常用的植入骨材料有自体骨、同种异体骨和人工骨。由于自体骨移植物具有较难保存、易降解、易感染等诸多弊端,利用自体骨移植进行骨缺损修补在临床上的应用已经越来越少,对生物骨替代物的研究和开发则成为临床研究领域的新焦点[1]。颅骨缺损修补的材料甚多,而与人体骨胳成分最为相近的则为生物活性陶瓷材料羟基磷灰石(HA)和β-磷酸三钙(β-TCP)[2-3]。磷酸钙力学强度较好,但其脆性大、可塑性差的缺点使其很少被用作填充颅骨缺损的单一材料[4]。
BAM骨诱导人工骨(OICPC)是以化学合成的复合磷酸钙盐经特殊工艺制备而成的仿生多孔骨修复材料,具有优良的生物相容性、生物活性和生物安全性[5-6]。尽管BAM骨诱导人工骨拥有众多的优点,但其较大的生物学脆性使其在临床上很难成型[7]。颅骨修补材料的临床应用,既要保证适当的抗压强度,又要维持头颅外形的美观。BAM骨诱导人工骨由于脆性过大的原因无法通过直接填塞就能成型,故无法应用于诸如颅骨这种扁骨的修补过程中。有学者提出对BAM骨诱导人工骨进行增强可朔性、机械强度等方面的改进[8-11]。因此本研究提出了一种新的修补颅骨缺损的方法,以离体灭活多孔骨瓣作为支架,结合BAM骨诱导人工骨进行颅骨修补,这在该材料的应用方法中尚未见报道。通过离体灭活多孔骨瓣结合BAM骨诱导人工骨修复颅骨缺损的研究,从而解决BAM骨诱导人工骨在颅骨修复过程中不能成型的难题,为BAM骨诱导人工骨在颅骨修补术中的使用提供实验基础和理论依据。
1 材料和方法 1.1 实验对象及分组选择8周龄、体质量200~220 g的雄性Wistar大鼠72只,随机分为4组:骨缺损未修补组(对照组)、灭活自体骨瓣支架组(AB组)、BAM骨诱导人工骨材料组(BAM组)、灭活自体骨瓣支架结合BAM骨诱导人工骨材料组(BAM+AB组)。
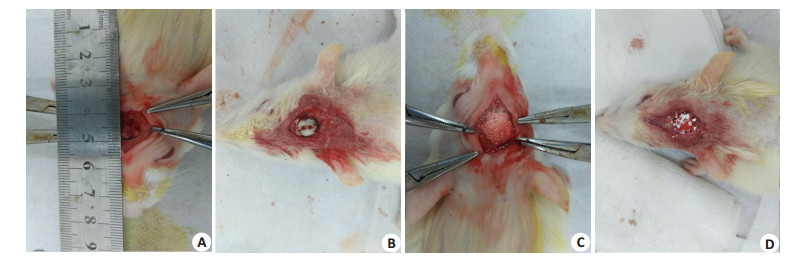
1.2 动物模型构建10%水合氯醛按l00 mg/kg(2 mL/200 g)经腹腔麻醉,手术区域碘伏消毒2遍。沿大鼠头颅中线(矢状缝)做一纵行皮肤切口,长约1.5 cm。充分显露大鼠矢状缝、双侧顶骨及部分额骨及枕骨,手术刀纵行切开顶骨骨膜,神经剥离子钝性分离骨膜,8 mm口径的环形切骨刀在大鼠顶骨正中位置预留痕迹,环形切骨刀后端连接骨钻,切取8 mm直径的颅骨。注意钻取过程中生理盐水持续滴注降温。牙科钻于骨瓣上钻取若干小孔,沸水中煮沸30 min,冷却消毒后备用。根据手术分组情况,于颅骨缺损处空缺、灭活自体骨瓣支架植入(AB组)、BAM骨诱导人工骨材料植入(BAM组)、BAM骨诱导人工骨材料+灭活自体骨瓣支架植入(BAM+AB组)(图 1)。术后1,2,3个月用断颈法分别处死大鼠,收集保存大鼠颅骨标本,行组织学染色及形态计量学分析。BAM骨诱导人工骨由四川大学生物材料工程研究中心提供。
|
