2. 南方医科大学南方医院健康管理科,广东 广州 510515;
3. 华南师范大学体育科学学院,广东 广州 510631
2. Department of Health Management, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China;
3. College of Sports Science and Physical Education, South China Normal University, Guangzhou 510631, China
氧化应激是新陈代谢生成的活性氧(ROS)与机体的抗氧化系统之间失去平衡所导致的一系列应激反应。众所周知,适宜强度的运动可以延缓肌肉损伤及老化[1-2],而长时间力竭运动导致机体ROS水平急剧增加,加速肌肉的疲劳损伤及老化[3],严重影响运动员的成绩。从ROS的角度出发,通过调控ROS缓解氧化损伤可能是依赖运动处方进行抗疲劳损伤的重要方法之一,其中的机制值得我们去探讨。5′-磷酸腺苷活化蛋白激酶(AMPK)及过氧化物酶体增殖物激活受体辅助激活因子(PGC1),对于细胞内的氧化应激水平具有重要的调节效应[4-6],能减少活性氧自由基的生成,改善细胞的生存环境[7-8]。
运动训练可通过活化多种转录因子,增强肌肉代谢、提高运动能力[9]。AMPK是一种由催化亚基α、β和γ三个亚基组成的异源三聚体蛋白。运动过程中,ROS引起AMPK异源三聚体的结构异变[10],但不同ROS水平导致AMPK的活化位点目前没有定论。AMPKα可通过亚基Thr172和Ser485磷酸化发挥调节作用,目前研究最多的是Thr172[11-12],而关于Ser485的研究相对较少[13-14]。多数研究支持,骨骼肌中ROS的上升导致AMPKThr172磷酸化而激活AMPK[15-17],而AMPK-Ser485拮抗AMPK-Thr172[18-19]。也有研究显示,AMPK-Ser485与Thr172变化一致,其活性的调节作用取决于实验环境和组织[11]。因此,不同运动时间后,ROS与AMPKThr172及AMPK-Ser485的关系仍值得研究。
本文基于AMPKα的Thr172和Ser485,研究不同时间电刺激模拟不同强度运动对C2C12肌管线粒体氧化应激水平的影响,并进一步探讨AMPK信号通路在不同运动强度过程中所发挥的作用,以期对后续的运动处方开具提供更有力的分子靶标。C2C12细胞是小鼠骨骼肌肌母细胞株,分化的C2C12肌管可完成与在体骨骼肌细胞同样的收缩[20],同时避免了在体实验中其他器官、组织、细胞和激素的影响,能更实时的研究不同运动刺激对骨骼肌线粒体功能的影响,为运动损伤及恢复提供理论依据。
1 材料和方法 1.1 研究材料C2C12细胞(南方医科大学解剖教研室);DMSO、DEPC、L-谷氨酰胺(sigma);4-羟乙基哌嗪乙磺酸(HEPES,MBCHEM公司);胎牛血清(四季青);DMEM培养基、马血清(GIBCO);MDA、SOD试剂盒(建成);AMPK、AMPK-Ser485、AMPK-Thr172、p-ULK(CST),PGC1(abcam);四甲基罗丹明甲酯TMRM染料(哈灵)。
