2. 吉林大学第二医院 外科,吉林 长春 130041
2. Department of General Surgery, Second Hospital of Jilin University, Changchun 130041, China
脊髓损伤是临床上常见的一种致残率较高的疾病,常常给社会家庭及个人带来沉重的负担,目前仍没有有效地治疗方法[1]。脊髓损伤包括原发性损伤和继发性损伤[2]。原发性损伤是不可逆不可控的,继发性损伤是可控的,因此如何有效的控制继发性损伤成为脊髓损伤治疗的焦点[3-5]。脂质过氧化是继发性脊髓损伤中的重要组成部分,在脊髓损伤的发生发展中起关键作用[6]。
淫羊藿苷为小檗科植物淫羊藿中的主要活性成分,具有抗炎[7]、抗氧化和抗凋亡[8]、抗肿瘤[9]、抗骨质疏松[10]、增强免疫功能[11]、改善心脑血管功能[12]等多种药理学作用。大量研究已经证实淫羊藿苷是一种有效的抗氧化剂,能够抑制多种脏器损伤中的脂质过氧化,发挥组织和细胞保护作用[13-15]。研究证实淫羊藿苷能够抑制中枢神经系统疾病中的脂质过氧化,发挥神经保护作用[16-17]。然而,淫羊藿苷能否抑制脊髓损伤后的脂质过氧化,发挥神经保护作用尚不清楚。为此本实验研究淫羊藿苷对大鼠脊髓损伤后脂质过氧化的影响,同时观察损伤脊髓组织超微结构改变及脊髓损伤大鼠的运动功能改变,探讨淫羊藿苷在脊髓损伤中的神经保护作用。
1 材料和方法 1.1 实验材料健康成年清洁级雄性SD大鼠,由吉林大学实验动物中心提供[许可证号为SCXK-(吉)2011-0003]。本实验所涉及的实验动物方案均按照吉林大学动物实验基本原则进行,并得到了吉林大学实验动物管理委员会的许可。淫羊藿苷购自南京泽朗医药有限公司,临用前溶于生理盐水中。MDA检测试剂盒和SOD检测试剂盒均购于南京建成生物工程研究所。LKB-5型超薄切片机购于瑞典LKB。JEM-1200EX透射电镜购于日本电子株式会社。
1.2 动物模型制备采用改良Allen's方法[18]制作脊髓重物打击损伤模型。健康成年SD大鼠称重后,1%戊巴比妥钠(40 mg/kg)腹腔麻醉,麻醉起效后将大鼠俯卧并固定于鼠板手术台上,剪掉颈背部鼠毛,严格按照无菌手术要求,用10%强力碘消毒,铺无菌洞巾。以T10棘突为中心取背部后正中纵行切口,长约5 cm,逐层进入,依次切开皮肤、皮下组织,剥离椎旁肌肉,显露棘突及椎板。咬除T10棘突及全椎板至椎弓根部,部分咬除T9和T11椎板扩大椎管,暴露待损伤脊髓长约20 mm。用自制的改良Allen打击装置以50 g/cm(冲击量为5 g重物从10 cm高度自由落下)致伤能量造成大鼠脊髓损伤。撞击成功标准为:损伤处脊髓外观出血、水肿,大鼠尾巴痉挛性摆动、双下肢及躯体回缩扑动后,双下肢瘫。脊髓损伤成功后,逐层缝合切口。
1.3 动物分组及给药健康成年清洁级雄性SD大鼠72只,体质量280±20 g,随机数字表法分为3组:淫羊藿苷(ICA)组:椎板切除后,进行重物打击,造成脊髓损伤后立即给予淫羊藿苷(100 mg/kg)灌胃,1次/d,连续灌胃。对照(Control)组:椎板切除后,进行重物打击,造成脊髓损伤后立刻给予等量的生理盐水灌胃,1次/d,连续灌胃。假手术(Sham)组:仅仅行椎板切除,不进行重物打击,无脊髓损伤,给予等量的生理盐水灌胃,1次/d,连续灌胃。自然光照周期,自由进食粉状饲料及饮用自来水,室温25~27 ℃。精心饲养,不限饮水及进食,人工膀胱排尿3次/d,直至形成反射性膀胱。本研究的淫羊藿苷的用量参照以前的实验研究[19-20]。