图 1 大鼠颅骨骨缺损手术示意图 Figure 1 Surgery for skull defect repair in rats. A: Cut the skin and the bone flap. Caution should be taken to protect the dura. B: Implantation of inactivated bone flap in the defect; C: Bone defect repaired with BAM bone induced artificial bone; D: Bone defect repaired with inactivated bone flap+BAM bone-induced artificial bone. |
术后3个月腹腔注射过量水合氯醛处死大鼠,收集大鼠颅骨标本行影像学分析。颅骨样本取材后浸于4%多聚甲醛固定24 h后,进行micro-CT扫描三维重建后评估颅骨缺损处的骨愈合情况。Micro-CT三维重建使用ZKKSMCT-Sharp-Ⅲ scanner系统*广州中科恺盛生物有限公司)。
1.4 颅骨骨缺损修复情况的组织免疫学观察大鼠颅骨缺损样本浸泡于4%多聚甲醛24 h,EDTA甘油脱钙14 d,脱水后石蜡包埋,做5 μm厚切片,行HE染色、Masson染色及VEGF免疫组化染色。
1.5 统计学分析统计学分析采用IBM SPSS statistics 20统计软件分析处理。计量资料统计描述用均数±标准差表示。单独效应分析采用单因素方差分析法(One-Way ANOVA),方差不齐时使用Welch检验结果。两两比较方差齐时采用LSD法,方差不齐时使用Dunnett's T3法。P < 0.05表明差异有统计学意义。
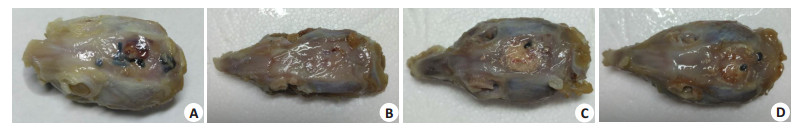
2 结果 2.1 离体灭活多孔骨瓣结合BAM骨诱导人工骨能更好恢复解剖原貌建模成功大鼠术区出现不同程度肿胀,术后1周症状消失,伤口愈合良好,未见红肿感染现象,体重无明显减轻,无局部和全身并发症发生。大鼠均存活至取材时间。建模术后3个月,BAM组和BAM+AB组骨瓣内均可见有纤维结缔组织长入,骨缺损表面被增厚的骨膜覆盖,与骨瓣结合紧密,植入骨瓣与颅骨骨性愈合,骨瓣表面可见与正常颅骨外观相似的新生骨。外观上,BAM组颅骨缺损表面凹凸不平,美观程度较差。而BAM+ AB组因为有离体骨瓣支撑,颅骨缺损愈合良好(图 2)。AB组和对照组则未见明显成骨,只有骨缺损边缘少量骨形成,缺损处以纤维结缔组织填充。
|
图 2 大鼠颅骨大体标本 Figure 2 Observation of 8 mm rat skull defects 3 months after surgery. A: Negative control (untreated defects); B: Inactivated porous bone flap; C: BAM bone-induced artificial bone material group; D: Inactivated porous bone flap +BAM bone-induced artificial bone material group. |
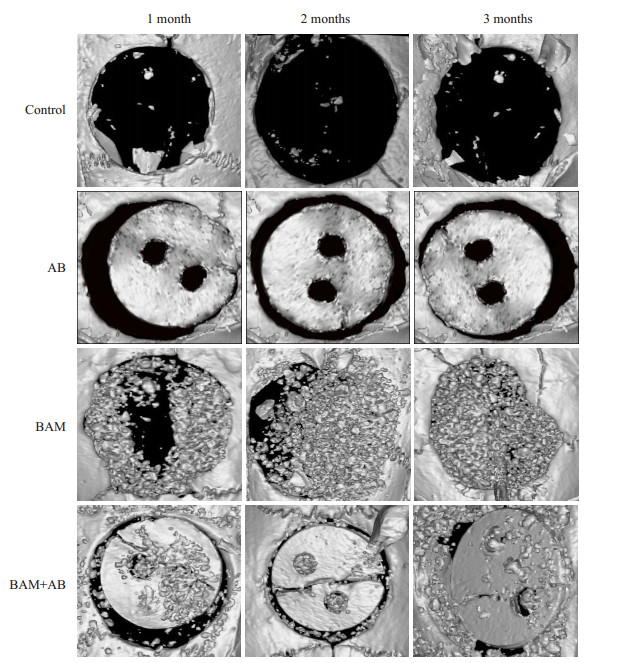