1.2 电刺激方案及分组C2C12细胞常规培养,取生长状态好的细胞传代于35 cm的6孔板培养皿中,加入含10%胎牛血清的生长培养基培养,待细胞铺满皿底面积的90%以上时,加入含2%马血清的分化培养基分化。采用GRASS S48 Stimulator型电刺激器电刺激共分化7 d的C2C12肌管,强度为15 V,30 ms,3 Hz,电刺激时间为60、120、180 min。实验共4组,对照组(Con);电刺激60 min组(E60),120 min(E120)和180 min(E180)。
1.3 MDA和ROS检测 1.3.1 MDA测量细胞收集后,按照试剂盒说明书加试剂,旋涡混匀器混匀。管口用保鲜膜扎紧,用针头刺一小孔,95 ℃水浴40 min,取出后流水冷却,3500 r/min离心10 min。移取200 μL至96T板中,532 nm处测定吸光度(A)。
1.3.2 ROS检测细胞收集后悬浮于稀释好的DCFHDA(10 μmoL/L)中,37 ℃细胞培养箱内孵育20 min。每隔3~5 min颠倒混匀一下,使探针和细胞充分接触。用无血清细胞培养液洗涤细胞3次,以充分去除未进入细胞内的DCFH-DA。使用488 nm激发波长,525 nm发射波长,荧光酶标仪实时或逐时间点检测刺激前后荧光的强弱。
1.4 免疫蛋白印迹取处理后的6孔板,冰PBS冲洗2遍,每孔加入150μL的裂解抽提液比例,混合并匀浆。取上清2~4 μL,进行蛋白定量。取蛋白样品,加入上样缓冲液混匀,煮沸10 min后,上样,蛋白样品经8%或10%的聚丙烯酰胺凝胶电泳分离后,运用湿转法将凝胶上的蛋白条带转移至聚偏二氟乙烯(PVDF)膜。用3%的牛血清白蛋白(BSA)封闭PVDF膜,置于脱色摇床上,室温封闭2 h。加入相应的一抗,4 ℃孵育过夜。所用抗体分别为AMPK、AMPKSer485、AMPK-Thr172、PGC1;TBST冲洗,二抗敷育2 h,TBST冲洗,常规显色曝光。
1.5 流式细胞仪测定肌管线粒体ROS按照线粒体提取试剂盒说明提取线粒体,加入荧光探针10 μmol DCFH-DA避光染色30 min;11 000 g离心,10 min,4 ℃,弃上清;翘液清洗2遍,每次11 000 g离心,10 min,4 ℃,弃上清;加入翘液重悬,上机检测线粒体ROS。
1.6 流式细胞仪测定线粒体膜电位加入荧光探针为100 nmol TMRM,避光染色30 min;11 000 g离心,10 min,4 ℃,弃上清;翘液清洗2遍,每次11 000 g离心,10 min,4 ℃,弃上清;加入翘液重悬,上机检测线粒体膜电位。
1.7 数据统计处理研究数据用SPSS 16.0统计软件进行统计学处理,各数据以均数±标准差表示,并用单因素方差分析进行各组间的比较。P < 0.05为差异有统计学意义。