1.4 MDA和SOD的测定术后24 h各组取6只大鼠麻醉后处死,取损伤段脊髓,以损伤点为中心切取脊髓组织10 mm,称质量后制备脊髓组织匀浆。采用硫代巴比妥酸法,根据MDA检测试剂盒说明方法测定MDA含量(nmol/mg);采用黄嘌呤氧化酶法,根据超氧化物歧化酶检测试剂盒说明方法测定SOD活性(U/mg)。
1.5 脊髓水肿的评价用干湿重法[21]测定脊髓组织的含水量,评估脊髓组织水肿程度。术后24 h各组分别取6只大鼠麻醉后处死,取损伤段脊髓,以损伤点为中心低温切取10 mm脊髓组织冻存,称湿重(W);然后于80 ℃烤箱干燥48 h,称干重(D);重复3次后,取均值,按以下公式计算脊髓组织含水量:[(W-D)/W]×100%,数值大小与脊髓水肿程度成正相关。
1.6 超微结构观察及超微结构评分术后48 h各组分别取6只大鼠麻醉后处死,按透射电镜标本制作要求分步骤进行标本处理后,用LKB-5型超薄切片机切片,片厚50~60 nm,用枸橼酸铅电子进行染色,JEM-1200EX透射电镜观察。采用Kaptanoglu评分法[22]在5000倍放大率下,对电镜标本进行超微结构评分,每个样本分别评估20个神经细胞、20个轴突、20个线粒体的超微结构改变,计算各组细胞质内水肿、细胞核损伤、轴突变性、髓鞘分解和线粒体损伤的评分。数值大小与脊髓损伤严重程度成正相关。由熟悉该评分标准的2名实验人员进行双盲法评分。
1.7 运动功能评价各组分别取6只大鼠,于术后7、14、21、28 d,进行BBB运动评分[23]。BBB运动评分是将动物放入一固定场地,观察动物的臀、膝、踝关节、行走步态、躯干运动及其协调情况,根据BBB运动评分法标准,观察期为5 min,动物要保持在中心活动范围内。由熟悉该评分标准的2名实验人员进行双盲法评分。
1.8 统计学处理所有数据以均数±标准差表示,采用SPSS 20.0统计软件进行分析,多组独立样本比较采用单因素方差分析,两两比较采用LSD检验,P < 0.05为差异有统计学意义。
2 结果本实验过程中,72只SD大鼠全部存活,淫羊藿苷组、对照组和假手术组各24只,均纳入结果分析中。
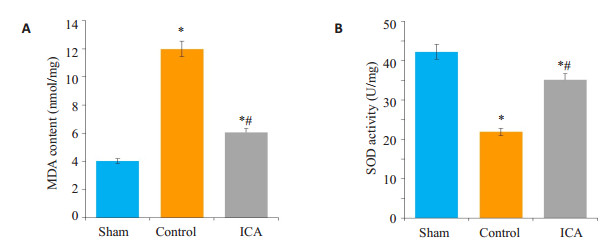
2.1 MDA含量和SOD活性变化对照组和淫羊藿苷组脊髓组织中MDA的含量高于假手术组,差异有统计学意义(P < 0.05),SOD的活性低于假手术组,差异有统计学意义(P < 0.05);淫羊藿苷组脊髓组织中MDA的含量低于对照组,差异有统计学意义(P < 0.05),SOD活性高于对照组,差异有统计学意义(P < 0.05,图 1)。
|
图 1 各组大鼠MDA的含量和SOD的活性比较 Figure 1 Comparison of MDA content and SOD activity among the 3 groups. *P < 0.05 vs sham group; #P < 0.05 vs control group. A: MDA Content; B: SOD activity. |
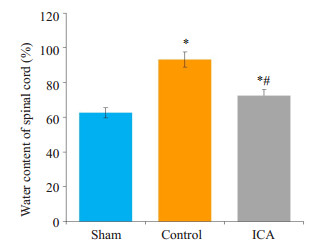
对照组和淫羊藿苷组脊髓组织含水量高于假手术组,差异有统计学意义(P < 0.05);淫羊藿苷组脊髓组织含水量低于对照组,差异有统计学意义(P < 0.05,图 2)。