建模术后3个月,micro-CT扫描三维重建后观察颅骨骨缺损处新骨形成(图 3)。新骨形成最明显的是BAM+AB组,同时我们观察到BAM组可见不透X线的组织广泛填充于颅骨缺损处,而对照组几乎没有新生骨生成。进一步进行新骨形成定量分析,BAM组和BAM+AB组中BMD(骨密度)分别为446.15±8.27 mg/cm3和434.52 ± 6.62 mg/cm3,明显高于对照组的244.94 ± 5.61 mg/cm3(P < 0.01)和AB组的278.60±6.68 mg/cm3 (P < 0.001)。所测得的Tb.Th(骨小梁厚度)结果与BMD结果也表现出同样的趋势。
|
图 3 micro-CT扫描三维重建 Figure 3 3D reconstructed micro-CT images of 8-mm rat skull defects at1, 2, and 3 months after surgery. Control: Negative control (untreated defects). AB: Inactivated porous bone flap; BAM: BAM bone-induced artificial bone material group; BAM + AB: Inactivated porous bone flap+BAM bone-induced artificial bone material group. |
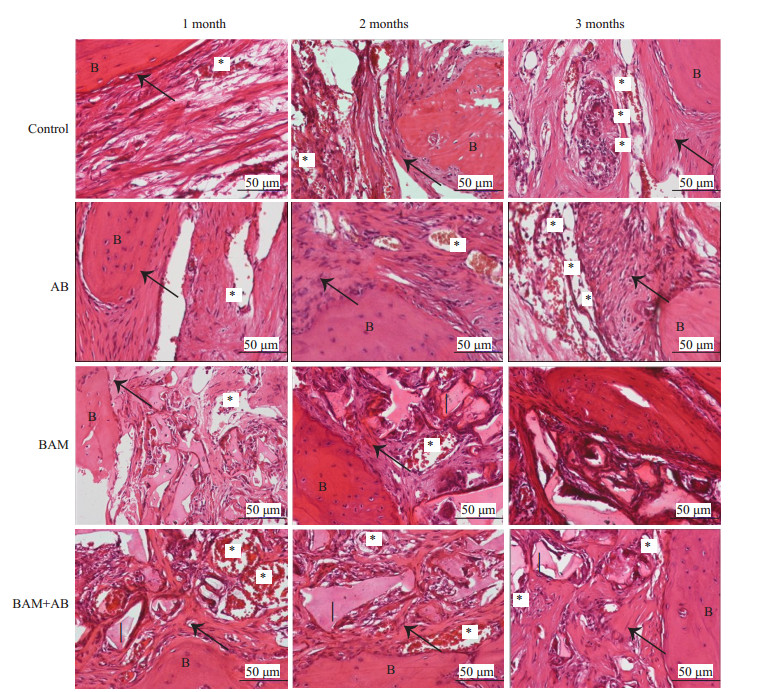
建模3个月后(图 4),BAM组和BAM+AB组骨缺损边缘新生骨小梁向骨瓣生长,骨瓣内大量编织骨生成,未降解的生物材料被纤维组织包绕,材料的降解速度与新生组织生长速度较一致,材料周围大量新生血管长入,周围未见炎性细胞浸润。对照组及AB组骨缺损边缘新生骨形成,骨缺损大量纤维组织填充,纤维组织内少量新生血管长入。血管计数结果形态计量学分析示,对照组几乎没有新生血管形成,而BAM+AB组和BAM组均有多量的新生血管形成成,两组无明显差异,分别为24.30±2.17个、22.40±2.72个,明显高于对照组的13.60±3.71个(P < 0.01)与AB组的15.40±3.48个(P < 0.001),提示离体灭活多孔骨瓣+BAM骨诱导人工骨材料组在诱导颅骨缺损修复的同时可以有效的刺激血管再生。
|
图 4 HE染色结果 Figure 4 HE staining showing the new bone (arrows) and blood vessel (asterisks) formation in the defects at 1, 2, and 3 months. Control: Negative control (untreated defects). AB: Inactivated porous bone flap. BAM: BAM bone- induced artificial bone material group. BAM + AB: Inactivated porous bone flap + BAM bone induced artificial bone material group. I: implant. B: bone. |