2 结果 2.1 电刺激对C2C12肌管形态的影响分化7 d的C2C12肌管作为电刺激对象,此时的肌管已分化完全,给予外源性刺激后具有在体性质的收缩能力。不同时间的电刺激后,10×的每组图片中均含有大量C2C12肌管;40×放大后,可清晰看到骨骼肌肌管的精细形态,但肉眼无法辨别其显著差异(图 1)。
|
图 1 电刺激对C2C12肌管形态的影响 Figure 1 Effects of electrical stimulation on C2C12 tube phenotypes. |
分化7 d的C2C12肌管给予一次模拟在体运动模式的电刺激,电刺激时间为60 min,120 min和180 min,分别模拟在体中等时间、长时间及力竭运动模式。收集各组肌管后,分别检测MDA和ROS水平,评价氧化应激水平。电刺激60 min ROS及MDA上升(P < 0.05),电刺激120 min和180 min ROS及MDA亦上升(P < 0.01) (表 1)。
| 表 1 电刺激对C2C12肌管MDA和ROS影响 Table 1 Effect of electrical stimulations on MDA and ROS in C2C12 myotubes |
电刺激60 min线粒体ROS稍有上升,但无显著差异(P>0.05);电刺激120 min的情况下线粒体ROS显著上升(P < 0.05),电刺激180 min的情况下线粒体ROS稍有上升(图 2)。
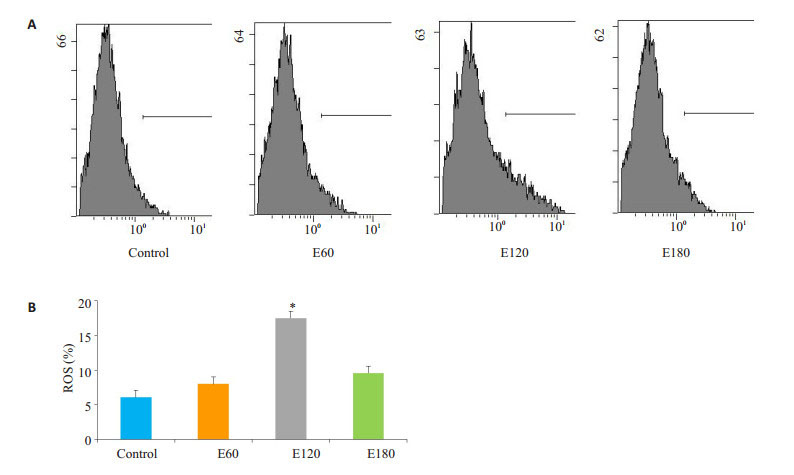
|
图 2 不同时间电刺激对C2C12肌管线粒体ROS影响 Figure 2 Effect of electrical stimulation on ROS in C2C12 myotubes (*P < 0.05 vs Control). A: Flow cytometry of DCFH; B: Quantification of the results. |
电刺激60 min线粒体膜电位稍有下降,但无显著差异(P>0.05);电刺激120 min的情况下线粒体膜电位下降(P < 0.05),电刺激180 min的情况下线粒体膜电位亦下降(P < 0.01,图 3)。
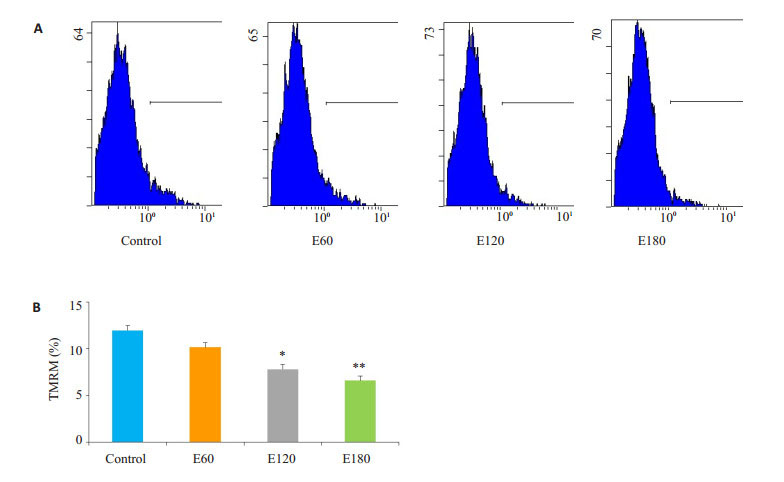
|
图 3 不同时间电刺激对C2C12肌管线粒体膜电位影响 Figure 3 Effect of electrical stimulation on TMRM in C2C12 myotubes (*P < 0.05; **P < 0.01 vs Control). A: Flow cytometry of the TMRM; B: Quantification of the results. |
电刺激60 min和120 min可以显著激活AMPKSer485(P < 0.05),而AMPK-Thr172仅在电刺激60 min显著活化(P < 0.01),PGC1在电刺激120 min显著上升(P < 0.01,图 4)。
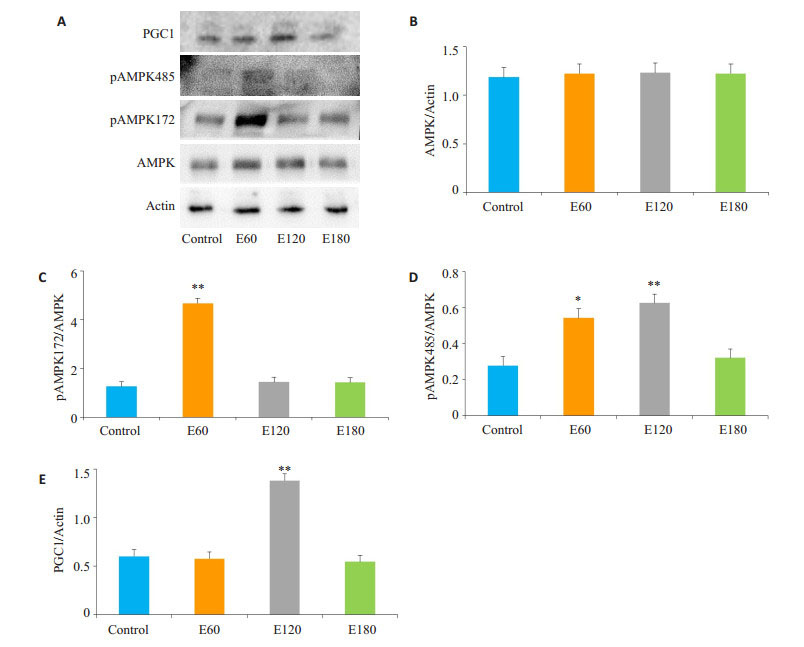
