺脑复苏、心肌梗死患者的溶栓治疗、动脉搭桥术后以及体外循环心内直视手术等均不可避免存在MI/R过程[12-13]。与非糖尿病患者相比较,糖尿病患者不仅MI/R损伤发病率高,且损伤程度更为严重[14]。目前,糖尿病加重MI/R损伤的机制尚不明确。Schneider等[15]通过对自发糖尿病大鼠MI/R模型的研究,表明与正常心肌的超微结构(肌节、空泡、线粒体)相比,糖尿病大鼠心肌更容易受到缺血再灌注损伤的影响。通过糖尿病大鼠MI/R损伤模型的建立,探寻损伤加重的机制,为减轻或防治临床糖尿病加重MI/ R损伤提供思路。本研究对2型糖尿病大鼠冠状动脉左前降支先结扎再灌注的方法建立心肌缺血再灌注模型,检测作为诊断心肌损伤“金标准”的cTnI及其它血清标志酶CK、LDH等,结果MI/R模型组cTnI、CK、LDH值明显升高,心肌细胞凋亡比例增加,凋亡蛋白cleaved caspase-3表达显著,出现明显的心肌梗死灶。上述指标符合糖尿病大鼠MI/R损伤的造模标准。
在急性MI/R状态中氧化应激发挥了显著的破坏作用[16-18]。当糖尿病患者MI/R损伤时,ROS爆发性产生[19],超过了机体捕获清除的能力,它攻击生物膜上的不饱和脂肪酸,发生脂质过氧化,从而造成膜流动性与离子通透性增加,破坏膜结构完整性,导致细胞损伤或死亡,最终造成MI/R损伤。亦有研究表明糖尿病患者本身就存在氧化应激,再灌注后产生的过量ROS又进一步加重了氧化应激,从而导致心肌损伤程度加重[20]。PARP-1是细胞内应激反应的枢纽(包括炎症反应、氧化应激、基因毒性反应、代谢等)[21]。PARP-1在多种生物功能上都扮演重要角色:包括DNA修复、基因表达、基因组稳态、细胞周期以及长期记忆[22-23]。PARP-1的活性在DNA损伤时被提高,催化受体蛋白的聚ADP核糖基化反应,参与DNA的修复。另一方面广泛的DNA损伤导致PARP-1过度活化从而消耗细胞内的NAD储备,降低NAD水平并通过氧化磷酸化抑制ATP的产生,造成细胞功能失调和坏死[24-25]。大约有超过90%的NAD包含在线粒体内,心肌组织中NAD的含量能用于评估PARP-1的活化程度[26]。因此PARP-1可能在MI/R损伤发病机制过程中发挥重要的作用。有研究表明抑制PARP-1活性可以部分逆转休克、高血压、心衰及缺血再灌注损伤的心肌内在功能[27]。MRL-45696作为新型的PARP-1特异性抑制剂,依靠去乙酰化酶通过触发线粒体展开蛋白反应提高线粒体呼吸链的功能,在糖尿病大鼠脑缺血再灌注损伤中发挥了保护性作用[28]。
而同样由于PARP-1过渡激活导致了糖尿病大鼠MI/R后损伤加重。本研究中使用MRL-45696对糖尿病大鼠MI/R损伤前预先喂食一周进行干预,结果显示,与MI/R组相比较,MRL组血清标志酶cTnI、CK、LDH和心肌细胞凋亡比例明显降低,同时作为能反映心肌细胞脂质过氧化程度与氧自由基活性的氧化代谢产物MDA[29]显著降低,而作为可对抗与阻断氧自由基对细胞损害的抗氧化酶SOD和NAD含量明显升高;作为检测DNA双链断裂损伤最敏感的检测指标γ-H2AX[30]和凋亡蛋白cleaved caspase3表达同时降低,PARP-1蛋白和其活化产物PAR蛋白表达均受到明显抑制,心肌梗死范围显著缩小。
综上所述,本研究表明糖尿病加重大鼠MI/R后DNA损伤,PARP-1及其下游产物PAR表达增加,MRL- 45696喂食治疗有效减弱了糖尿病大鼠MI/R损伤后心肌细胞PARP-1过度活化,从而避免细胞内NAD的消耗,避免ATP产生障碍,减轻了心肌细胞DNA的损伤,减少心肌细胞凋亡,缩小了心肌梗死灶。本研究不足之处表现为:(1)本研究未对早期和晚期2型糖尿病大鼠模型分别进行相关研究;(2)没有进一步阐明MRL- 45696的最低有效剂量。下一步在转基因小鼠上完善上述研究,进一步明确上述作用机制,得出更加科学的结论,为减轻糖尿病患者MI/R损伤提供思路。
| [1] |
Dieuzeide G, Puchulu F, Sanabria H, et al. Cardiovascular effects of new non-insulinic anti-diabetes drugs[J].