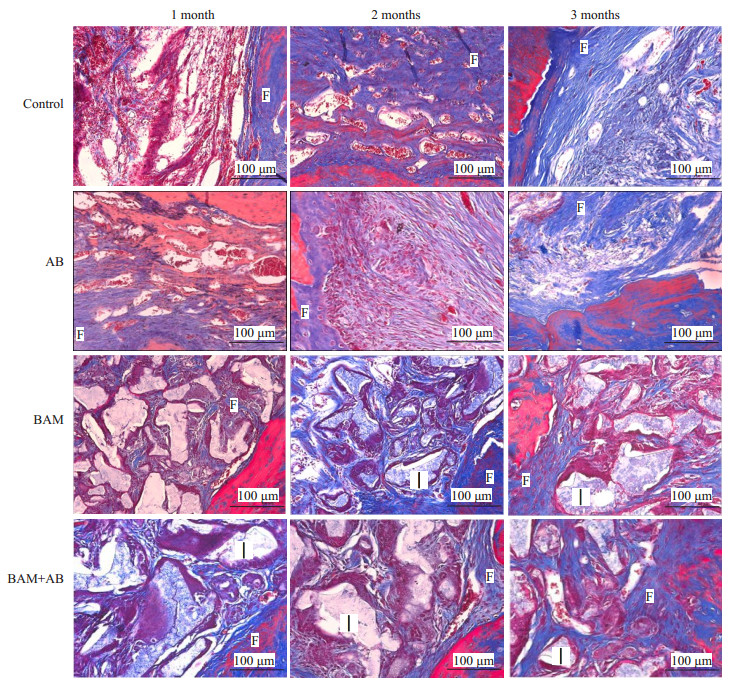
通过Masson染色可见(图 5),未治疗(对照组),AB组骨缺损边缘有蓝色的新生骨组织生长,骨缺损内部被蓝色纤维组织填充。单纯植入BAM骨诱导人工骨材料组(BAM)和离体灭活多孔骨瓣+BAM骨诱导人工骨材料组(AB+BAM)组可见大量淡红色未降解的材料被蓝色纤维组织包绕,材料周围纤维组织内大量新生血管长入,材料周围未见炎性细胞浸润,骨缺损边缘大量蓝色或红色新生骨组织形成,由骨缺损边缘向骨缺损内部生长。
|
图 5 Masson染色结果 Figure 5 Masson staining showing new bone and blood vessel formation in BAM and BAM+AB groups at 1, 2, and 3 months. Control: Negative control (untreated defects). AB: Inactivated porous bone flap. BAM: BAM bone-induced artificial bone material group. BAM +AB: Inactivated porous bone flap +BAM bone- induced artificial bone material group. I: Implant. F: Fibrous connective tissue. |
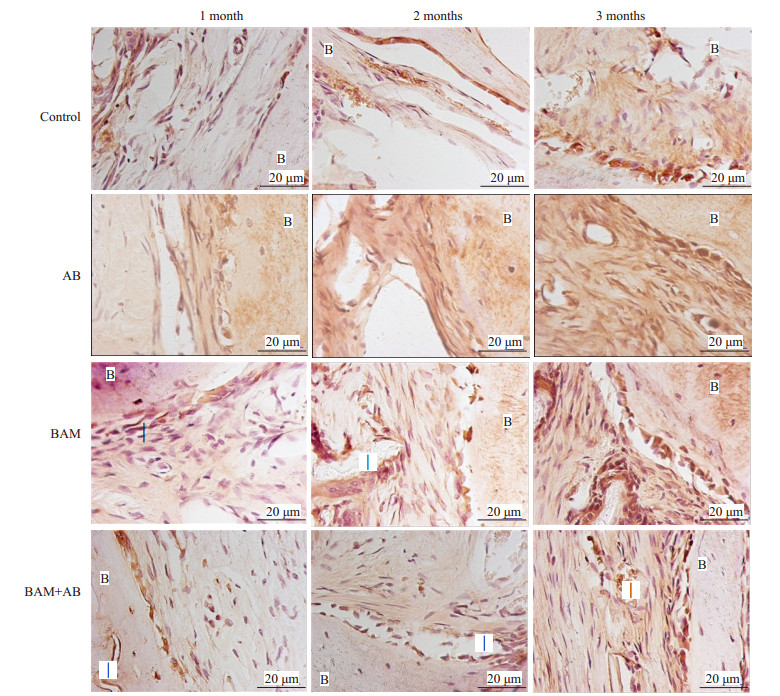
从免疫组化结果中我们可以看出,未治疗(CON组)及离体灭活多孔骨瓣组(AB组)成纤维细胞和炎性细胞明显少于治疗组,同时血管内皮生长因子(VEGF)免疫组化染色也表现为低表达。然而在BAM+AB组和单纯BAM组治疗中,新生骨小梁可见大量VEGF阳性表达的成熟成骨细胞密集分布,提示成骨细胞活动十分活跃。明显地,在BAM组和BAM+AB组中,可见VEGF免疫组化染色呈现髙表达水平(图 6)。
|
图 6 免疫组化染色结果 Figure 6 Immunohistochemical staining showing new bone and blood vessel formation in BAM and BAM+AB groups at1, 2, and 3 months. Control: Negative control (untreated defects). AB: Inactivated porous bone flap. BAM: BAM bone-induced artificial bone material group. BAM+AB: Inactivated porous bone flap+BAM bone induced artificial bone material group I: Implant. B: Bone. |
本研究中,我们以BAM骨诱导人工骨为研究对象,探讨其在颅骨缺损中的骨修复作用,在没有其他异体材料复合的前提下,利用离体灭活多孔骨瓣与BAM骨诱导人工骨相结合,结果表明颅骨缺损修复后形态美观,颅骨愈合良好。