|
图 4 不同时间电刺激后C2C12肌管AMPK表达 Figure 4 Expression of AMPK proteins in C2C12 myotube after electrical stimulations (*P < 0.05; **P < 0.01 vs Control). A: Immunoblotting of the related proteins; B-E: Quantification of the results. |
众所周知,不同的运动方案引发不同的细胞和组织表型变化[21-23]。耐力运动的特点是高摄氧量、低收缩力和线粒体依赖功能,运动可刺激骨骼肌线粒体生物发生,促进线粒体融合与分裂[24-25]。此外,最近研究表明肌肉是运动中产生自由基的重要来源[26-27],因此耐力运动一定程度上提高了骨骼肌的氧化能力和线粒体功能[28-30]。本研究发现,随着电刺激时间的延长,虽然肌管的形态没有显著变化,但是肌管MDA、ROS和线粒体ROS都有上升,线粒体膜电位逐渐下降。表明本实验的电刺激强度模拟的是有氧耐力运动,而且成功导致运动氧化应激和线粒体功能下降。但是,不同的电刺激时间导致的运动氧化应激和线粒体功能的变化不同,因此需要开展其机制研究。
我们的实验结果显示,电刺激60 min,AMPKSer485显著上升,AMPK-Thr172极显著上升;电刺激120 min,只有AMPK-Ser485极显著上升,电刺激180 min,两者均无变化。分析结果可知,首先,中等时间电刺激后,虽然两个AMPK两个位点都被磷酸化,但AMPKThr172磷酸化占绝对优势,此时AMPK处于活化状态。因此,C2C12肌管电刺激60 min后,即使ROS造成了显著的氧化应激,但线粒体膜电位虽有下降,并无显著差异。其次,中长时间电刺激后,AMPK-Ser485磷酸化占绝对优势,AMPK活化受到抑制,此时ROS造成的氧化应激引起明显的线粒体膜损伤。最后,超长时间电刺激后氧化应激水平较高,肌管损伤非常严重,AMPK两个位点的磷酸化均未显著上升。
综上所述,不同时间的电刺激后导致肌管不同程度的氧化应激,且随时间延长,氧化应激程度越强烈,线粒体损伤越严重。不同时间电刺激引起的氧化应激由AMPK不同位点的磷酸化调控,AMPK-Thr172调控中长时间电刺激引起的氧化应激,而AMPK-Ser485及PGC1调控超长时间电刺激引起的线粒体氧化应激。本文研究了不同强度的运动训练对ROS及AMPK信号通路的影响,探索最新的分子靶点,为调控骨骼肌细胞稳态及运动后肌肉疲劳损伤及老化。
| [1] |
Berlin K, Kruger T, Klenosky DB. A mixed-methods investigation of successful aging among older women engaged in sports- based versus exercise-based leisure time physical activities[J].