|
图 2 各组大鼠脊髓组织含水量比较 Figure 2 Comparison of water content in the spinal cord among the 3 groups. *P < 0.05 vs sham group; #P < 0.05 vs control group. |
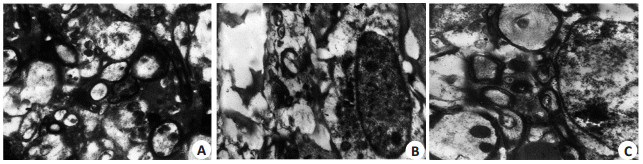
假手术组:脊髓组织无出血、水肿,脊髓灰质和白质呈现正常的超微结构,神经细胞细胞膜完整、线粒体结构正常(图 3A)。对照组:脊髓组织明显出血、水肿;神经细胞细胞核和胞质细胞器显著变性。有髓神经纤维出现髓鞘分裂和轴突细胞器变性(图 3B)。毛细血管周围水肿,毛细血管内皮细胞细胞器变性。淫羊藿苷组:脊髓组织局部偶可见出血、水肿,较对照组程度轻;神经细胞核核膜完整,染色质密集,部分有髓神经纤维髓鞘板层部分分离,大多数神经纤维呈现正常的超微结构(图 3C)。部分毛细血管周围水肿,但毛细血管内皮细胞结构正常。
|
图 3 各组大鼠超微结构的改变 Figure 3 Ultrastructural changes of rats in each group (Original magnification: × 5000). A: Sham group; B: Control group; C: ICA group. |
假手术组超微结构评分为0。对照组的超微结构评分为9.86±0.42,淫羊藿苷组的超微结构评分为4.24±0.21,对照组和淫羊藿苷组的超微结构评分较假手术组明显升高,差异有统计学意义(P < 0.05);淫羊藿苷组的超微结构评分均较对照组明显降低,差异有统计学意义(P < 0.05)。
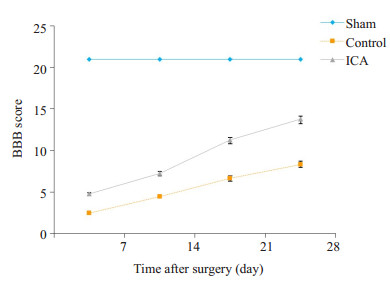
2.4 运动功能的评价-BBB评分对照组和淫羊藿苷组各时间点大鼠BBB评分较假手术组明显降低,差异有统计学意义(P < 0.05),淫羊藿苷组各时间点大鼠BBB评分较对照组明显升高,差异有统计学意义(P < 0.05,图 4)。
|
图 4 各组大鼠BBB评分比较 Figure 4 Comparison of BBB scores among the 3 groups. *P < 0.05 vs sham group; #P < 0.05 vs control group. |
脊髓损伤是一个复杂的病理生理过程,分为原发性损伤和继发性损伤[2]。原发性损伤是指脊髓受到暴力破坏后,神经细胞破碎、轴索断裂,细胞质膜破裂,内溶酶释放致细胞直接死亡。继发性损伤是在原发性损伤基础上发生的多种因素参与的序列性组织自毁性破坏的过程。原发性损伤是不可控的,而继发性损伤是可控的,因此,如何有效控制继发性脊髓损伤已成为脊髓损伤治疗的重点和关键点[24-26]。脊髓损伤急性期产生的过量的氧自由基引发的自由基损伤和脂质过氧化在继发性损伤中起关键作用[27-29]。自由基对细胞膜双磷脂结构进行过氧化作用,生成多种脂质过氧化物,损伤细胞膜,并引起溶酶体及线粒体的破裂。由于脂质过氧化物很难检测,目前通常通过检测脂质过氧化的终产物MDA来间接反应机体细胞受自由基攻击的严重程度。SOD是机体重要的抗氧化酶,是机体清除自由基的首要物质,可对抗与阻断氧自由基对细胞的损害,修复受损细胞,复原因自由基造成的对细胞伤害。