BAM骨诱导人工骨较少应用于颅骨缺损修补中,一般在骨科应用比较广泛。檀臻炜[12]等应用锁定钢板结合生物活性陶瓷(BAM)骨诱导人工骨植骨治疗胫骨平台粉碎性骨折,对26例胫骨平台粉碎性骨折均采用锁定钢板内固定并植入BAM骨诱导人工骨填充骨折复位后的骨缺损。随访9~12月,术后疗效按Rasmussen评分标准评价优良率达92.3%。李康养[13]等人将BAM骨诱导人工骨与自体骨髓细胞植入经髓芯减压的股骨头内。全部病例随访12~24月,按髋关节Harris评分,总优良率为86.11%。X线片显示股骨头部位有较好的骨修复,MRI显示病灶区信号无明显降低。本研究以离体灭活多孔骨瓣作支架,结合BAM骨诱导人工骨,探讨其在颅骨缺损中的骨修复作用,结果显示BAM骨诱导人工骨与灭活自体多孔骨瓣相结合后,在完全没有种子细胞与生物因子附着的情况下不仅可以促进颅骨愈合,而且避免了材料脆性过大不能成型的弊端,可使颅骨外观恢复原貌,是颅骨缺损修补切实可行的方法。
本研究结果表明,BAM骨诱导人工骨生物材料具有良好的骨再生诱导性,可有效刺激颅骨缺损时新生骨的再生并最终促进颅骨愈合,灭活多孔骨瓣的支撑作用则可达到颅骨外观重建要求。肉眼观可见BAM组由于材料脆性太大而未能成型,颅骨缺损表面凹凸不平,美观程度差。而BAM+AB组因为有离体骨瓣支撑,颅骨缺损愈合良好。术后3个月,BAM组和BAM+AB组的BMD值均显著增高(P < 0.05),反映了其可在一定程度诱导局部钙磷水平增高,可能与成骨细胞局部形成而钙磷沉积有关[14-26]。同时,我们发现,Tb.Th值结果与BMD值结果表现出同样的趋势。根据HE染色结果表明,缺损区生长的主要是纤维结缔组织和脂肪细胞,软组织的增长阻碍了成骨细胞向缺损区内迁移生长,进而影响了新生骨的形成[27-29]。BAM+离体灭活自体骨不但为新生骨提供支架支持,而且对于颅骨缺损处软组织的生长起到了阻碍作用。与此同时,BAM组和BAM+AB组可见多量的新生血管生成,显著高于对照组(P < 0.05)。通过进一步观察发现,Masson染色、VEGF免疫组织化学染色结果表现与HE染色结果相一致。据此可推断,BAM骨诱导人工骨生物材料与灭活多孔骨瓣结合后具有促进骨和血管再生的能力。
由于聚酯类具有良好的韧性和延展性,这或是降低支架脆性、增加可塑性的不错选择。数字化3D塑形技术也可以成为材料塑形的新的尝试[30]。在下一步研究中,我们将在材料中附着种子细胞与生物因子,以进一步评估作为骨移植物的BAM骨诱导人工骨生物材料骨学诱导性,为BAM骨诱导人工骨生物材料的临床应用打下基础。
综上所述,应用离体灭活自体骨瓣结合BAM骨诱导人工骨复合材料修补颅骨缺损,可有效促进颅骨愈合,且避免了材料脆性过大不能成型的弊端,使颅骨外观恢复原貌,是颅骨缺损修复有效可行的方法。
| [1] |
Yadla S, Campbell PG, Chitale R, et al. Effect of early surgery, material, and method of ap preservation on cranioplasty infections: a systematic review[J].