J Women Aging, 2018, 30(1): 27-37.
DOI: 10.1080/08952841.2016.1259439. |
| [2] |
Seo DY, Lee SR, Kim N, et al. Age-related changes in skeletal muscle mitochondria: the role of exercise[J].
Integr Med Res, 2016, 5(3): 182-6.
DOI: 10.1016/j.imr.2016.07.003. |
| [3] |
Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, et al. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats[J].
Dis Model Mech, 2016, 9(10): 1221-9.
DOI: 10.1242/dmm.026591. |
| [4] |
Nylén C, Aoi W, Abdelmoez AM, et al. IL6 and LIF mRNA expression in skeletal muscle is regulated by AMPK and the transcription factors NFYC, ZBTB14 and SP1[J].
Am J Physiol Endocrinol Metab, 2018, [Epub ahead of print].
|
| [5] |
Lee SG, Wu HM, Lee CG, et al. Binge alcohol intake after hypergravity stress sustainably decreases AMPK and transcription factors necessary for hepatocyte survival[J].
Alcohol Clin Exp Res, 2017, 41(1): 76-86.
DOI: 10.1111/acer.13265. |
| [6] |
Chang HW, Pisano S, Chaturbedi A, et al. Transcription factors CEP- 1/p53 and CEH-23 collaborate with AAK-2/AMPK to modulate longevity in Caenorhabditis elegans[J].
Aging Cell, 2017, 16(4): 814-24.
DOI: 10.1111/acel.2017.16.issue-4. |
| [7] |
Lo VF, Carnio S, Vainshtein A, et al. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity[J].
Autophagy, 2014, 10(11): 1883-94.
DOI: 10.4161/auto.32154. |
| [8] |
Shi L, Zhang T, Zhou Y, et al. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPKPGC-1α-Sirt3 signaling pathway[J].
Endocrine, 2015, 50(2): 378-89.
DOI: 10.1007/s12020-015-0599-5. |
| [9] |
Close GL, Hamilton DL, Philp A, et al. New strategies in sport nutrition to increase exercise performance[J].
Free Radic Biol Med, 2016, 98: 144-58.
DOI: 10.1016/j.freeradbiomed.2016.01.016. |
| [10] |
Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPKmediated signal transduction beyond energetic clues[J].
J Cell Sci, 2012, 125(Pt 9): 2115-25.
|
| [11] |
Kim JE, Song SE, Kim YW, et al. Adiponectin inhibits palmitateinduced apoptosis through suppression of reactive Oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase[J].
J Endocrinol, 2010, 207(1): 35-44.
DOI: 10.1677/JOE-10-0093. |
| [12] |
Ohsaka Y, Nishino H, Nomura Y. Adipose cells induce phospho-Thr- 172 AMPK production by epinephrine or CL316243 in mouse 3T3- L1 adipocytes or MAPK activation and G protein-associated PI3K responses induced by CL316243 or Aluminum fluoride in rat white adipocytes[J].
Folia Biol (Praha), 2014, 60(4): 168-79.
|
| [13] |
Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491[J].
J Biol Chem, 2006, 281(9): 5335-40.
DOI: 10.1074/jbc.M506850200. |
| [14] |
Kim H, Park M, Lee SK, et al. Phosphorylation of hypothalamic AMPK on serine(485/491) related to sustained weight loss by alphalipoic acid in mice treated with olanzapine[J].
Psychopharmacology (Berl), 2014, 231(20): 4059-69.
DOI: 10.1007/s00213-014-3540-3. |
| [15] |
Morales-Alamo D, Ponce-González JG, Guadalupe-Grau A, et al. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKα phosphorylation, in response to sprint exercise in severe acute hypoxia in humans[J].