SOD在生物体内的水平反映了机体清除自由基的抗氧化能力。机体内SOD水平与MDA水平负相关,因此二者结合起来更能精确反映机体的自由基损伤和脂质过氧化反应。在大鼠脊髓损伤模型中研究发现,脊髓损伤导致MDA水平显著升高,SOD、GSH活性显著下降,证实脊髓损伤诱发氧自由基的过度生成及脂质过氧化,下调机体的抗氧化酶活性[30-32]。本实验结果显示对照组和淫羊藿苷组脊髓组织中MDA含量显著高于假手术组(P < 0.05),SOD活性显著低于假手术组(P < 0.05),表明脊髓损伤诱发了氧自由基过度生成和脂质过氧化,下调了机体抗氧化酶活性,降低了机体抗氧化能力。对照组和淫羊藿苷组脊髓组织含水量、超微结构评分和大鼠BBB评分显著高于假手术组(P < 0.05);表明脊髓损伤诱发了脊髓水肿、脊髓超微结构损伤和大鼠的运动功能障碍。
淫羊藿苷药理学研究表明,淫羊藿苷具有抗炎、抗氧化、抗凋亡、抗肿瘤、增强免疫功能等多种药理学作用[7-9]。大量文献已经证实淫羊藿苷是一种有效的抗氧化剂,能够提高机体的抗氧化能力,抑制氧化应激,提高SOD活性,降低MDA水平,发挥组织和细胞保护作用[13-15]。研究表明淫羊藿苷能够抑制中枢神经系统疾病中的脂质过氧化,发挥神经保护作用[16-17]。Luo等[16]在铝诱导的大鼠学习记忆障碍模型中发现,淫羊藿苷能够提高海马SOD活性,降低MDA含量,减少β淀粉样蛋白肽Abeta (1-40)含量,提高大鼠海马的抗氧化能力,降低脂质过氧化,改善铝中毒大鼠的空间学习和记忆能力,发挥神经保护作用。He等[17]在快速老化模型小鼠SAMP8中证实,淫羊藿苷能够提高的SOD和谷胱甘肽过氧化物酶(GSH-Px)的活性,降低MDA的含量,降低一氧化氮合酶活性和一氧化氮含量,同时,淫羊藿苷抑制乙酰胆碱酯酶的活性,抑制氧化损伤,降低乙酰胆碱酯酶活性,增加单胺水平,改善SAMP8小鼠的认知障碍,发挥神经保护作用。本实验结果显示淫羊藿苷组脊髓组织中SOD的活性显著高于对照组(P < 0.05),MDA的含量显著低于对照组(P < 0.05),表明淫羊藿苷增强了机体的抗氧化酶活性,提高了机体抗氧化能力,抑制了脊髓损伤诱发的氧自由基过度生成和脂质过氧化。淫羊藿苷组脊髓组织含水量、超微结构评分、大鼠BBB评分显著低于对照组(P < 0.05)。表明淫羊藿苷能够明显减轻脊髓水肿、脊髓的组织病理学损伤,改善脊髓损伤大鼠的运动功能,有效保护脊髓组织,具有明显的神经保护作用。
综上所述,本研究结果揭示了淫羊藿苷能够提高机体抗氧化酶SOD的活性,减少脊髓组织中的MDA含量,提高了机体抗氧化能力,抑制脊髓损伤诱发的氧自由基过度生成和脂质过氧化,减轻氧自由基对神经细胞的损伤,减轻脊髓水肿和脊髓的组织病理学损伤,增强脊髓组织对缺血的抗损伤能力,促进脊髓损伤大鼠的运动功能的恢复,有效地保护脊髓组织,具有良好的神经保护作用。本实验为淫羊藿苷临床治疗脊髓损伤提供了理论基础,但其对脊髓损伤的神经保护作用仍需进一步的探讨。
| [1] |
Xun C, Mamat M, Guo H, et al. Tocotrienol alleviates inflammation and oxidative stress in a rat model of spinal cord injury via suppression of transforming growth factor-β[J].