Neurosurgery, 2011, 68: 1124-30.
DOI: 10.1227/NEU.0b013e31820a5470. |
| [2] |
Kwarcinski J, Boughton P, Ruys A, et al. Cranioplasty and craniofacial reconstruction: a review of implant material, manufacturing method and infection risk[J].
Appl Sci, 2017, 7(3): 1-17.
|
| [3] |
Junior ACA, Hamamoto Filho PT, Neto AAP, et al. Biomaterials for reconstruction of cranial defects[J].
Arq Bras Neurocir, 2016, 35(4): 291-5.
DOI: 10.1055/s-0036-1592411. |
| [4] |
Bhattacharya S, Khanna V, Kohli R. Cleft lip the historical perspective[J].
Indian J Plast Surg, 2009, 42(Suppl): S4-S8.
|
| [5] |
Yang Z, Yuan H, Tong W, et al. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals[J].
Biomaterials, 1996, 17: 2131.
DOI: 10.1016/0142-9612(96)00044-0. |
| [6] |
Cong Z, Jian W, Z Xingdong. Repairing bone defects using bioactive ceramics: A clinical reports of 40 cases[J].
J Porous Media, 2001, 4(1): 89.
|
| [7] |
Costantino PD, Chaplin JM, Wolpoe ME, et al. Applications of fastsetting hydroxy- apatite cement: cranioplasty[J].
Otolaryngol Head Neck Surg, 2000, 123: 409-12.
DOI: 10.1067/mhn.2000.107679. |
| [8] |
Kao CT, Huang TH, Chen YJ, et al. Using calcium silicate to regulate the physicochemical and biological properties when using beta-tricalcium phosphate as bone cement[J].
Mater Sci Eng C Mater Biol Appl, 2014, 43: 126-34.
DOI: 10.1016/j.msec.2014.06.030. |
| [9] |
Lee EU, Lim HC, Hong JY, et al. Bone regenerative efficacy of biphasic calcium phosphate collagen composite as a carrier of rhBMP-2[J].
Clin Oral Implants Res, 2016, 27(11): e91-9.
DOI: 10.1111/clr.12568. |
| [10] |
Charles-Harris M, Koch MA, Navarro M, et al. A PLA/calcium phosphate degradable composite material for bone tissue engineering: an in vitro study[J].
J Mater Sci Mater Med, 2008, 19(4): 1503-13.
DOI: 10.1007/s10856-008-3390-9. |
| [11] |
Zhao X, Lui YS, Choo CK, et al. Calcium phosphate coated KeratinPCL scaffolds for potential bone tissue regeneration[J].
Mater Sci Eng C Mater Biol Appl, 2015, 49: 746-53.
DOI: 10.1016/j.msec.2015.01.084. |
| [12] |
檀臻炜, 姚一民, 娄延举, 等. 锁定钢板结合BAM人工骨治疗胫骨平台 粉碎性骨折[J].
中国骨与关节损伤杂志, 2012, 27(10): 940-1.
|
| [13] |
李康养, 黄永翔, 李艳玲, 等. 镜下髓芯减压BAM骨诱导人工骨治疗 股骨头坏死的临床研究[J].
海南医学院学报, 2012, 18(11): 1557-1559.
|
| [14] |
MacMillan AK, Lamberti FV Moulton JN, et al. Similar healthy osteoclast and osteoblast activity on nanocrystalline hydroxyapatite and nanoparticles of tri-calcium phosphate[J].
Int J Nanomedicine, 2014, 9: 5627-37.
|
| [15] |
Choy J, Albers CE, Siebenrock KA, et al. Incorporation of RANKL promotes osteoclast formation and osteoclast activity on β-TCP ceramics[J].