J Appl Physiol (1985), 2012, 113(6): 917-28.
DOI: 10.1152/japplphysiol.00415.2012. |
| [16] |
Miura S, Kai Y, Tadaishi M, et al. Marked phenotypic differences of endurance performance and exercise-induced Oxygen consumption between AMPK and LKB1 deficiency in mouse skeletal muscle: changes occurring in the diaphragm[J].
Am J Physiol Endocrinol Metab, 2013, 305(2): E213-29.
DOI: 10.1152/ajpendo.00114.2013. |
| [17] |
Morales-Alamo D, Calbet JA. Free radicals and sprint exercise in humans[J].
Free Radic Res, 2014, 48(1): 30-42.
DOI: 10.3109/10715762.2013.825043. |
| [18] |
Dagon Y, Hur E, Zheng B, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake[J].
Cell Metab, 2012, 16(1): 104-12.
DOI: 10.1016/j.cmet.2012.05.010. |
| [19] |
Hawley SA, Ross FA, Gowans GJ, et al. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells[J].
Biochem J, 2014, 459(2): 275-87.
DOI: 10.1042/BJ20131344. |
| [20] |
Irving M. Regulation of contraction by the thick filaments in skeletal muscle[J].
Biophys J, 2017, 113(12): 2579-94.
DOI: 10.1016/j.bpj.2017.09.037. |
| [21] |
Gibb AA, Epstein PN, Uchida S, et al. Exercise-Induced changes in glucose metabolism promote physiological cardiac growth[J].
Circulation, 2017, 136(22): 2144-57.
DOI: 10.1161/CIRCULATIONAHA.117.028274. |
| [22] |
Short AK, Yeshurun S, Powell R, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety[J].
Transl Psychiatry, 2017, 7(5): e1114.
DOI: 10.1038/tp.2017.82. |
| [23] |
Brown B, Somauroo J, Green DJ, et al. The complex phenotype of the athlete's heart: implications for preparticipation screening[J].
Exerc Sport Sci Rev, 2017, 45(2): 96-104.
DOI: 10.1249/JES.0000000000000102. |
| [24] |
Gioscia-Ryan RA, Battson ML, Cuevas LM, et al. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice[J].
Aging (Albany NY), 2016, 8(11): 2897-914.
|
| [25] |
Carpentieri A, Gamberi T, Modesti A, et al. Profiling carbonylated proteins in heart and skeletal muscle mitochondria from trained and untrained mice[J].
J Proteome Res, 2016, 15(10): 3666-78.
DOI: 10.1021/acs.jproteome.6b00475. |
| [26] |
Chen PB, Yang JS, Park Y. Adaptations of skeletal muscle mitochondria to obesity, exercise, and polyunsaturated fatty acids[J].
Lipids, 2018, 53(3): 271-8.
DOI: 10.1002/lipd.2018.53.issue-3. |
| [27] |
Maeda A, Shirao T, Shirasaya D, et al. Piperine promotes glucose uptake through ROS-Dependent activation of the CAMKK/AMPK signaling pathway in skeletal muscle[J].
Mol Nutr Food Res, 2018, 62(11): e1800086.
DOI: 10.1002/mnfr.v62.11. |
| [28] |
Sadeghi A, Rostamirad A, Seyyedebrahimi S, et al. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production[J].
Inflammopharmacology, 2018, [Epub ahead of print].
|
| [29] |
Zhou T, Prather ER, Garrison DE, et al. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle[J].
Int J Mol Sci, 2018, 19(2): E417.
DOI: 10.3390/ijms19020417. |
| [30] |
Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources[J].
J Endocrinol, 2017, 233(1): R15-42.
DOI: 10.1530/JOE-16-0598. |
 2018, Vol. 38
2018, Vol. 38