Medicina (B Aires), 2018, 78(3): 185-93.
|
| [2] |
方跃亭, 张军能, 张焕棕. 尿微量白蛋白与老年2型糖尿病合并缺血性心脏病关系的研究[J].
中国医药指南, 2016, 14(21): 112-3.
|
| [3] |
Lirk P, Verhamme C, Boeckh R, et al. Effects of early and late diabetic neuropathy on sciatic nerve block duration and neurotoxicity in Zucker diabetic fatty rats[J].
Br JAnaesth, 2015, 114(2): 319-26.
DOI: 10.1093/bja/aeu270. |
| [4] |
Stratton IM, Cull CA, Adler AI, et al. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75)[J].
Diabetologia, 2006, 49(8): 1761-9.
DOI: 10.1007/s00125-006-0297-1. |
| [5] |
Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes[J].
N Engl J Med, 2005, 353(25): 2643-53.
DOI: 10.1056/NEJMoa052187. |
| [6] |
Patel A, Macmahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes[J].
N Engl J Med, 2008, 358(24): 2560-72.
DOI: 10.1056/NEJMoa0802987. |
| [7] |
Liu M, Li Z, Chen GW, et al. AG-690/11026014, a novel PARP- 1 inhibitor, protects cardiomyocytes from AngⅡ-induced hypertrophy[J].
Mol Cell Endocrinol, 2014, 392(1/2): 14-22.
|
| [8] |
Pirinen E, Cantó C, Jo YS, et al. Pharmacological inhibition of poly (ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle[J].
Cell Metab, 2014, 19(6): 1034-41.
DOI: 10.1016/j.cmet.2014.04.002. |
| [9] |
熊年, 韦晟. 银杏内酯B对大鼠心肌缺血再灌注损伤的保护作用及机制研究[J].
中国现代应用药学, 2015, 32(3): 289-94.
|
| [10] |
Sugano M, Yamato H, Hayashi T, et al. High-fat diet in low-dosestreptozotocin-treated heminephrectomized rats induces all features of human type 2 diabetic nephropathy: a new rat model of diabetic nephropathy[J].
Nutr Metab Cardiovasc Dis, 2006, 16(7): 477-84.
DOI: 10.1016/j.numecd.2005.08.007. |
| [11] |
杨阳. 2型糖尿病患者踝肱指数与心血管疾病危险因素的相关性分析[J].
中国医学创新, 2012, 9(8): 145-6.
|
| [12] |
Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial[J].
Lancet, 2013, 382(9892): 597-604.
DOI: 10.1016/S0140-6736(13)61450-6. |
| [13] |
Devereaux PJ, Goldman L, Cook DJ, et al. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk[J].
CMAJ, 2005, 173(6): 627-34.
DOI: 10.1503/cmaj.050011. |
| [14] |
Buntaine AJ, Shah B, Lorin JD, et al. Revascularization strategies in patients with diabetes mellitus and acute coronary syndrome[J].