Bone, 2014, 69: 80-8.
DOI: 10.1016/j.bone.2014.09.013. |
| [16] |
Bernhardt A, Dittrich R, Lode A, et al. Nanocrystalline spherical hydroxyapatite granules for bone repair: in vitro evaluation with osteoblast-like cells and osteoclasts[J].
J Mater Sci Mater Med, 2013, 24(7): 1755-66.
DOI: 10.1007/s10856-013-4933-2. |
| [17] |
Udagawa N, Takahashi N, Jimi E, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colonystimulating factor: receptor activator of NF-kappa B ligand[J].
Bone, 1999, 25(5): 517-23.
DOI: 10.1016/S8756-3282(99)00210-0. |
| [18] |
Banava S, Houshyari M, Safaie T. The effect of casein phosphopeptide amorphous calcium phosphate fluoride paste (CPPACPF) on oral and salivary conditions of patients undergoing chemotherapy: A randomized controlled clinical trial[J].
Electron Physician, 2015, 7(7): 1535-41.
DOI: 10.19082/1535. |
| [19] |
Xie Y, Rustom LE, McDermott AM, et al. Net shape fabrication of calcium phosphate scaffolds with multiple material domains[J].
Biofabrication, 2016, 8(1): 015005.
DOI: 10.1088/1758-5090/8/1/015005. |
| [20] |
AbdulQader ST, Rahman IA, Thirumulu KP, et al. Effect of biphasic calcium phosphate scaffold porosities on odontogenic differentiation of human dental pulp cells[J].
J Biomater Appl, 2016, 30(9): 1300-11.
DOI: 10.1177/0885328215625759. |
| [21] |
Bolander J, Ji W, Geris L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells[J].
Eur Cell Mater, 2016, 30: 11-25.
|
| [22] |
Kovar FM, Endler G, Wagner OF, et al. Basal elevated serum calcium phosphate product as an independent risk factor for mortality in patients with fractures of the proximal femur-A 20 year observation study[J].
Injury, 2016, 47(3): 728-32.
DOI: 10.1016/j.injury.2015.11.033. |
| [23] |
Zhang HX, Zhang XP, Xiao GY, et al. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head[J].
Mater Sci Eng C Mater Biol Appl, 2016, 60: 298-307.
DOI: 10.1016/j.msec.2015.11.055. |
| [24] |
Zheng J, Xiao Y, Gong T, et al. Fabrication and characterization of a novel carbon fiber-reinforced calcium phosphate silicate bone cement with potential osteo-inductivity[J].
Biomed Mater, 2015, 11(1): 015003.
DOI: 10.1088/1748-6041/11/1/015003. |
| [25] |
Frede A, Neuhaus B, Klopfleisch R, et al. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo[J].
J Control Release, 2015, 222: 86-96.
|
| [26] |
Wen B, Li Z, Nie R, et al. Influence of biphasic calcium phosphate surfaces coated with enamel matrix derivative on vertical bone growth in an extra-oral rabbit model[J].
Clin Oral Implants Res, 2016, 27(10): 1297-1304.
DOI: 10.1111/clr.2016.27.issue-10. |
| [27] |
Dahlin C, Alberius P, Linde A. Osteopromotion for cranioplasty. An experimental study in rats using a membrane technique[J].
J Neurosurg, 1991, 74(3): 487-91.
DOI: 10.3171/jns.1991.74.3.0487. |
| [28] |
Mardas N, Kostopoulos L, Karring T. Bone and suture regeneration in calvarial defects by e-PTFE-membranes and demineralized bone matrix and the impact on calvarial growth: an experimental study in the rat[J].
J Craniofac Surg, 2002, 13(3): 453-62.
DOI: 10.1097/00001665-200205000-00017. |
| [29] |
Donos N, Lang NP, Karoussis IK, et al. Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects[J].
Clin Oral Implants Res, 2004, 15(1): 101-11.
DOI: 10.1111/clr.2004.15.issue-1. |
| [30] |
Przekora A, Ginalska G. Enhanced differentiation of osteoblastic cells on novel chitosan/β-1, 3-glucan/bioceramic scaffolds for bone tissue regeneration[J].
Biomed Mater, 2015, 10(1): 015009.
DOI: 10.1088/1748-6041/10/1/015009. |
 2018, Vol. 38
2018, Vol. 38