Exp Ther Med, 2017, 14(1): 431-8.
DOI: 10.3892/etm.2017.4505. |
| [2] |
Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury[J].
Spine J, 2004, 4(4): 451-64.
DOI: 10.1016/j.spinee.2003.07.007. |
| [3] |
Lim PA, Tow AM. Recovery and regeneration after spinal cord injury: a review and summary of recent literature[J].
Ann Acad Med Singapore, 2007, 36(1): 49-57.
|
| [4] |
Tederko P, Krasuski M, Kiwerski J, et al. Strategies for neuroprotection following spinal cord injury[J].
Ortop Traumatol Rehabil, 2009, 11(2): 103-10.
|
| [5] |
Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods[J].
Neurosci Lett, 2017, 652: 3-10.
DOI: 10.1016/j.neulet.2016.12.004. |
| [6] |
Hall ED, Wang JA, Bosken JM, et al. Lipid peroxidation in brain or spinal cord mitochondria after injury[J].
J Bioenerg Biomembr, 2016, 48(2): 169-74.
DOI: 10.1007/s10863-015-9600-5. |
| [7] |
Sun X, Deng X, Cai W, et al. Icariin inhibits LPS-induced cell inflammatory response by promoting GRα nuclear translocation and upregulating GRα expression[J].
Life Sci, 2018, 195: 33-43.
DOI: 10.1016/j.lfs.2018.01.006. |
| [8] |
Song YH, Cai H, Zhao ZM, et al. Icariin attenuated oxidative stress induced-cardiac apoptosis by mitochondria protection and ERK activation[J].
Biomed Pharmacother, 2016, 83: 1089-94.
DOI: 10.1016/j.biopha.2016.08.016. |
| [9] |
Tan HL, Chan KG, Pusparajah P, et al. Anti-cancer properties of the naturally occurring aphrodisiacs: icariin and its derivatives[J].
Front Pharmacol, 2016, 7: 191, 1-18.
|
| [10] |
Liu Y, Zuo H, Liu X, et al. The antiosteoporosis effect of icariin in ovariectomized rats: a systematic review and meta-analysis[J].
Cell Mol Biol (Noisy-le-grand), 2017, 63(11): 124-31.
DOI: 10.14715/cmb/2017.63.11.22. |
| [11] |
Shen R, Deng W, Li C, et al. A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis[J].
Int Immunopharmacol, 2015, 24(2): 224-31.
DOI: 10.1016/j.intimp.2014.12.015. |
| [12] |
Meng X, Pei H, Lan C. Icariin exerts protective effect against myocardial ischemia/reperfusion injury in rats[J].
Cell Biochem Biophys, 2015, 73(1): 229-35.
DOI: 10.1007/s12013-015-0669-6. |
| [13] |
Xiong W, Zhang W, Yuan W, et al. Phosphorylation of icariin can alleviate the oxidative stress caused by the duck hepatitis virus a through Mitogen-Activated protein kinases signaling pathways[J].
Front Microbiol, 2017, 8: 1850, 1-13.
|
| [14] |
Ke Z, Liu J, Xu P, et al. The cardioprotective effect of icariin on Ischemia-Reperfusion injury in isolated rat heart: potential involvement of the PI3K-Akt signaling pathway[J].
Cardiovasc Ther, 2015, 33(3): 134-40.
DOI: 10.1111/cdr.2015.33.issue-3. |
| [15] |
Hu Y, Sun B, Liu K, et al. Icariin attenuates high-cholesterol Diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway[J].
Inflammation, 2016, 39(1): 228-36.
DOI: 10.1007/s10753-015-0242-x. |
| [16] |
Luo Y, Nie J, Gong QH, et al. Protective effects of icariin against learning and memory deficits induced by aluminium in rats[J].
Clin Exp Pharmacol Physiol, 2007, 34(8): 792-5.
DOI: 10.1111/cep.2007.34.issue-8. |
| [17] |
He XL, Zhou WQ, Bi MG, et al. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice[J].