Curr Cardiol Rep, 2016, 18(8): 79.
DOI: 10.1007/s11886-016-0756-3. |
| [15] |
Schneider R, Welt K, Aust W, et al. Cardiac ischemia and reperfusion in spontaneously diabetic rats with and without application of EGb 761: Ⅱ. Interstitium and microvasculature[J].
Histol Histopathol, 2009, 24(5): 587-98.
|
| [16] |
Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation[J].
Nat Med, 2011, 17(11): 1391-401.
DOI: 10.1038/nm.2507. |
| [17] |
Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury[J].
Cardiovasc Res, 2006, 70(2): 181-90.
DOI: 10.1016/j.cardiores.2006.02.025. |
| [18] |
Xie N, Zhang W, Li J, et al. α-Linolenic acid intake attenuates myocardial ischemia/reperfusion injury through anti-inflammatory and anti-oxidative stress effects in diabetic but not normal rats[J].
Arch Med Res, 2011, 42(3): 171-81.
DOI: 10.1016/j.arcmed.2011.04.008. |
| [19] |
Ansley DM, Wang BH. Oxidative stress and myocardial injury in the diabetic heart[J].
J Pathol, 2013, 229(2): 232-41.
DOI: 10.1002/path.4113. |
| [20] |
Di Filippo C, Marfella R, Cuzzocrea S, et al. Hyperglycemia in streptozotocin- induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion[J].
Diabetes, 2005, 54(3): 803-10.
DOI: 10.2337/diabetes.54.3.803. |
| [21] |
Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1[J].
Genes Dev, 2012, 26(5): 417-32.
DOI: 10.1101/gad.183509.111. |
| [22] |
Rouleau M, Patel A, Hendzel MJ, et al. PARP inhibition: PARP1 and beyond[J].
Nat Rev Cancer, 2010, 10(4): 293-301.
DOI: 10.1038/nrc2812. |
| [23] |
Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer- binding, coregulation, and insulation[J].
Curr Opin Cell Biol, 2008, 20(3): 294-302.
DOI: 10.1016/j.ceb.2008.03.006. |
| [24] |
Bock FJ, Chang P. Stress response: PARP1 911[J].
Nat Chem Biol, 2015, 11(3): 179-80.
DOI: 10.1038/nchembio.1756. |
| [25] |
Meloche J, Pflieger A, Vaillancourt M, et al. Role for DNA damage signaling in pulmonary arterial hypertension[J].
Circulation, 2014, 129(7): 786-97.
DOI: 10.1161/CIRCULATIONAHA.113.006167. |
| [26] |
Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/ sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling[J].
Cell, 2013, 154(2): 430-41.
DOI: 10.1016/j.cell.2013.06.016. |
| [27] |
Liu M, Li Z, Chen GW, et al. AG-690/11026014, a novel PARP- 1 inhibitor, protects cardiomyocytes from AngⅡ-induced hypertrophy[J].
Mol Cell Endocrinol, 2014, 392(1-2): 14-22.
DOI: 10.1016/j.mce.2014.05.010. |
| [28] |
Giansanti V, Donà F, Tillhon M, et al. PARP inhibitors: new tools to protect from inflammation[J].
Biochem Pharmacol, 2010, 80(12): 1869-77.
DOI: 10.1016/j.bcp.2010.04.022. |
| [29] |
郭佳, 边云飞, 肖传实, 等. 脂联素通过增强糖尿病大鼠心肌缺血/再灌注损伤后心肌抗氧化能力减少细胞凋亡[J].
中国药理学通报, 2014, 30(5): 623-7.
|
| [30] |
Kuo LJ, Yang LX. Gamma-H2AX-a novel biomarker for DNA double-strand breaks[J].
In Vivo, 2008, 22(3): 305-9.
|
 2018, Vol. 38
2018, Vol. 38