Brain Res, 2010, 1334: 73-83.
DOI: 10.1016/j.brainres.2010.03.084. |
| [18] |
Jiang JL, Guo XD, Zhang SQ, et al. Repetitive magnetic stimulation affects the microenvironment of nerve regeneration and evoked potentials after spinal cord injury[J].
Neural Regen Res, 2016, 11(5): 816-22.
DOI: 10.4103/1673-5374.182710. |
| [19] |
Li L, Sun J, Xu C, et al. Icariin ameliorates cigarette smoke induced inflammatory responses via suppression of NF-κB and modulation of GR in vivo and in vitro[J].
PLoS One, 2014, 9(8): e102345, 1-9.
|
| [20] |
Chen M, Hao J, Yang Q, et al. Effects of icariin on reproductive functions in male rats[J].
Molecules, 2014, 19(7): 9502-14.
DOI: 10.3390/molecules19079502. |
| [21] |
Hu J, Yang Z, Li X, et al. C-C motif chemokine ligand 20 regulates neuroinflammation following spinal cord injury via Th17 cell recruitment[J].
J Neuroinflammation, 2016, 13(1): 162, 1-14.
|
| [22] |
Gokce EC, Kahveci R, Gokce A, et al. Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord Ischemia-Reperfusion injury in rats[J].
J Stroke Cerebrovasc Dis, 2016, 25(5): 1196-207.
DOI: 10.1016/j.jstrokecerebrovasdis.2016.01.008. |
| [23] |
Tian DS, Jing JH, Qian J, et al. Effect of oscillating electrical field stimulation on motor function recovery and myelin regeneration after spinal cord injury in rats[J].
J Phys Ther Sci, 2016, 28(5): 1465-71.
DOI: 10.1589/jpts.28.1465. |
| [24] |
Li R, Shang J, Zhou W, et al. Overexpression of HIPK2 attenuates spinal cord injury in rats by modulating apoptosis, oxidative stress, and inflammation[J].
Biomed Pharmacother, 2018, 103(5): 127-34.
|
| [25] |
Yang W, Yang Y, Yang JY, et al. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway[J].
Int J Mol Med, 2016, 37(4): 1075-82.
DOI: 10.3892/ijmm.2016.2498. |
| [26] |
Song HL, Zhang X, Wang WZ, et al. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and antiinflammation and inhibition of p38 mitogen activated protein kinase pathway[J].
Neural Regen Res, 2018, 13(1): 128-34.
DOI: 10.4103/1673-5374.217349. |
| [27] |
Wang X, Wu X, Liu Q, et al. Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats[J].
Neuroscience, 2017, 366: 36-43.
DOI: 10.1016/j.neuroscience.2017.09.056. |
| [28] |
Fu S, Lv R, Wang L, et al. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway[J].
Saudi J Biol Sci, 2018, 25(2): 259-66.
DOI: 10.1016/j.sjbs.2016.10.019. |
| [29] |
Hu W, Wang H, Liu Z, et al. Neuroprotective effects of lycopene in spinal cord injury in rats via antioxidative and anti-apoptotic pathway[J].
Neurosci Lett, 2017, 642: 107-12.
DOI: 10.1016/j.neulet.2017.02.004. |
| [30] |
Zhou KL, Chen DH, Jin HM, et al. Effects of calcitriol on experimental spinal cord injury in rats[J].
Spinal Cord, 2016, 54(7): 510-6.
DOI: 10.1038/sc.2015.217. |
| [31] |
Cong L, Chen W. Neuroprotective Effect of ginsenoside Rd in spinal cord injury rats[J].
Basic Clin Pharmacol Toxicol, 2016, 119(2): 193-201.
DOI: 10.1111/bcpt.2016.119.issue-2. |
| [32] |
Karatas Y, Cengiz SL, Esen H, et al. Effect of carvedilol on secondary damage in experimental spinal cord injury in rats[J].
Turk Neurosurg, 2015, 25(6): 930-5.
|
 2018, Vol. 38
2018, Vol. 38
